Enhancing Oxygen Permeation via the Incorporation of Silver Inside Perovskite Oxide Membranes
Abstract
:1. Introduction
2. Experimental
2.1. Preparation of LCF/LCFA Powders and Hollow Fibre Membranes
2.2. Oxygen Permeation Measurement
2.3. Characterization
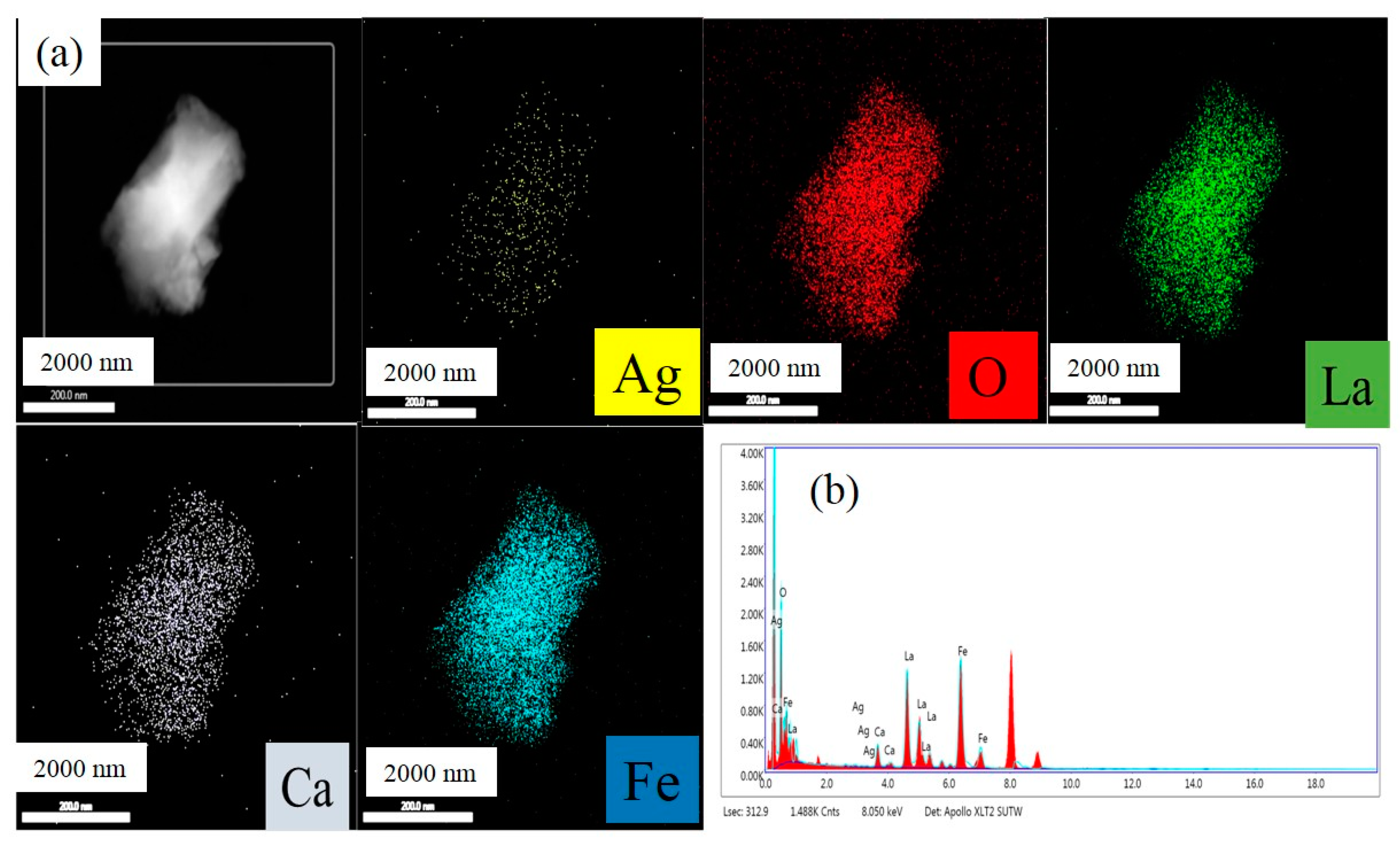
3. Results and Discussion
3.1. Phase Structure and Thermal Shrinkage Behavior of LCF and LCFA
3.2. Morphology of LCF and LCFA Hollow Fiber Membranes
3.3. Oxygen Permeation Test
3.4. Oxygen Permeation Performance under CO2 Atmosphere
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Liu, N.; Tan, X.; Meng, B.; Liu, S. Honeycomb-structured perovskite hollow fibre membranes with ultra-thin densified layer for oxygen separation. Sep. Purif. Technol. 2011, 80, 396–401. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, X.; Li, M.; O’Hayre, R.P.; Yang, W. Nanoparticles at grain boundaries inhibit the phase transformation of perovskite membrane. Nano Lett. 2015, 15, 7678–7683. [Google Scholar] [CrossRef]
- Wei, Y.; Liao, Q.; Li, Z.; Wang, H. Enhancement of oxygen permeation through U-shaped K2NiF4-type oxide hollow fiber membranes by surface modifications. Sep. Purif. Technol. 2013, 110, 74–80. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, G.; Liu, Z.; Chu, Z.; Jin, W.; Xu, N. Unprecedented perovskite oxyfluoride membranes with high-efficiency oxygen ion transport paths for low-temperature oxygen permeation. Adv. Mater. 2016, 28, 3511–3515. [Google Scholar] [CrossRef]
- Wang, H.; Tablet, C.; Caro, J. Oxygen production at low temperature using dense perovskite hollow fiber membranes. J. Membr. Sci. 2008, 322, 214–217. [Google Scholar] [CrossRef]
- Han, N.; Wei, Q.; Tian, H.; Zhang, S.; Zhu, Z.; Liu, J.; Liu, S. Highly stable dual-phase membrane based on Ce0.9Gd0.1O2-δ-La2NiO4+δ for oxygen permeation under pure CO2 atmosphere. Energy Technol. 2018. [Google Scholar] [CrossRef]
- Chen, C.-S.; Ran, S.; Liu, W.; Yang, P.; Peng, D.; Bouwmeester, H.J.M. YBa2Cu3O6+δ as an oxygen separation membrane. Angew. Chem. Int. Ed. 2001, 40, 784–786. [Google Scholar] [CrossRef]
- Yoon, K.J.; Marina, O.A. Highly stable dual-phase Y0.8Ca0.2Cr0.8Co0.2O3-Sm0.2Ce0.8O1.9 ceramic composite membrane for oxygen separation. J. Membr. Sci. 2016, 499, 301–306. [Google Scholar] [CrossRef]
- Shao, X.; Dong, D.; Parkinson, G.; Li, C.-Z. Improvement of oxygen permeation through microchanneled ceramic membranes. J. Membr. Sci. 2014, 454, 444–450. [Google Scholar] [CrossRef]
- Sunarso, J.; Baumann, S.; Serra, J.M.; Meulenberg, W.A.; Liu, S.; Lin, Y.; Diniz da Costa, J.C. Mixed ionic-electronic conducting ceramic-based membranes for oxygen separation. J. Membr. Sci. 2008, 320, 13–41. [Google Scholar] [CrossRef]
- Li, K.; Tan, X.; Liu, Y. Single-step fabrication of ceramic hollow fibers for oxygen permeation. J. Membr. Sci. 2006, 272, 1–5. [Google Scholar] [CrossRef]
- Tan, X.; Liu, N.; Meng, B.; Liu, S. Morphology control of the perovskite hollow fibre membranes for oxygen separation using different bore fluids. J. Membr. Sci. 2011, 378, 308–318. [Google Scholar] [CrossRef]
- An, R.; Song, J.; Li, Y.; Tan, X.; Sunarso, J.; Zhang, C.; Wang, S.; Liu, S. Bundling strategy to simultaneously improve the mechanical strength and oxygen permeation flux of the individual perovskite hollow fiber membranes. J. Membr. Sci. 2017, 527, 137–142. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, G.; Liu, G.; Liu, Z.; Jin, W.; Xu, N. Perovskite Hollow Fibers with Precisely Controlled Cation Stoichiometry via One-Step Thermal Processing. Adv. Mater. 2017, 29, 1606377. [Google Scholar] [CrossRef] [PubMed]
- Leo, A.; Motuzas, J.; Yacou, C.; Liu, S.; Serra, J.; Navarrete, L.; Drennan, J.; Julbe, A.; Diniz da Costa, J.C. Copper oxide-perovskite mixed matrix membranes delivering very high oxygen fluxes. J. Membr. Sci. 2017, 526, 323–333. [Google Scholar] [CrossRef]
- Jin, W.; Li, S.; Huang, P.; Xu, N.; Shi, J.; Lin, Y.S. Tubular lanthanum cobaltite perovskite-type membrane reactors for partial oxidation of methane to syngas. J. Membr. Sci. 2000, 66, 13–22. [Google Scholar] [CrossRef]
- Zhuang, S.; Han, N.; Wang, T.; Meng, X.; Meng, B.; Li, Y.; Sunarso, J.; Liu, S. Enhanced CO selectivity for reverse water-gas shift reaction using Ti4O7-doped SrCe0.9Y0.1O3-δ hollow fiber membrane reactor. Can. J. Chem. Eng. 2018, 9999, 1–8. [Google Scholar] [CrossRef]
- Czyperek, M.; Zapp, P.; Bouwmeester, H.J.M.; Modigell, M.; Ebert, K.; Voigt, I.; Meulenberg, W.A.; Singheiser, L.; Stöver, D. Gas separation membranes for zero-emission fossil power plants: MEM-BRAIN. J. Membr. Sci. 2010, 359, 149–159. [Google Scholar] [CrossRef] [Green Version]
- Pei, S.; Kleefisch, M.S.; Kobylinski, T.P.; Faber, J.; Udovich, C.A.; Zhang-McCoy, V.; Dabrowski, B.; Balachandran, U.; Mieville, R.L.; Poeppel, R.B. Failure mechanisms of ceramic membrane reactors in partial oxidation of methane to synthesis gas. Catal. Lett. 1995, 30, 201–212. [Google Scholar] [CrossRef]
- Kneer, R.; Toporov, D.; Förster, M.; Christ, D.; Broeckmann, C.; Pfaff, E.; Zwick, M.; Engels, S.; Modigell, M. OXYCOAL-AC: Towards an integrated coal-fired power plant process with ion transportmembrane-based oxygen supply. Energy Environ. Sci. 2010, 3, 198–207. [Google Scholar] [CrossRef]
- Waindich, A.; Möbius, A.; Müller, M. Corrosion of Ba1-xSrxCo1-yFeyO3-δ and La0.3Ba0.7Co0.2Fe0.8O3-δ materials for oxygen separating membranes under Oxycoal conditions. J. Membr. Sci. 2009, 337, 182–187. [Google Scholar] [CrossRef]
- Zhu, J.; Guo, S.; Chu, Z.; Jin, W. Effects of polymer binders on separation performance of the perovskite-type 4-bore hollow fiber membranes. Sep. Purif. Technol. 2017, 187, 294–302. [Google Scholar]
- Lin, Y.S.; Wang, W.; Han, J. Oxygen permeation through thin mixed-conducting solid oxide membranes. AIChE J. 1994, 40, 786–798. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Zhu, X.; Cong, Y.; Xu, S.; Xu, W.; Yang, W. Oxygen permeation through Ca-contained dual-phase membranes for oxyfuel CO2 capture. Sep. Purif. Technol. 2013, 114, 31–37. [Google Scholar] [CrossRef]
- Tan, X.; Shi, L.; Hao, G.; Meng, B.; Liu, S. La0.7Sr0.3FeO3-δ perovskite hollow fiber membranes for oxygen permeation and methane conversion. Sep. Purif. Technol. 2012, 96, 89–97. [Google Scholar] [CrossRef]
- Engels, S.; Markus, T.; Modigell, M.; Singheiser, L. Oxygen permeation and stability investigations on MIEC membrane materials under operating conditions for power plant processes. J. Membr. Sci. 2011, 370, 58–69. [Google Scholar] [CrossRef]
- Gao, J.; Lun, Y.; Han, N.; Tan, X.; Fan, C.; Liu, S. Influence of nitric oxide on the oxygen permeation behavior of La0.6Sr0.4Co0.2Fe0.8O3-δ perovskite membranes. Sep. Purif. Technol. 2019, 210, 900–906. [Google Scholar] [CrossRef]
- Efimov, K.; Klande, T.; Juditzki, N.; Feldhoff, A. Ca-containing CO2-tolerant perovskite materials for oxygen separation. J. Membr. Sci. 2012, 389, 205–215. [Google Scholar] [CrossRef]
- Zhang, K.; Shao, Z.; Li, C.; Liu, S. Novel CO2-tolerant ion-transporting ceramic membranes with an external short circuit for oxygen separation at intermediate temperatures. Energy Environ. Sci. 2012, 5, 5257–5264. [Google Scholar] [CrossRef]
- Fang, W.; Steinbach, F.; Chen, C.; Feldhoff, A. An approach to enhance the CO2 tolerance of fluorite-perovskite dual-phase oxygen-transporting membrane. Chem. Mater. 2015, 27, 7820–7826. [Google Scholar] [CrossRef]
- Yang, D.; Han, N.; Han, D.; Meng, B.; Wang, G.; Liu, S. Novel SrCo0.9W0.1O3-δ hollow fiber membrane with enhanced oxygen delivery performance and CO2 resistance ability. Chem. Sel. 2018, 3, 13700–13704. [Google Scholar]
- Li, Q.; Zhu, X.; He, Y.; Yang, W. Oxygen permeability and stability of BaCe0.1Co0.4Fe0.5O3-δ oxygen permeable membrane. Sep. Purif. Technol. 2010, 73, 38–43. [Google Scholar] [CrossRef]
- Han, N.; Zhang, S.; Meng, X.; Yang, N.; Meng, B.; Tan, X.; Liu, S. Effect of enhanced oxygen reduction activity on oxygen permeation of La0.6Sr0.4Co0.2Fe0.8O3-δ membrane decorated by K2NiF4-type oxide. J. Alloys Compd. 2016, 654, 280–289. [Google Scholar] [CrossRef]
- Chen, X.; Huang, L.; Wei, Y.; Wang, H. Tantalum stabilized SrCoO3-δ perovskite membrane for oxygen separation. J. Membr. Sci. 2011, 368, 159–164. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, Z.; Zhu, N.; Jiang, W.; Dong, X.; Jin, W. A novel Nb2O5-doped SrCo0.8Fe0.2O3-δ oxide with high permeability and stability for oxygen separation. J. Membr. Sci. 2012, 405, 300–309. [Google Scholar] [CrossRef]
- Ran, R.; Guo, Y.; Gao, D.; Liu, S.; Shao, Z. Effect of foreign oxides on the phase structure, sintering and transport properties of Ba0.5Sr0.5Co0.8Fe0.2O3-δ as ceramic membranes for oxygen separation. Sep. Purif. Technol. 2011, 81, 384–391. [Google Scholar] [CrossRef]
- Zeng, P.; Ran, R.; Chen, Z.; Gu, H.; Shao, Z.; Liu, S. Novel mixed conducting SrSc0.05Co0.95O3-δ ceramic membrane for oxygen separation. AICHE J. 2007, 53, 3116–3124. [Google Scholar] [CrossRef]
- He, B.; Ding, D.; Ling, Y.; Xu, J.; Zhao, L. Efficient modification for enhancing surface activity of Ba0.5Sr0.5Co0.8Fe0.2O3-δ oxygen permeation membrane. J. Membr. Sci. 2015, 477, 7–13. [Google Scholar] [CrossRef]
- Tan, X.; Liu, N.; Meng, B.; Sunarso, J.; Zhang, K.; Liu, S. Oxygen permeation behavior of La0.6Sr0.4Co0.2Fe0.8O3-δ hollow fibre membranes with highly concentrated CO2 exposure. J. Membr. Sci. 2012, 389, 216–222. [Google Scholar] [CrossRef]
- Zhang, C.; Sunarso, J.; Liu, S. Designing CO2-resistant oxygen-selective mixed ionic-electronic conducting membranes: Guidelines, recent advances and future directions. Chem. Soc. Rev. 2017, 46, 2941–3005. [Google Scholar] [CrossRef]
- Yang, D.; Yang, N.; Meng, B.; Tan, X.; Zhang, C.; Sunarso, J.; Zhu, Z.; Liu, S. A-Site Excess (La0.8Ca0.2)1.01FeO3-δ (LCF) Perovskite Hollow Fiber Membrane for Oxygen Permeation in CO2-Containing Atmosphere. Energy Fuels 2017, 31, 4531–4538. [Google Scholar] [CrossRef]
- Zhu, X.; Cong, Y.; Yang, W. Oxygen permeability and structural stability of BaCe0.15Fe0.85O3-δ membranes. J. Membr. Sci. 2006, 283, 38–44. [Google Scholar] [CrossRef]
- Price, P.M.; Butt, D.P. Stability and Decomposition of Ca-Substituted Lanthanum Ferrite in Reducing Atmospheres. J. Am. Ceram. Soc. 2015, 98, 2881–2886. [Google Scholar] [CrossRef] [Green Version]
- Leo, A.; Smart, S.; Liu, S.; Diniz da Costa, J.C. High Performance Perovskite Hollow Fibres for Oxygen Separation. J. Membr. Sci. 2011, 368, 64–68. [Google Scholar] [CrossRef]
- Leo, A.; Liu, S.; Diniz da Costa, J.C.; Shao, Z. Oxygen permeation through perovskite membranes and the improvement of oxygen flux by surface modification. Sci. Technol. Adv. Mater. 2007, 7, 819–825. [Google Scholar] [CrossRef]
- Tan, X.; Wang, Z.; Liu, H.; Liu, S. Enhancement of oxygen permeation through La0.6Sr0.4Co0.2Fe0.8O3-δ hollow fiber membranes by surface modifications. J. Membr. Sci. 2008, 324, 128–135. [Google Scholar] [CrossRef]
- Savinskaya, O.A.; Nemudry, A.P. Oxygen permeability and structural features of SrFe1−xWxO3−δ membranes. J. Membr. Sci. 2014, 459, 45–51. [Google Scholar] [CrossRef]
- Ovalle-Encinia, O.; Pfeiffer, H.; Ortiz-Landeros, J. CO2 Separation improvement produced on a ceramic–carbonate dense membrane superficially modified with Au–Pd. Ind. Eng. Chem. Res. 2018, 57, 9261–9268. [Google Scholar] [CrossRef]
- Chu, Y.; Tan, X.; Shen, Z.; Liu, P.; Han, N.; Kang, J.; Duan, X.; Wang, S.; Liu, L.; Liu, S. Efficient removal of organic and bacterial pollutants by Ag-La0.8Ca0.2Fe0.94O3-δ perovskite via catalytic peroxymonosulfate activation. J. Hazard. Mater. 2018, 356, 53–60. [Google Scholar] [CrossRef]
- Tan, X.; Liu, Y.; Li, K. Preparation of LSCF ceramic hollow-fiber membranes for oxygen production by a phase-inversion/sintering technique. Ind. Eng. Chem. Res. 2005, 44, 61–66. [Google Scholar] [CrossRef]
- Han, N.; Zhang, S.; Meng, B.; Tan, X. The effect of microstructure and surface decoration with K2NiF4-type oxide upon the oxygen permeability of perovskite-type La0.7Sr0.3FeO3-δ hollow fiber membranes. RSC Adv. 2015, 5, 88602–88611. [Google Scholar] [CrossRef]
- Tan, X.; Liu, Y.; Li, K. Mixed conducting ceramic hollow-fiber membranes for air separation. AIChE J. 2005, 51, 1991–2000. [Google Scholar] [CrossRef]
- Han, N.; Meng, B.; Yang, N.; Sunarso, J.; Zhu, Z.; Liu, S. Enhancement of oxygen permeation fluxes of La0.6Sr0.4CoO3-δ hollow fiber membrane via macrostructure modification and (La0.5Sr0.5)2CoO4+δ decoration. Chem. Eng. Res. Des. 2018, 134, 487–496. [Google Scholar] [CrossRef]
- Wang, H.; Tablet, C.; Yang, W.; Caro, J. In situ high temperature X-ray diffraction studies of mixed ionic and electronic conducting perovskite-type membranes. Mater. Lett. 2005, 59, 3750–3755. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.; Ran, R.; Shao, Z.; Jin, W.; Xu, N. Evaluation of A-site cation-deficient (Ba0.5Sr0.5)1-xCo0.8Fe0.2O3-δ (x > 0) perovskite as a solid-oxide fuel cell cathode. J. Power Sources 2008, 182, 24–31. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Y.; Tade, M.O.; Hao, Y.; Liu, S.; Shao, Z. Tin-doped perovskite mixed conducting membrane for efficient air separation. J. Mater. Chem. A 2014, 2, 9666–9674. [Google Scholar] [CrossRef]
- Tan, X.; Liu, S.; Li, K. Preparation and characterization of inorganic hollow fiber membranes. J. Membr. Sci. 2001, 188, 87–95. [Google Scholar] [CrossRef] [Green Version]
- Zeng, P.; Ran, R.; Chen, Z.; Gu, H.; Shao, Z.; Diniz da Costa, J.C.; Liu, S. Significant effects of sintering temperature on the performance of La0.6Sr0.4Co0.2Fe0.8O3-δ oxygen selective membranes. J. Membr. Sci. 2007, 302, 171–179. [Google Scholar] [CrossRef]
- Liu, S.; Tan, X.; Li, K.; Hughes, R. Preparation and characterisation of SrCe0.95Yb0.05O2.975 hollow fibre membranes. J. Membr. Sci. 2001, 193, 249–260. [Google Scholar] [CrossRef]
- Ge, L.; Shao, Z.; Zhang, K.; Ran, R.; Diniz da Costa, J.C.; Liu, S. Evaluation of mixed-conducting lanthanum-strontium-cobaltite ceramic membrane for oxygen separation. AIChE J. 2009, 55, 2603–2613. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, H.; Cong, Y.; Yang, W. Permeation model and experimental investigation of mixed conducting membranes. AIChE J. 2012, 58, 1744–1754. [Google Scholar] [CrossRef]
- Leo, A.; Liu, S.; Diniz da Costa, J.C. The enhancement of oxygen flux on Ba0.5Sr0.5Co0.8Fe0.2O3-δ (BSCF) hollow fibers using silver surface modification. J. Membr. Sci. 2009, 340, 148–153. [Google Scholar] [CrossRef]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, T.; Han, N.; Meng, B.; Yang, N.; Zhu, Z.; Liu, S. Enhancing Oxygen Permeation via the Incorporation of Silver Inside Perovskite Oxide Membranes. Processes 2019, 7, 199. https://doi.org/10.3390/pr7040199
Ma T, Han N, Meng B, Yang N, Zhu Z, Liu S. Enhancing Oxygen Permeation via the Incorporation of Silver Inside Perovskite Oxide Membranes. Processes. 2019; 7(4):199. https://doi.org/10.3390/pr7040199
Chicago/Turabian StyleMa, Teng, Ning Han, Bo Meng, Naitao Yang, Zhonghua Zhu, and Shaomin Liu. 2019. "Enhancing Oxygen Permeation via the Incorporation of Silver Inside Perovskite Oxide Membranes" Processes 7, no. 4: 199. https://doi.org/10.3390/pr7040199





