Process Optimization by a Response Surface Methodology for Adsorption of Congo Red Dye onto Exfoliated Graphite-Decorated MnFe2O4 Nanocomposite: The Pivotal Role of Surface Chemistry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Instruments
2.2. Synthesis of EG
2.3. Synthesis of MnFe2O4
2.4. Synthesis of EG@MnFe2O4
2.5. Experimental Batch
2.6. Experimental Design with RSM
3. Results
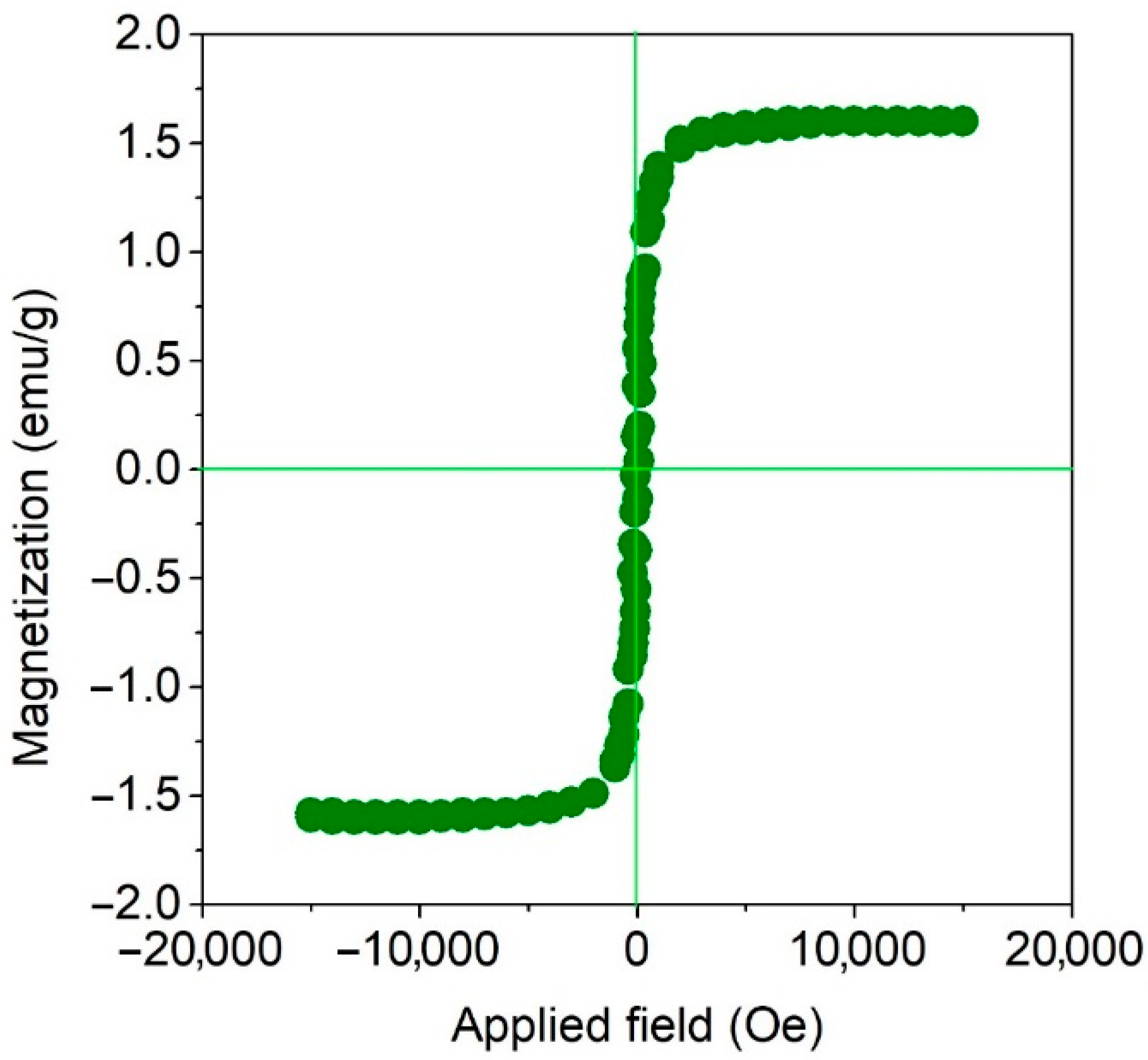
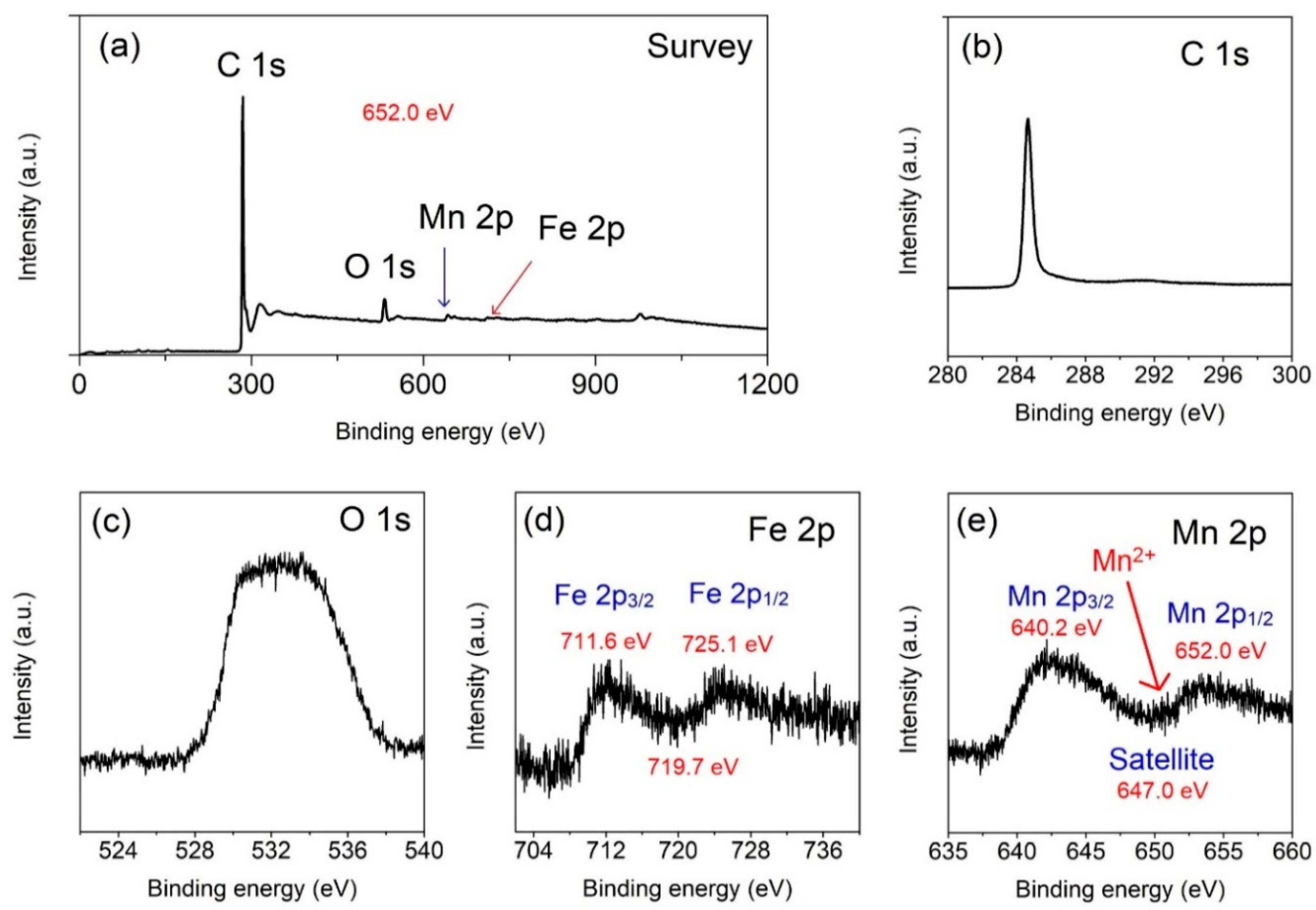
3.1. Structural Characterization
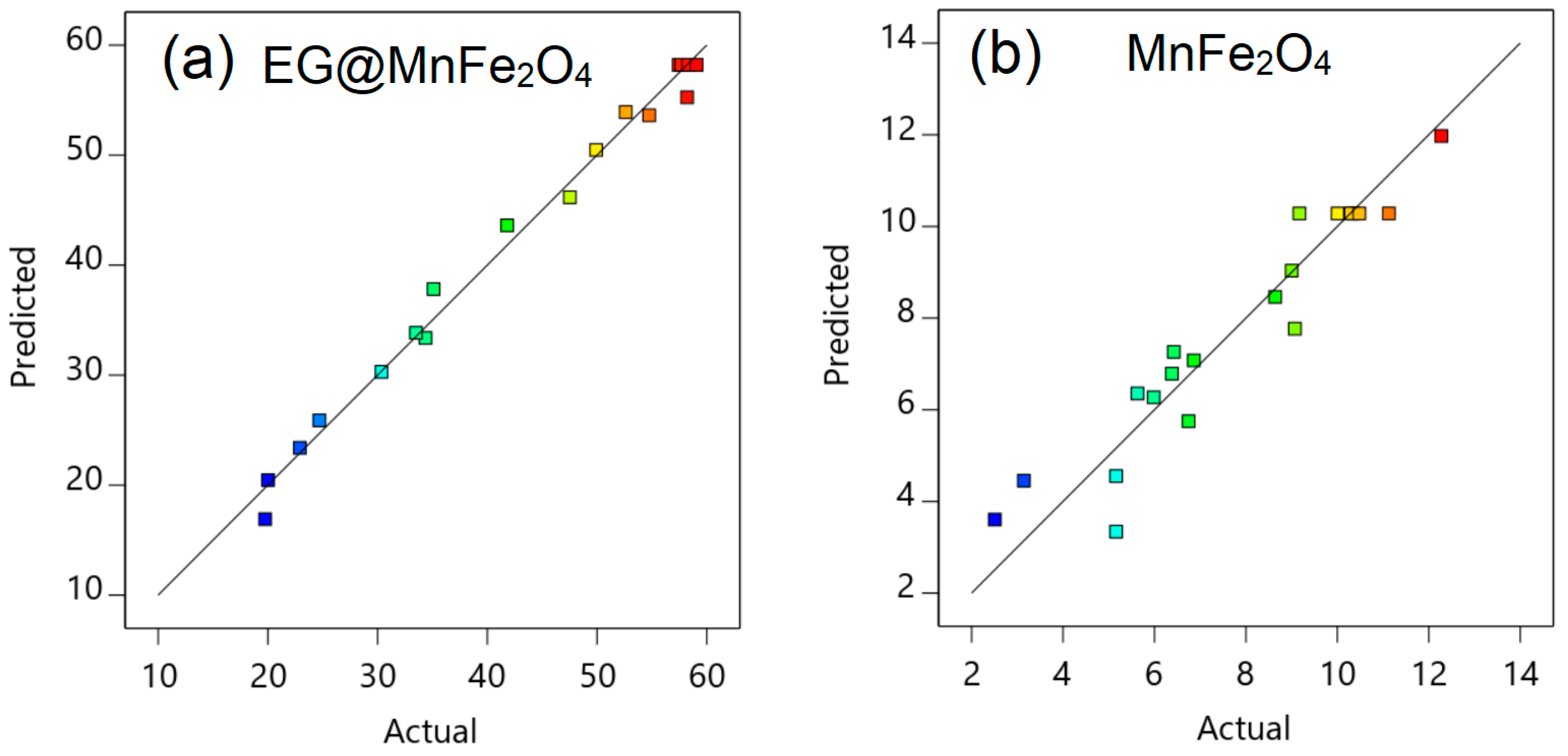
3.2. Optimization with RSM
3.3. Proposed Mechanism
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Forgacs, E.; Cserhati, T.; Oros, G. Removal of synthetic dyes from wastewaters: A review. Environ. Int. 2004, 30, 953–971. [Google Scholar] [CrossRef]
- Ali, H. Biodegradation of synthetic dyes—A review. Water Air Soil Pollut. 2010, 213, 251–273. [Google Scholar] [CrossRef]
- Liu, W.; Liu, L.; Liu, C.; Hao, Y.; Yang, H.; Yuan, B.; Jiang, J. Methylene blue enhances the anaerobic decolorization and detoxication of azo dye by Shewanella onediensis MR-1. Biochem. Eng. J. 2016, 110, 115–124. [Google Scholar] [CrossRef]
- Zhang, Q.; Xie, X.; Liu, Y.; Zheng, X.; Wang, Y.; Cong, J.; Yu, C.; Liu, N.; Liu, J.; Sand, W. Fructose as an Additional Co-Metabolite Promotes Refractory Dye Degradation: Performance and Mechanism. Bioresour. Technol. 2019, 280, 430–440. [Google Scholar] [CrossRef]
- Raj, R.A.; Manimozhi, V.; Saravanathamizhan, R. Adsorption studies on removal of Congo red dye from aqueous solution using petroleum coke. Pet. Sci. Technol. 2019, 37, 913–924. [Google Scholar] [CrossRef]
- Si, Y.; Samulski, E.T. Exfoliated graphene separated by platinum nanoparticles. Chem. Mater. 2008, 20, 6792–6797. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon N. Y. 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Wang, H.; Robinson, J.T.; Li, X.; Dai, H. Solvothermal reduction of chemically exfoliated graphene sheets. J. Am. Chem. Soc. 2009, 131, 9910–9911. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.-H.; Zheng, X.; Lv, W.; Wang, M.; Yang, Q.-H.; Kang, F. Adsorption of Lead(II) Ions from Aqueous Solution on Low-Temperature Exfoliated Graphene Nanosheets. Langmuir 2011, 27, 7558–7562. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, Z.H.; Coomes, A.; Haghseresht, F.; Lu, G.Q. The physical and surface chemical characteristics of activated carbons and the adsorption of methylene blue from wastewater. J. Colloid Interface Sci. 2005, 284, 440–446. [Google Scholar] [CrossRef]
- Zeng, H.; Rice, P.M.; Wang, S.X.; Sun, S. Shape-controlled synthesis and shape-induced texture of MnFe2O4 nanoparticles. J. Am. Chem. Soc. 2004, 126, 11458–11459. [Google Scholar] [CrossRef]
- Hu, J.; Lo, I.M.C.; Chen, G. Fast removal and recovery of Cr, VI, using surface-modified jacobsite, MnFe2O4, nanoparticles. Langmuir 2005, 213, 11173–11179. [Google Scholar] [CrossRef]
- Tran, T.V.; Nguyen, U.T.T.; Nguyen, T.T.; Hoang, B.N.; Tran, H.T.; Nguyen, N.P.T.; Ho, V.T.T.; Nguyen, M.T.; Bach, L.G.; Nguyen, T.D. Synthesis and magnetic properties of graphene oxide-decorated cobalt, manganese and nickel ferrite nanoparticles prepared by polymerized route. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Nice, France, 22–24 February 2019; IOP Publishing: Bristol, UK, 2019; p. 12114. [Google Scholar]
- Bach, L.G.; van Tran, T.; Nguyen, T.D.; van Pham, T.; Do, S.T. Enhanced adsorption of methylene blue onto graphene oxide-doped XFe2O4, X = Co, Mn, Ni, nanocomposites: Kinetic, isothermal, thermodynamic and recyclability studies. Res. Chem. Intermed. 2018, 44, 1661–1687. [Google Scholar] [CrossRef]
- Nguyen, D.T.C. Metal-Organic Framework MIL-53(Fe, as an Adsorbent for Ibuprofen Drug Removal from Aqueous Solutions: Response Surface Modeling and Optimization. J. Chem. 2019. [Google Scholar] [CrossRef]
- Shao, L.; Ren, Z.; Zhang, G.; Chen, L. Facile synthesis, characterization of a MnFe2O4/activated carbon magnetic composite and its effectiveness in tetracycline removal. Mater. Chem. Phys. 2012, 135, 16–24. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Y.; Tan, Q.; Zhong, Z.; Su, F. Facile solvothermal synthesis of mesoporous manganese ferrite, MnFe2O4, microspheres as anode materials for lithium-ion batteries. J. Colloid Interface Sci. 2013, 398, 185–192. [Google Scholar] [CrossRef]
- Han, A.; Liao, J.; Ye, M.; Li, Y.; Peng, X. Preparation of Nano-MnFe2O4 and Its Catalytic Performance of Thermal Decomposition of Ammonium Perchlorate. Chin. J. Chem. Eng. 2011, 19, 1047–1051. [Google Scholar] [CrossRef]
- Şimşek, T.; Akansel, S.; Özcan, Ş.; Ceylan, A. Synthesis of MnFe2O4 nanocrystals by wet-milling under atmospheric conditions. Ceram. Int. 2014, 40, 7953–7956. [Google Scholar] [CrossRef]
- Aslibeiki, B.; Kameli, P.; Ehsani, M.H.; Salamati, H.; Muscas, G.; Agostinelli, E.; Foglietti, V.; Casciardi, S.; Peddis, D. Solvothermal synthesis of MnFe2O4 nanoparticles: The role of polymer coating on morphology and magnetic properties. J. Magn. Magn. Mater. 2016, 399, 236–244. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, Y.; Kang, Z. A low temperature synthesis of MnFe2O4 nanocrystals by microwave-assisted ball-milling. Chem. Eng. J. 2013, 215–216, 235–239. [Google Scholar] [CrossRef]
- Yao, Y.; Cai, Y.; Lu, F.; Wei, F.; Wang, X.; Wang, S. Magnetic recoverable MnFe2O4 and MnFe2O4-graphene hybrid as heterogeneous catalysts of peroxymonosulfate activation for efficient degradation of aqueous organic pollutants. J. Hazard. Mater. 2014, 270, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, N.U.; Bergamasco, R.; Hamoudi, S. Magnetic MnFe2O4-graphene hybrid composite for efficient removal of glyphosate from water. Chem. Eng. J. 2016, 295, 391–402. [Google Scholar] [CrossRef]
- Li, S.; Wang, B.; Li, B.; Liu, J.; Yu, M.; Wu, X. Self-assembly of 2D sandwich-structured MnFe2O4/graphene composites for high-performance lithium storage. Mater. Res. Bull. 2015, 61, 369–374. [Google Scholar] [CrossRef]
- Pham, M.C.; Piro, B.; Bazzaoui, E.A.; Hedayatullah, M.; Lacroix, J.-C.; Novák, P.; Haas, O. Anodic oxidation of 5-amino-1, 4-naphthoquinone, ANQ, and synthesis of a conducting polymer, PANQ). Synth. Met. 1998, 92, 197–205. [Google Scholar] [CrossRef]
- Tran, T.V.; Le, H.T.N.; Ha, H.Q.; Duong, X.N.T.; Nguyen, L.H.-T.; Doan, T.L.H.; Nguyen, H.L.; Truong, T. A five coordination Cu(ii) cluster-based MOF and its application in the synthesis of pharmaceuticals via sp3 C-H/N-H oxidative coupling. Catal. Sci. Technol. 2017, 7, 3453–3458. [Google Scholar] [CrossRef]
- Gharib, M.; Safarifard, V.; Morsali, A. Ultrasound assisted synthesis of amide functionalized metal-organic framework for nitroaromatic sensing. Ultrason. Sonochem. 2018, 42, 112–118. [Google Scholar] [CrossRef]
- Tran, V.T.; Nguyen, D.T.; Ho, V.T.T.; Hoang, P.Q.H.; Bui, P.Q.; Bach, L.G. Efficient removal of Ni 2 ions from aqueous solution using activated carbons fabricated from rice straw and tea waste. J. Mater. Environ. Sci. 2017, 8, 426–437. [Google Scholar]
- Le, H.T.N.; Tran, T.V.; Phan, N.T.S.; Truong, T. Efficient and recyclable Cu2(BDC)2(BPY)-catalyzed oxidative amidation of terminal alkynes: Role of bipyridine ligand. Catal. Sci. Technol. 2015, 5, 851–859. [Google Scholar] [CrossRef]
- Bodirlau, R.; Teaca, C.A. Fourier transform infrared spectroscopy and thermal analysis of lignocellulose fillers treated with organic anhydrides. Rom. J. Phys. 2009, 54, 93–104. [Google Scholar]
- Jia, X.-J.; Wang, J.; Wu, J.; Du, Y.; Zhao, B.; den Engelsen, D. Bouquet-like calcium sulfate dihydrate: A highly efficient adsorbent for Congo red dye. RSC Adv. 2015, 5, 72321–72330. [Google Scholar] [CrossRef]
- Mittal, A.; Thakur, V.; Mittal, J.; Vardhan, H. Process development for the removal of hazardous anionic azo dye Congo red from wastewater by using hen feather as potential adsorbent. Desalin. Water Treat. 2014, 52, 227–237. [Google Scholar] [CrossRef]
- Van Tran, T.; Nguyen, D.T.C.; Le, H.T.N.; Bach, L.G.; Vo, D.-V.N.; Hong, S.S.; Phan, T.-Q.T.; Nguyen, T.D. Tunable Synthesis of Mesoporous Carbons from Fe3O(BDC)3 for Chloramphenicol Antibiotic Remediation. Nanomaterials 2019, 9, 237. [Google Scholar] [CrossRef]
- Guedidi, H.; Reinert, L.; Lévêque, J.-M.; Soneda, Y.; Bellakhal, N.; Duclaux, L. The effects of the surface oxidation of activated carbon, the solution pH and the temperature on adsorption of ibuprofen. Carbon N. Y. 2013, 54, 432–443. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Wang, H.; Yao, Q.; Wang, C.; Fan, B.; Sun, Q.; Jin, C.; Xiong, Y.; Chen, Y. A simple, one-step hydrothermal approach to durable and robust superparamagnetic, superhydrophobic and electromagnetic wave-absorbing wood. Sci. Rep. 2016, 6, 35549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Tran, T.; Nguyen, D.T.C.; Le, H.T.N.; Nguyen, O.T.K.; Nguyen, V.H.; Nguyen, T.T.; Bach, L.G.; Nguyen, T.D. A hollow mesoporous carbon from metal-organic framework for robust adsorbability of ibuprofen drug in water. R. Soc. Open Sci. 2019, 6, 190058. [Google Scholar] [CrossRef] [Green Version]
- Goertzen, S.L.; Thériault, K.D.; Oickle, A.M.; Tarasuk, A.C.; Andreas, H.A. Standardization of the Boehm titration. Part I. CO2 expulsion and endpoint determination. Carbon N. Y. 2010, 48, 1252–1261. [Google Scholar] [CrossRef]
- Van Tran, T.; Nguyen, D.T.C.; Le, H.T.N.; Duong, C.D.; Bach, L.G.; Nguyen, H.-T.T.; Nguyen, T.D. Facile synthesis of manganese oxide-embedded mesoporous carbons and their adsorbability towards methylene blue. Chemosphere 2019, 227, 455–461. [Google Scholar] [CrossRef]
- Van Tran, T.; Bui, Q.T.P.; Nguyen, T.D.; Le, N.T.H.; Bach, L.G. A comparative study on the removal efficiency of metal ions, Cu2+, Ni2+, and Pb2+, using sugarcane bagasse-derived ZnCl2-activated carbon by the response surface methodology. Adsorpt. Sci. Technol. 2017, 35, 72–85. [Google Scholar] [CrossRef]
- Van Thuan, T.; Quynh, B.T.P.; Nguyen, T.D.; Ho, V.T.T.; Bach, L.G. Response surface methodology approach for optimization of Cu2+, Ni2+, and Pb2+ adsorption using KOH-activated carbon from banana peel. Surf. Interfaces 2017, 6, 209–217. [Google Scholar] [CrossRef]
- Van Tran, T.; Bui, Q.T.P.; Nguyen, T.D.; Ho, V.T.T.; Bach, L.G. Application of response surface methodology to optimize the fabrication of ZnCl2-activated carbon from sugarcane bagasse for the removal of Cu2+. Water Sci. Technol. 2017, 75, 2047–2055. [Google Scholar] [CrossRef] [PubMed]
- Hadi, A.; Karimi-Sabet, J.; Moosavian, S.M.A.; Ghorbanian, S. Optimization of graphene production by exfoliation of graphite in supercritical ethanol: A response surface methodology approach. J. Supercrit. Fluids. 2016, 107, 92–105. [Google Scholar] [CrossRef]
- Ghasemi, F.A.; Ghasemi, I.; Menbari, S.; Ayaz, M.; Ashori, A. Optimization of mechanical properties of polypropylene/talc/graphene composites using response surface methodology. Polym. Test. 2016, 53, 283–292. [Google Scholar] [CrossRef]
- Ölmez, T. The optimization of Cr(VI) reduction and removal by electrocoagulation using response surface methodology. J. Hazard. Mater. 2009, 162, 1371–1378. [Google Scholar] [CrossRef]
- Grace, M.N.; Wilson, G.M.; Leslie, P.F. Statistical testing of input factors in the carbonation of brine impacted fly ash. J. Environ. Sci. Heal. Part A 2012, 47, 245–259. [Google Scholar] [CrossRef] [Green Version]
- Van Tran, T.; Nguyen, D.T.C.; Le, H.T.N.; Bach, L.G.; Vo, D.-V.N.; Kwon, L.T.; Nong, L.X.; Nguyen, T.D. Combined Minimum-Run Resolution IV and Central Composite Design for Optimized Removal of the Tetracycline Drug Over Metal–Organic Framework-Templated Porous Carbon. Molecules 2019, 24, 1887. [Google Scholar] [CrossRef]
- Ilaiyaraja, N.; Likhith, K.R.; Babu, G.R.S.; Khanum, F. Optimisation of extraction of bioactive compounds from Feronia limonia, wood apple, fruit using response surface methodology (RSM). Food Chem. 2015, 173, 348–354. [Google Scholar] [CrossRef]
- Van Tran, T.; Nguyen, D.T.C.; Le, H.T.N.; Tu, T.T.K.; Le, N.D.; Lim, K.T.; Bach, L.G.; Nguyen, T.D. MIL-53(Fe)-directed synthesis of hierarchically mesoporous carbon and its utilization for ciprofloxacin antibiotic remediation. J. Environ. Chem. Eng. 2019, 7, 102881. [Google Scholar] [CrossRef]
- Pavan, F.A.; Dias, S.L.P.; Lima, E.C.; Benvenutti, E.V. Removal of Congo red from aqueous solution by anilinepropylsilica xerogel. Dye. Pigment. 2008, 76, 64–69. [Google Scholar] [CrossRef]
- Vimonses, V.; Lei, S.; Jin, B.; Chow, C.W.K.; Saint, C. Kinetic study and equilibrium isotherm analysis of Congo Red adsorption by clay materials. Chem. Eng. J. 2009, 148, 354–364. [Google Scholar] [CrossRef]
- Namasivayam, C.; Muniasamy, N.; Gayatri, K.; Rani, M.; Ranganathan, K. Removal of dyes from aqueous solutions by cellulosic waste orange peel. Bioresour. Technol. 1996, 57, 37–43. [Google Scholar] [CrossRef]
- Akl, M.A.; Youssef, A.M.; Al-Awadhi, M.M. Adsorption of acid dyes onto bentonite and surfactant-modified bentonite. J. Anal. Bioanal. Tech. 2013, 4, 3–7. [Google Scholar]
- Wang, L.; Wang, A. Adsorption characteristics of Congo Red onto the chitosan/montmorillonite nanocomposite. J. Hazard. Mater. 2007, 147, 979–985. [Google Scholar] [CrossRef]
- Zhu, H.-Y.; Fu, Y.-Q.; Jiang, R.; Jiang, J.-H.; Xiao, L.; Zeng, G.-M.; Zhao, S.-L.; Wang, Y. Adsorption removal of congo red onto magnetic cellulose/Fe3O4/activated carbon composite: Equilibrium, kinetic and thermodynamic studies. Chem. Eng. J. 2011, 173, 494–502. [Google Scholar] [CrossRef]
- Uchimiya, M.; Chang, S.; Klasson, K.T. Screening biochars for heavy metal retention in soil: Role of oxygen functional groups. J. Hazard. Mater. 2011, 190, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.; Jhung, S.H. Applications of metal-organic frameworks in adsorption/separation processes via hydrogen bonding interactions. Chem. Eng. J. 2017, 310, 197–215. [Google Scholar] [CrossRef]
- Song, J.Y.; Bhadra, B.N.; Jhung, S.H. Contribution of H-bond in adsorptive removal of pharmaceutical and personal care products from water using oxidized activated carbon. Microporous Mesoporous Mater. 2017, 243, 221–228. [Google Scholar] [CrossRef]
- Van Tran, T.; Cao, V.D.; Nguyen, V.H.; Hoang, B.N.; Vo, D.-V.N.; Nguyen, T.D.; Bach, L.G. MIL-53(Fe) derived magnetic porous carbon as a robust adsorbent for the removal of phenolic compounds under the optimized conditions. J. Environ. Chem. Eng. 2019, 102902. [Google Scholar] [CrossRef]





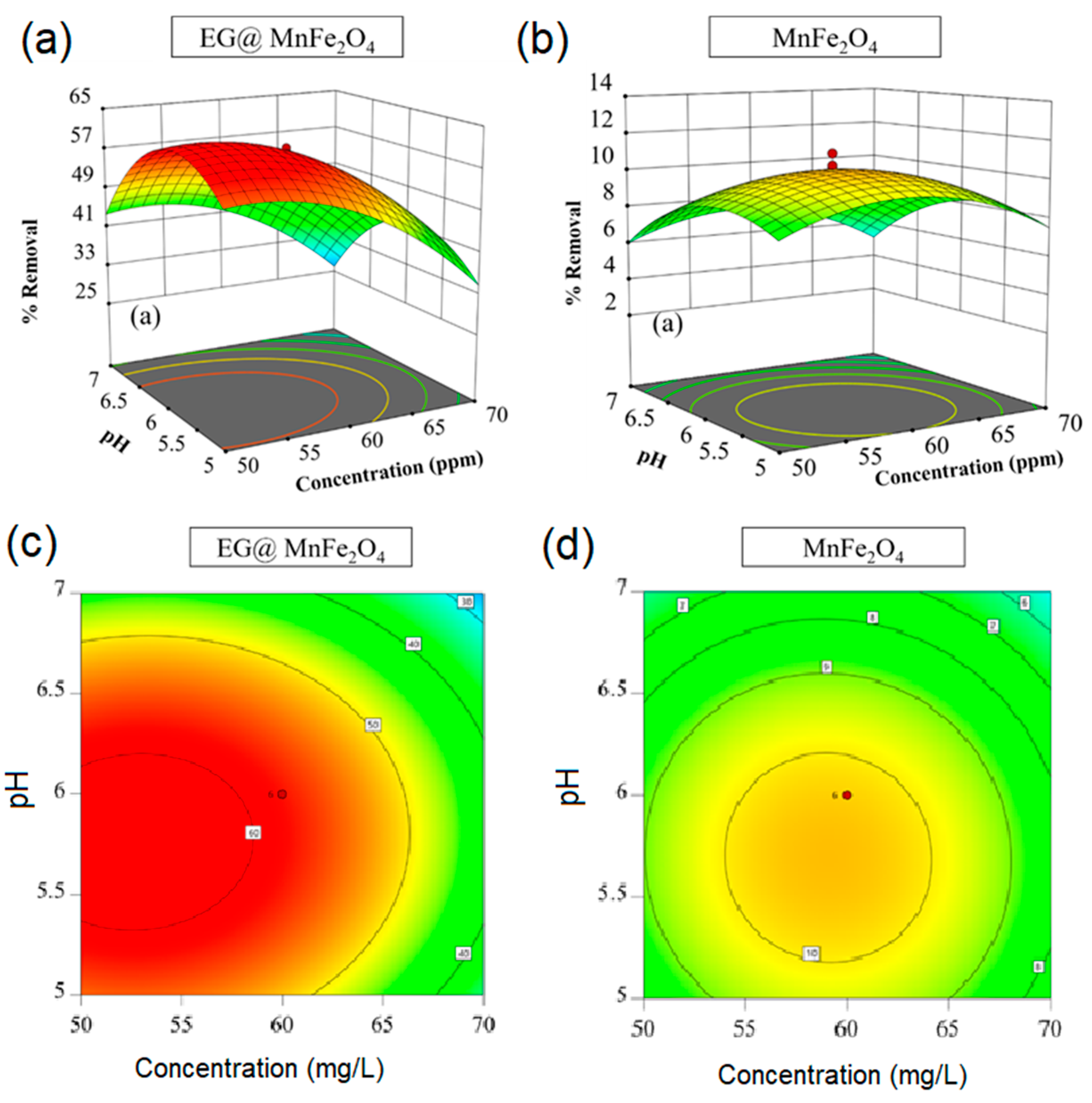

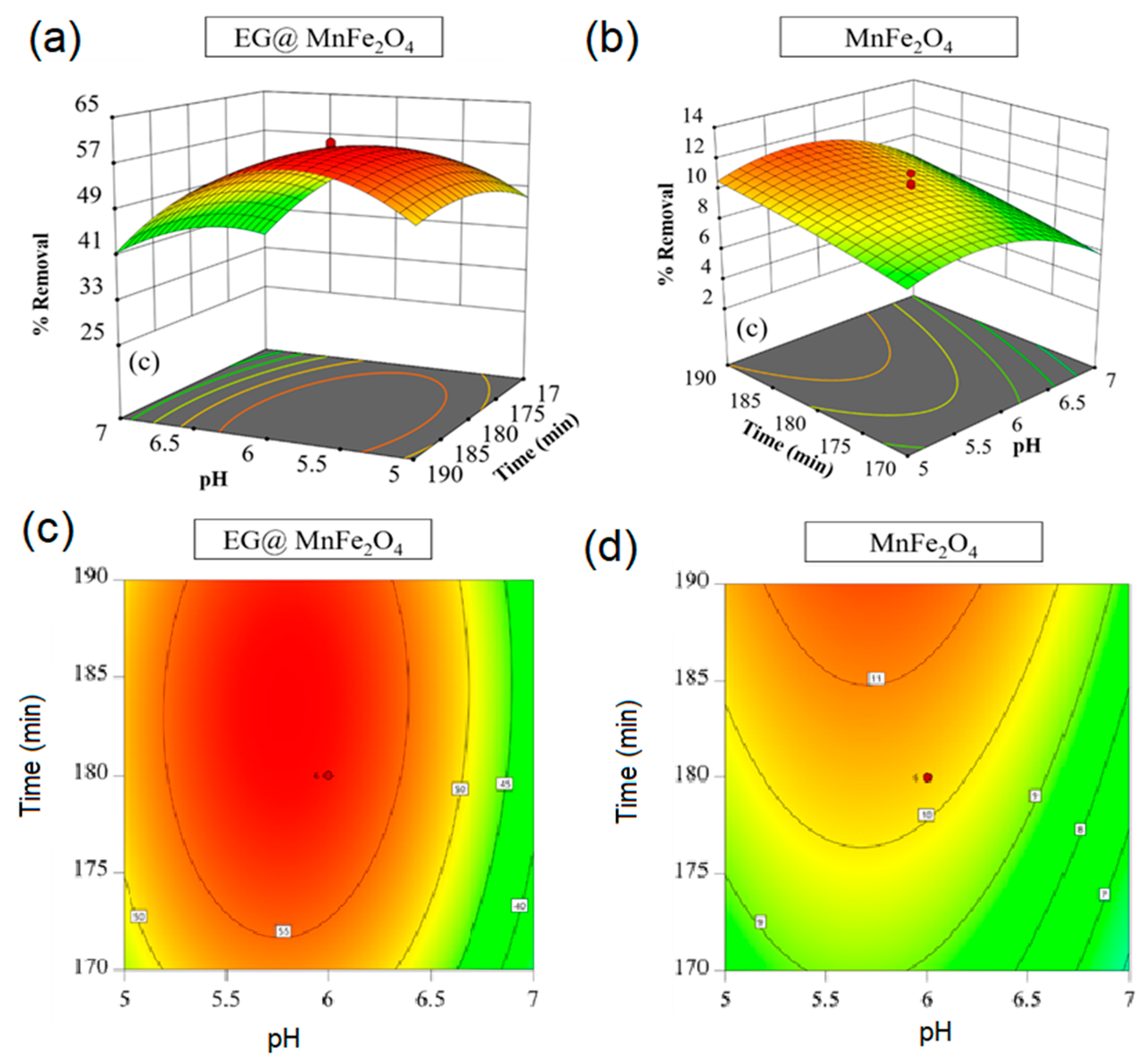

| No | Independent Factors | Unit | Code | Levels | ||||
|---|---|---|---|---|---|---|---|---|
| −1 | 0 | +1 | ||||||
| 1 | pH of solution (pH) | - | 4.3 | 5 | 6 | 7 | 7.7 | |
| 2 | Concentration (Co) | g/L | 43.2 | 50 | 60 | 70 | 76.8 | |
| 3 | Time | min | 163.2 | 170 | 180 | 190 | 196.8 | |
| Functional Groups | Experimental Frequency (cm−1) | Class | Ref. |
|---|---|---|---|
| O–H and N–H stretching | 3300–3500 (broad band) | Primary amines, secondary amines, hydroxyls of absorbed water. | [25] |
| C=O stretch | 1730 (very strong band) | Carbonyls of aldehydes (–CHO) or ketones (–C=O), lactone, esters (–COO–) or acid carboxylic (–COOH) | [26] |
| 1639 (very strong band) | Conjugation lowers frequency amides (–NHCO), or N–H stretching | [27] | |
| C=C bending | 1520 | Aromatic rings, alkenes | [28] |
| C–O stretch | 1195 (strong band) | Phenolic compounds or tertiary alcohol | [29] |
| 1076 | Primary alcohols | [30] |
| No | Materials | MnFe2O4 | EG@MnFe2O4 |
|---|---|---|---|
| 1 | Carboxylic groups (mmol/g) | 0 | 0.044 |
| 2 | Lactonic groups (mmol/g) | 0 | 0.032 |
| 3 | Phenolic groups (mmol/g) | 0 | 0.020 |
| 4 | Total oxygenated groups (mmol/g) | 0 | 0.096 |
| 5 | Total basic groups (mmol/g) | 0 | 0.156 |
| Run | Independent Factors | Onto EG@MnFe2O4 | Onto MnFe2O4 | ||||
|---|---|---|---|---|---|---|---|
| Actual (mg/g) | Predicted (mg/g) | Actual (mg/g) | Predicted (mg/g) | ||||
| 1 | 50 | 5 | 170 | 49.92 | 50.48 | 6.38 | 6.79 |
| 2 | 50 | 7 | 170 | 35.10 | 37.83 | 5.98 | 6.27 |
| 3 | 70 | 5 | 170 | 30.36 | 30.31 | 3.14 | 4.45 |
| 4 | 70 | 7 | 170 | 20.01 | 20.47 | 2.51 | 3.61 |
| 5 | 50 | 5 | 190 | 52.62 | 53.92 | 9.01 | 9.04 |
| 6 | 50 | 7 | 190 | 41.81 | 43.62 | 8.64 | 8.46 |
| 7 | 70 | 5 | 190 | 34.36 | 33.38 | 6.42 | 7.26 |
| 8 | 70 | 7 | 190 | 24.70 | 25.89 | 5.63 | 6.36 |
| 9 | 60 | 4.3 | 180 | 33.50 | 33.85 | 6.75 | 5.75 |
| 10 | 60 | 7.7 | 180 | 19.74 | 16.91 | 5.16 | 3.34 |
| 11 | 6 | 43.2 | 180 | 58.21 | 55.26 | 6.86 | 7.08 |
| 12 | 6 | 76.8 | 180 | 22.91 | 23.39 | 5.16 | 3.34 |
| 13 | 6 | 60 | 163.2 | 47.52 | 46.17 | 9.07 | 7.77 |
| 14 | 6 | 60 | 196.8 | 54.75 | 53.62 | 12.28 | 11.97 |
| 15 | 6 | 60 | 180 | 57.91 | 58.21 | 10.35 | 10.28 |
| 16 | 6 | 60 | 180 | 57.45 | 58.21 | 10.30 | 10.28 |
| 17 | 6 | 60 | 180 | 58.41 | 58.21 | 11.13 | 10.28 |
| 18 | 6 | 60 | 180 | 57.68 | 58.21 | 9.18 | 10.28 |
| 19 | 6 | 60 | 180 | 58.30 | 58.21 | 10.48 | 10.28 |
| 20 | 6 | 60 | 180 | 59.08 | 58.21 | 10.01 | 10.28 |
| Material | Source | Sum of Squares | Degree of Freedom | Mean Square | F-Value | Prob. > F | Comment |
|---|---|---|---|---|---|---|---|
| EG@ MnFe2O4 | Model | 4056.21 | 9 | 450.69 | 121.02 | <0.0001 | SD = 1.93 |
| 1225.91 | 1 | 1225.91 | 329.18 | <0.0001 | Mean = 43.72 | ||
| 346.28 | 1 | 346.28 | 92.98 | <0.0001 | CV(%) = 4.41 | ||
| 67.03 | 1 | 67.03 | 18.00 | 0.0017 | R2 = 0.9909 | ||
| 642.60 | 1 | 642.60 | 172.55 | <0.0001 | AP = 30.2649 | ||
| 1941.60 | 1 | 1941.60 | 521.36 | <0.0001 | |||
| 124.46 | 1 | 124.46 | 33.42 | 0.0002 | |||
| 3.94 | 1 | 3.94 | 1.06 | 0.3279 | |||
| 0.0655 | 1 | 0.0655 | 0.0176 | 0.8971 | |||
| 2.76 | 1 | 2.76 | 0.7406 | 0.4096 | |||
| MnFe2O4 | Mode | 125.91 | 9 | 13.99 | 10.75 | 0.0005 | SD = 1.14 |
| 1.73 | 1 | 1.73 | 1.33 | 0.2763 | Mean = 7.72 | ||
| 16.84 | 1 | 16.84 | 12.94 | 0.0049 | CV(%) = 14.78 | ||
| 21.34 | 1 | 21.34 | 16.39 | 0.0023 | R2 = 0.9063 | ||
| 47.42 | 1 | 47.42 | 36.43 | 0.0001 | AP = 10.6995 | ||
| 46.37 | 1 | 46.37 | 35.63 | 0.0001 | |||
| 0.3050 | 1 | 0.3050 | 0.2343 | 0.6388 | |||
| 0.0558 | 1 | 0.0588 | 0.0428 | 0.8402 | |||
| 0.0018 | 1 | 0.0018 | 0.0014 | 0.9713 | |||
| 0.1578 | 1 | 0.1578 | 0.1212 | 0.7349 |
| Sample | pH (-) | Concentration (mg/L) | Time (min) | Adsorption Capacity (mg/g) | Desirability | ||
|---|---|---|---|---|---|---|---|
| Predicted | Tested | Error | |||||
| EG@MnFe2O4 | 5.7 | 57.7 | 181 | 60.6 | 62.0 | 1.4 | 1.0000 |
| MnFe2O4 | 6.0 | 62.0 | 182 | 10.4 | 11.1 | 0.7 | 1.0000 |
| No. | Adsorbents | Adsorption Capacity (mg/g) | Reference |
|---|---|---|---|
| 1 | EG@MnFe2O4 | 62.0 | This work |
| 2 | MnFe2O4 | 11.1 | This work |
| 3 | Anilinepropylsilica xerogel | 22.62 | [50] |
| 4 | Kaolin | 5.44 | [51] |
| 5 | Waste orange peel | 22.4 | [52] |
| 6 | Bentonite | 40.4 | [53] |
| 7 | CTS powder | 74.7 | [54] |
| 8 | CTS-MMT | 54.5 | [54] |
| 9 | m-Cell/Fe3O4/ACCs | 60.5 | [55] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pham, V.T.; T. Nguyen, H.-T.; Thi Cam Nguyen, D.; T. N. Le, H.; Thi Nguyen, T.; Thi Hong Le, N.; Lim, K.T.; Duy Nguyen, T.; Tran, T.V.; Bach, L.G. Process Optimization by a Response Surface Methodology for Adsorption of Congo Red Dye onto Exfoliated Graphite-Decorated MnFe2O4 Nanocomposite: The Pivotal Role of Surface Chemistry. Processes 2019, 7, 305. https://doi.org/10.3390/pr7050305
Pham VT, T. Nguyen H-T, Thi Cam Nguyen D, T. N. Le H, Thi Nguyen T, Thi Hong Le N, Lim KT, Duy Nguyen T, Tran TV, Bach LG. Process Optimization by a Response Surface Methodology for Adsorption of Congo Red Dye onto Exfoliated Graphite-Decorated MnFe2O4 Nanocomposite: The Pivotal Role of Surface Chemistry. Processes. 2019; 7(5):305. https://doi.org/10.3390/pr7050305
Chicago/Turabian StylePham, Van Thinh, Hong-Tham T. Nguyen, Duyen Thi Cam Nguyen, Hanh T. N. Le, Thuong Thi Nguyen, Nhan Thi Hong Le, Kwon Teak Lim, Trinh Duy Nguyen, Thuan Van Tran, and Long Giang Bach. 2019. "Process Optimization by a Response Surface Methodology for Adsorption of Congo Red Dye onto Exfoliated Graphite-Decorated MnFe2O4 Nanocomposite: The Pivotal Role of Surface Chemistry" Processes 7, no. 5: 305. https://doi.org/10.3390/pr7050305





