Insights into the Fouling Propensities of Natural Derived Alginate Blocks during the Microfiltration Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Alginate Extraction from Raw Seaweed
2.2. Block Fractionation of Alginate
2.3. FTIR Spectroscopy of Alginate Blocks
2.4. Field Emission Scanning Electron Microscope (FESEM) Observations
2.5. TEP Determination
2.6. Dead-End Filtration Tests
3. Results and Discussion
3.1. Block Composition of Alginate
3.2. FTIR Spectrums of MG-, MM- and GG-Blocks
3.3. The Micro-Structures of the Alginate, MG-, MM- and GG-Blocks
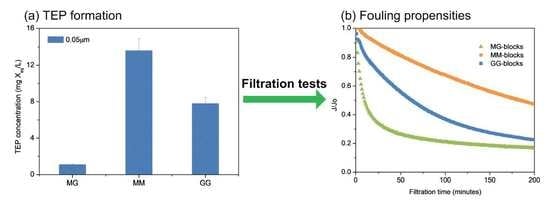
3.4. The TEP Formation from MG-, MM- and GG-Blocks
3.5. Filtration Behaviors of MG-, MM- and GG-Blocks
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Qu, C.; Lu, S.; Liang, D.; Chen, S.; Xiang, Y.; Zhang, S. Simultaneous electro-oxidation and in situ electro-peroxone process for the degradation of refractory organics in wastewater. J. Hazard. Mater. 2019, 364, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Cui, M.; Li, X.; Thuyet, D.Q.; Fan, W. Effects of hydrophobicity of titanium dioxide nanoparticles and exposure scenarios on copper uptake and toxicity in Daphnia magna. Water Res. 2019, 154, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Gwenzi, W.; Sipowo-Tala, V.R.; Noubactep, C. Water Treatment Using Metallic Iron: A Tutorial Review. Processes 2019, 7, 622. [Google Scholar] [CrossRef]
- Qu, C.; Soomro, G.S.; Ren, N.; Liang, D.-W.; Lu, S.-F.; Xiang, Y.; Zhang, S.-J. Enhanced electro-oxidation/peroxone (in situ) process with a Ti-based nickel-antimony doped tin oxide anode for phenol degradation. J. Hazard. Mater. 2019, 121398. [Google Scholar] [CrossRef] [PubMed]
- Elimelech, M.; Phillip, W.A. The Future of Seawater Desalination: Energy, Technology, and the Environment. Science 2011, 333, 712–717. [Google Scholar] [CrossRef]
- Zamora, S.; Sandoval, L.; Marín-Muñíz, J.L.; Fernández-Lambert, G.; Hernández-Orduña, M.G. Impact of Ornamental Vegetation Type and Different Substrate Layers on Pollutant Removal in Constructed Wetland Mesocosms Treating Rural Community Wastewater. Processes 2019, 7, 531. [Google Scholar] [CrossRef]
- Bis, M.; Montusiewicz, A.; Piotrowicz, A.; Łagód, G. Modeling of Wastewater Treatment Processes in Membrane Bioreactors Compared to Conventional Activated Sludge Systems. Processes 2019, 7, 285. [Google Scholar] [CrossRef]
- Song, Y.; Motuzas, J.; Wang, D.K.; Birkett, G.; Smart, S.; Diniz da Costa, J.C. Substrate Effect on Carbon/Ceramic Mixed Matrix Membrane Prepared by a Vacuum-Assisted Method for Desalination. Processes 2018, 6, 47. [Google Scholar] [CrossRef]
- Wagh, P.; Zhang, X.; Blood, R.; Kekenes-Huskey, P.M.; Rajapaksha, P.; Wei, Y.; Escobar, I.C. Increasing Salt Rejection of Polybenzimidazole Nanofiltration Membranes via the Addition of Immobilized and Aligned Aquaporins. Processes 2019, 7, 76. [Google Scholar] [CrossRef]
- Martin Vincent, N.; Tong, J.; Yu, D.; Zhang, J.; Wei, Y. Membrane Fouling Characteristics of a Side-Stream Tubular Anaerobic Membrane Bioreactor (AnMBR) Treating Domestic Wastewater. Processes 2018, 6, 50. [Google Scholar] [CrossRef]
- Berman, T.; Holenberg, M. Don’t fall foul of biofilm through high TEP levels. Filtr. Sep. 2005, 42, 30–32. [Google Scholar] [CrossRef]
- Passow, U. Transparent exopolymer particles (TEP) in aquatic environments. Prog. Oceanogr. 2002, 55, 287–333. [Google Scholar] [CrossRef]
- Wang, R.; Liang, D.; Liu, X.; Fan, W.; Meng, S.; Cai, W. Effect of magnesium ion on polysaccharide fouling. Chem. Eng. J. 2020, 379. [Google Scholar] [CrossRef]
- Bar-Zeev, E.; Passow, U.; Castrillon, S.R.; Elimelech, M. Transparent exopolymer particles: From aquatic environments and engineered systems to membrane biofouling. Environ. Sci. Technol. 2015, 49, 691–707. [Google Scholar] [CrossRef] [PubMed]
- Draget, K.I.; Smidsrød, O.; Skjåk-Bræk, G. Alginates from Algae. In Biopolymers Online; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2005. [Google Scholar] [CrossRef]
- Melvik, J.; Dornish, M. Alginate as a Carrier for Cell Immobilisation. In Fundamentals of Cell Immobilisation Biotechnology; Nedović, V., Willaert, R., Eds.; Springer: Amsterdam, The Netherlands, 2004; Volume 8A, pp. 33–51. [Google Scholar]
- Tønnesen, H.H.; Karlsen, J. Alginate in Drug Delivery Systems. Drug Dev. Ind. Pharm. 2002, 28, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Leal, D.; Matsuhiro, B.; Rossi, M.; Caruso, F. FT-IR spectra of alginic acid block fractions in three species of brown seaweeds. Carbohydr. Res. 2008, 343, 308–316. [Google Scholar] [CrossRef]
- Meng, S.; Liu, Y. New insights into transparent exopolymer particles (TEP) formation from precursor materials at various Na+/Ca2+ ratios. Sci. Rep. 2016, 6, 19747. [Google Scholar] [CrossRef]
- Meng, S.; Winters, H.; Liu, Y. Ultrafiltration behaviors of alginate blocks at various calcium concentrations. Water Res. 2015, 83, 248–257. [Google Scholar] [CrossRef]
- Chandía, N.P.; Matsuhiro, B.; Mejías, E.; Moenne, A. Alginic acids in Lessonia vadosa: Partial hydrolysis and elicitor properties of the polymannuronic acid fraction. J. Appl. Phycol. 2004, 16, 127–133. [Google Scholar] [CrossRef]
- Steyermark, A. Spectrometric Identification of Organic Compounds, 3rd ed.; Silverstein, R.M., Bassler, G.C., Morrill, T.C., Eds.; Wiley: New York, NY, USA, 1974; p. 340. [Google Scholar] [CrossRef]
- Mathlouthi, M.; Koenig, J.L. Vibrational Spectra of Carbohydrates. In Advances in Carbohydrate Chemistry and Biochemistry; Tipson, R.S., Derek, H., Eds.; Academic Press: Cambridge, MA, USA, 1987; Volume 44, pp. 7–89. [Google Scholar]
- Gómez-Ordóñez, E.; Rupérez, P. FTIR-ATR spectroscopy as a tool for polysaccharide identification in edible brown and red seaweeds. Food Hydrocoll. 2011, 25, 1514–1520. [Google Scholar] [CrossRef]
- Coimbra, M.A.; Barros, A.; Barros, M.; Rutledge, D.N.; Delgadillo, I. Multivariate analysis of uronic acid and neutral sugars in whole pectic samples by FT-IR spectroscopy. Carbohydr. Polym. 1998, 37, 241–248. [Google Scholar] [CrossRef]
- Synytsya, A.; Kim, W.-J.; Kim, S.-M.; Pohl, R.; Synytsya, A.; Kvasnička, F.; Čopíková, J.; Il Park, Y. Structure and antitumour activity of fucoidan isolated from sporophyll of Korean brown seaweed Undaria pinnatifida. Carbohydr. Polym. 2010, 81, 41–48. [Google Scholar] [CrossRef]
- Meng, S.; Liu, Y. Alginate block fractions and their effects on membrane fouling. Water Res. 2013, 47, 6618–6627. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Fan, W.; Li, X.; Liu, Y.; Liang, D.; Liu, X. Intermolecular interactions of polysaccharides in membrane fouling during microfiltration. Water Res. 2018, 143, 38–46. [Google Scholar] [CrossRef]
- Wang, X.; Fan, W.; Dong, Z.; Liang, D.; Zhou, T. Interactions of natural organic matter on the surface of PVP-capped silver nanoparticle under different aqueous environment. Water Res. 2018, 138, 224–233. [Google Scholar] [CrossRef]
- Berman, T.; Mizrahi, R.; Dosoretz, C.G. Transparent exopolymer particles (TEP): A critical factor in aquatic biofilm initiation and fouling on filtration membranes. Desalination 2011, 276, 184–190. [Google Scholar] [CrossRef]
- Villacorte, L.O.; Kennedy, M.D.; Amy, G.L.; Schippers, J.C. Measuring transparent exopolymer particles (TEP) as indicator of the (bio)fouling potential of RO feed water. Desalin. Water Treat. 2009, 5, 207–212. [Google Scholar] [CrossRef]
- Bar-Zeev, E.; Berman-Frank, I.; Liberman, B.; Rahav, E.; Passow, U.; Berman, T. Transparent exopolymer particles: Potential agents for organic fouling and biofilm formation in desalination and water treatment plants. Desalin. Water Treat. 2009, 3, 136–142. [Google Scholar] [CrossRef] [Green Version]








| MG | MM | GG | |
|---|---|---|---|
| Extracted alginate from the seaweed | 19.2 ± 2.1% | 62.4 ± 3.5% | 19.3 ± 0.9% |
| Commercial alginate | 13.8 ± 1.9% | 53.5 ± 1.3% | 32.7 ± 0.6% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, S.; Wang, R.; Zhang, M.; Meng, X.; Liu, H.; Wang, L. Insights into the Fouling Propensities of Natural Derived Alginate Blocks during the Microfiltration Process. Processes 2019, 7, 858. https://doi.org/10.3390/pr7110858
Meng S, Wang R, Zhang M, Meng X, Liu H, Wang L. Insights into the Fouling Propensities of Natural Derived Alginate Blocks during the Microfiltration Process. Processes. 2019; 7(11):858. https://doi.org/10.3390/pr7110858
Chicago/Turabian StyleMeng, Shujuan, Rui Wang, Minmin Zhang, Xianghao Meng, Hongju Liu, and Liang Wang. 2019. "Insights into the Fouling Propensities of Natural Derived Alginate Blocks during the Microfiltration Process" Processes 7, no. 11: 858. https://doi.org/10.3390/pr7110858