High-Throughput Microfiltration Membranes with Natural Biofouling Reducer Agent for Food Processing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Mixed Matrix Membranes Preparation
2.3. Membranes Thickness Measurement
2.4. Membranes Morphology
2.5. Tensile Strength and Elasticity
2.6. Flux Test
2.7. Anti-Bacterial Test
3. Results
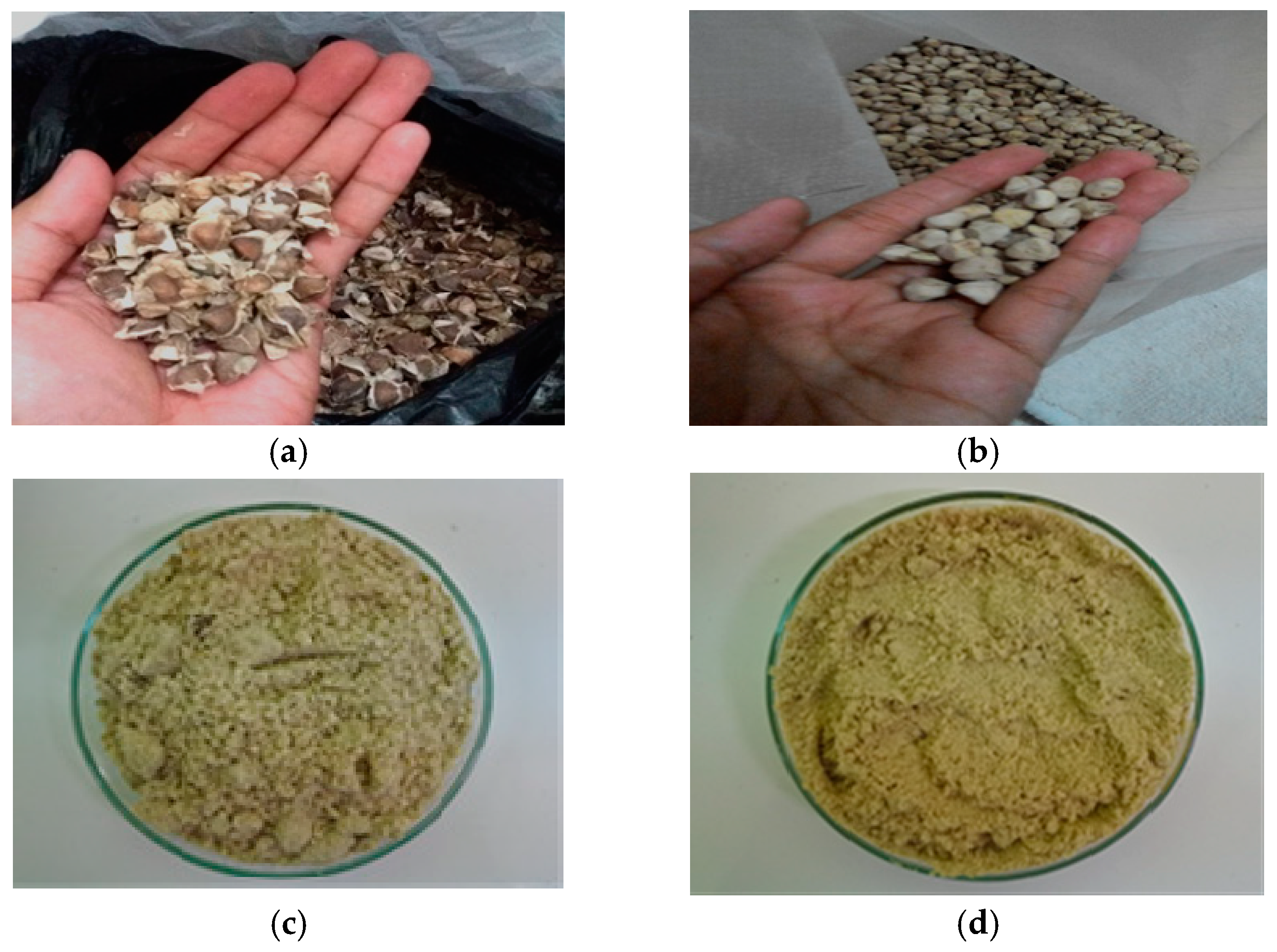
3.1. Moringa Oleifera Seeds
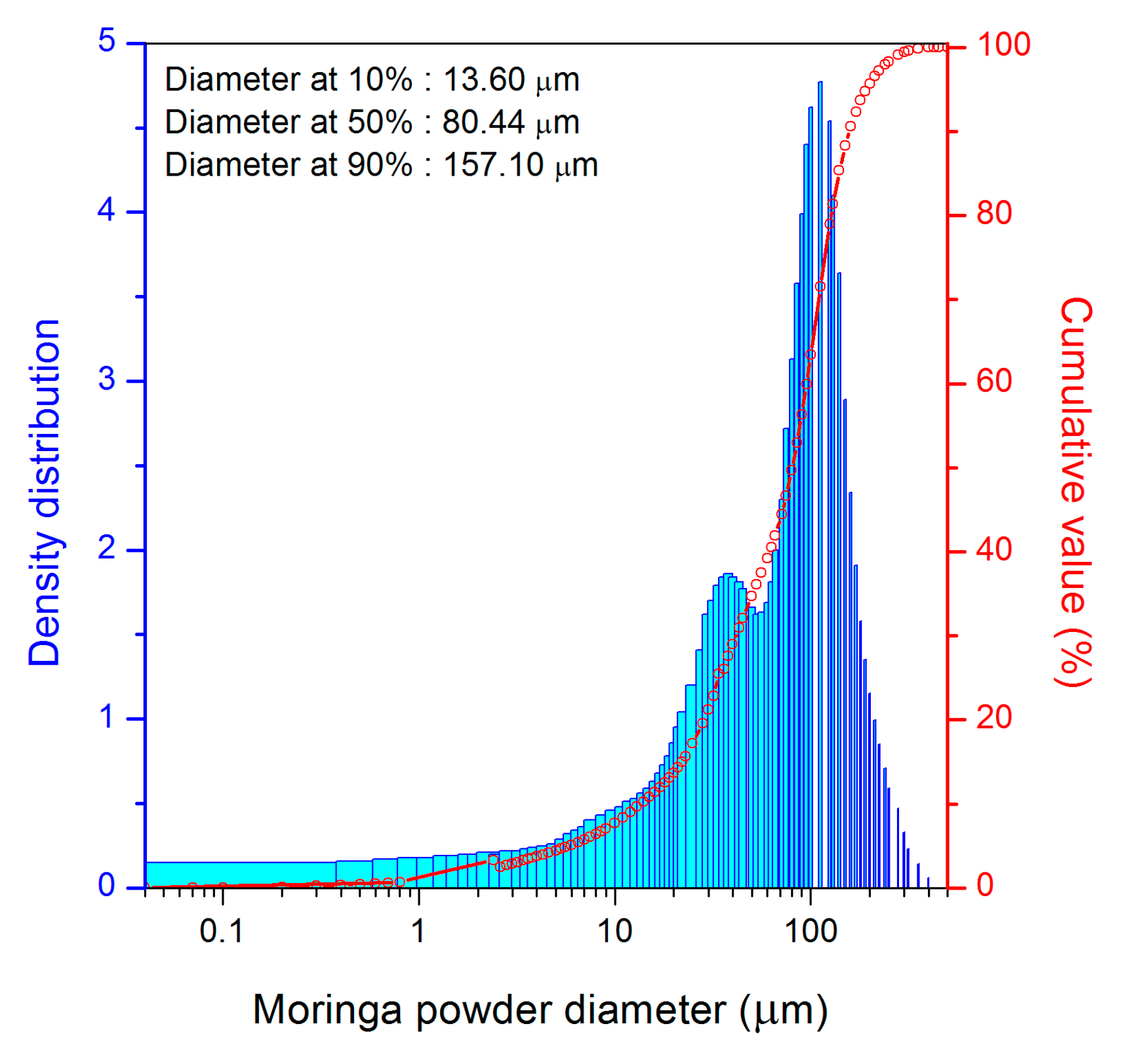
3.1.1. Physical Characteristics
3.1.2. Chemical Composition
3.2. Mechanical Properties of the Membranes
3.2.1. Membranes Thicknesses
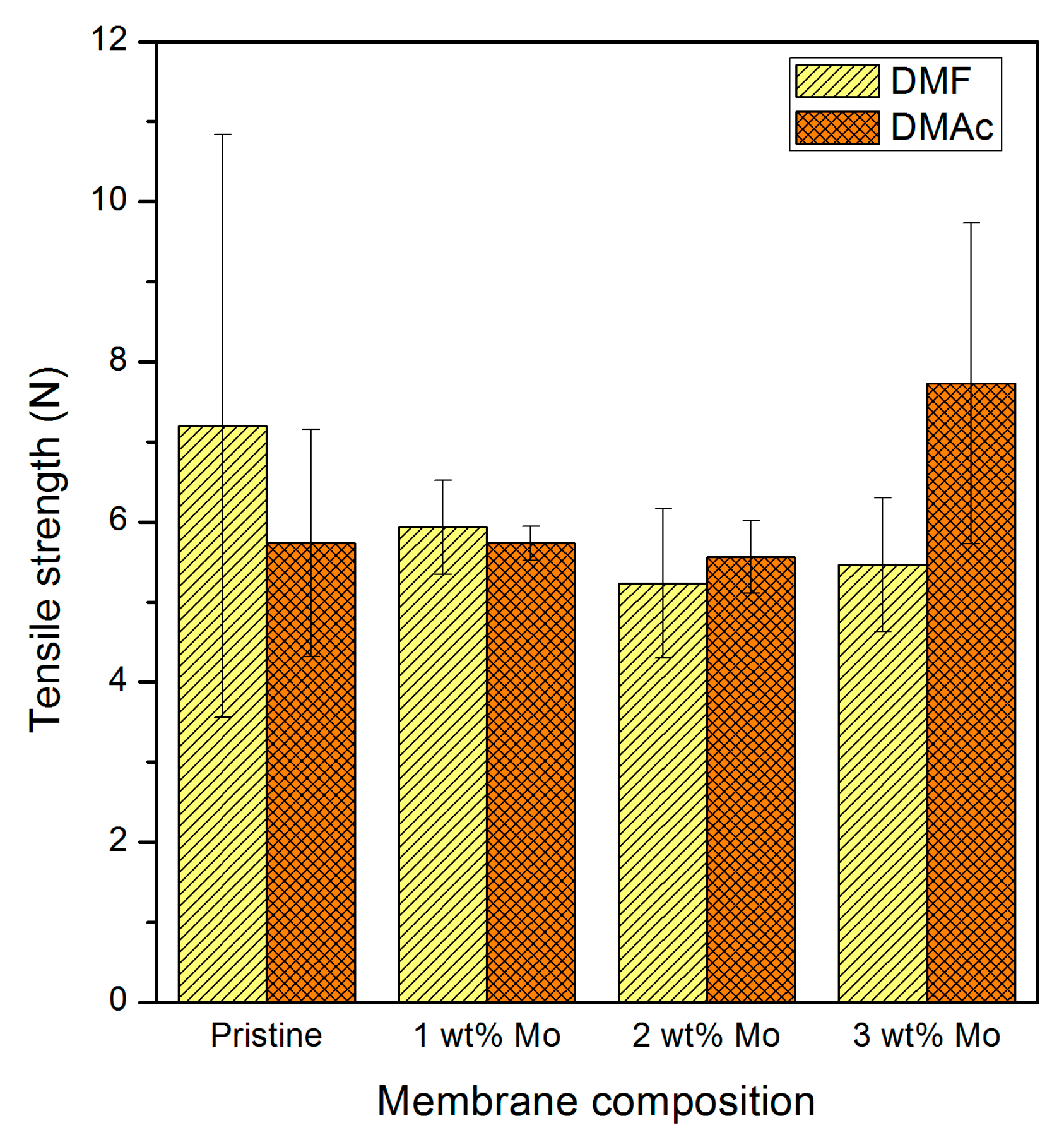
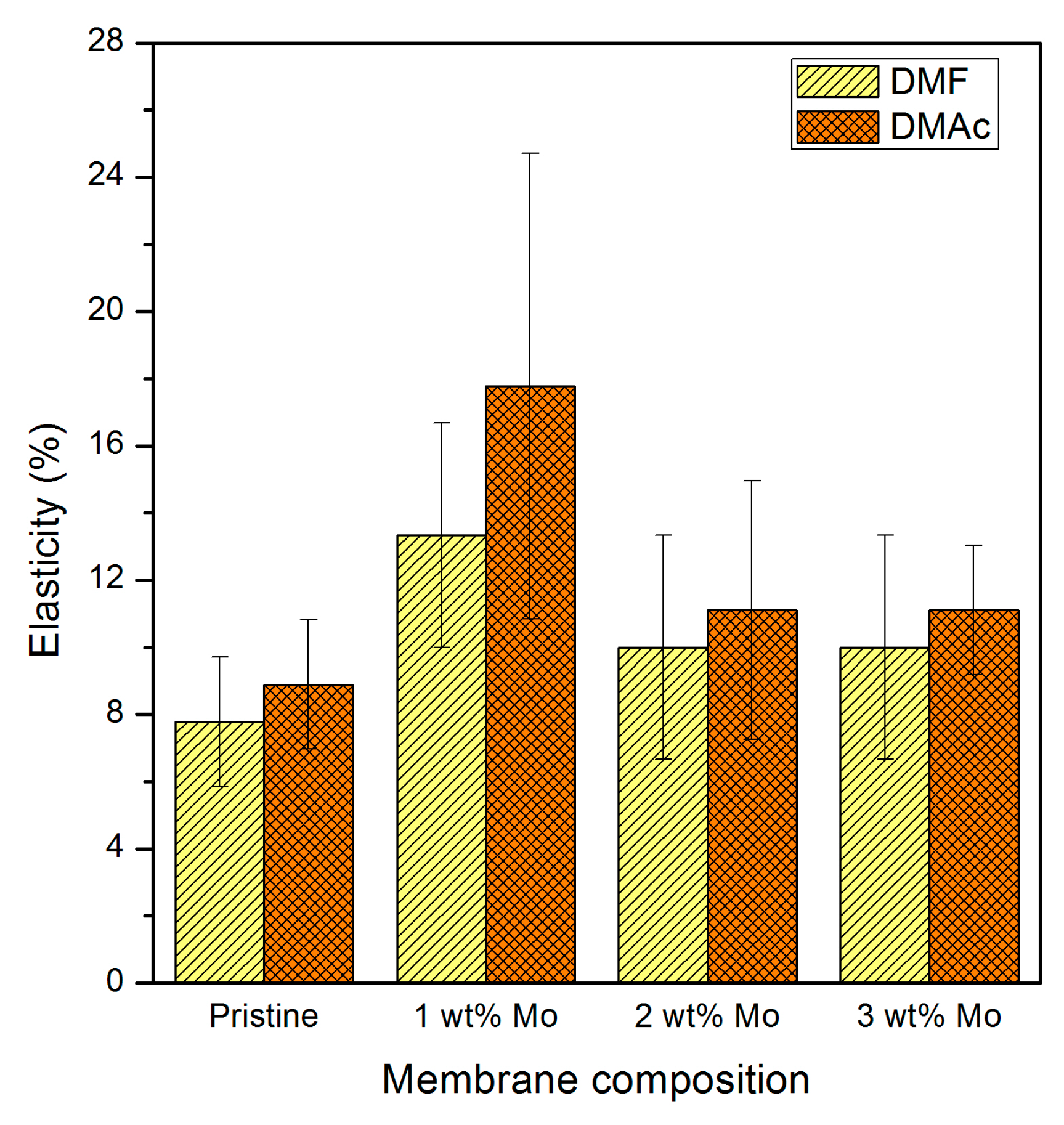
3.2.2. Membrane Sheets Tensile Strength and Elasticity
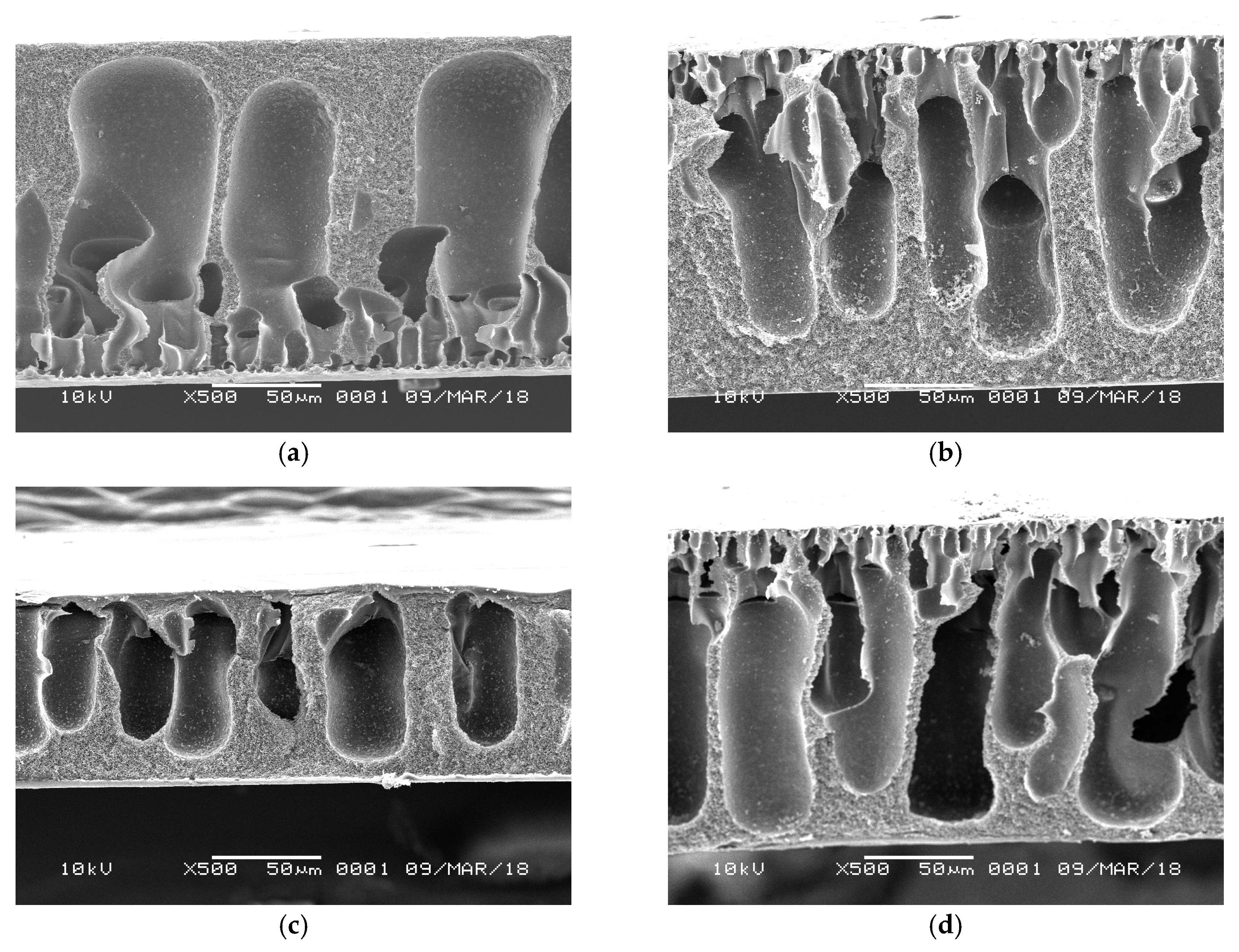
3.3. Membrane Pores and Morphology
3.4. Effect of Adding M. oleifera Seeds Powder on Membrane Flux
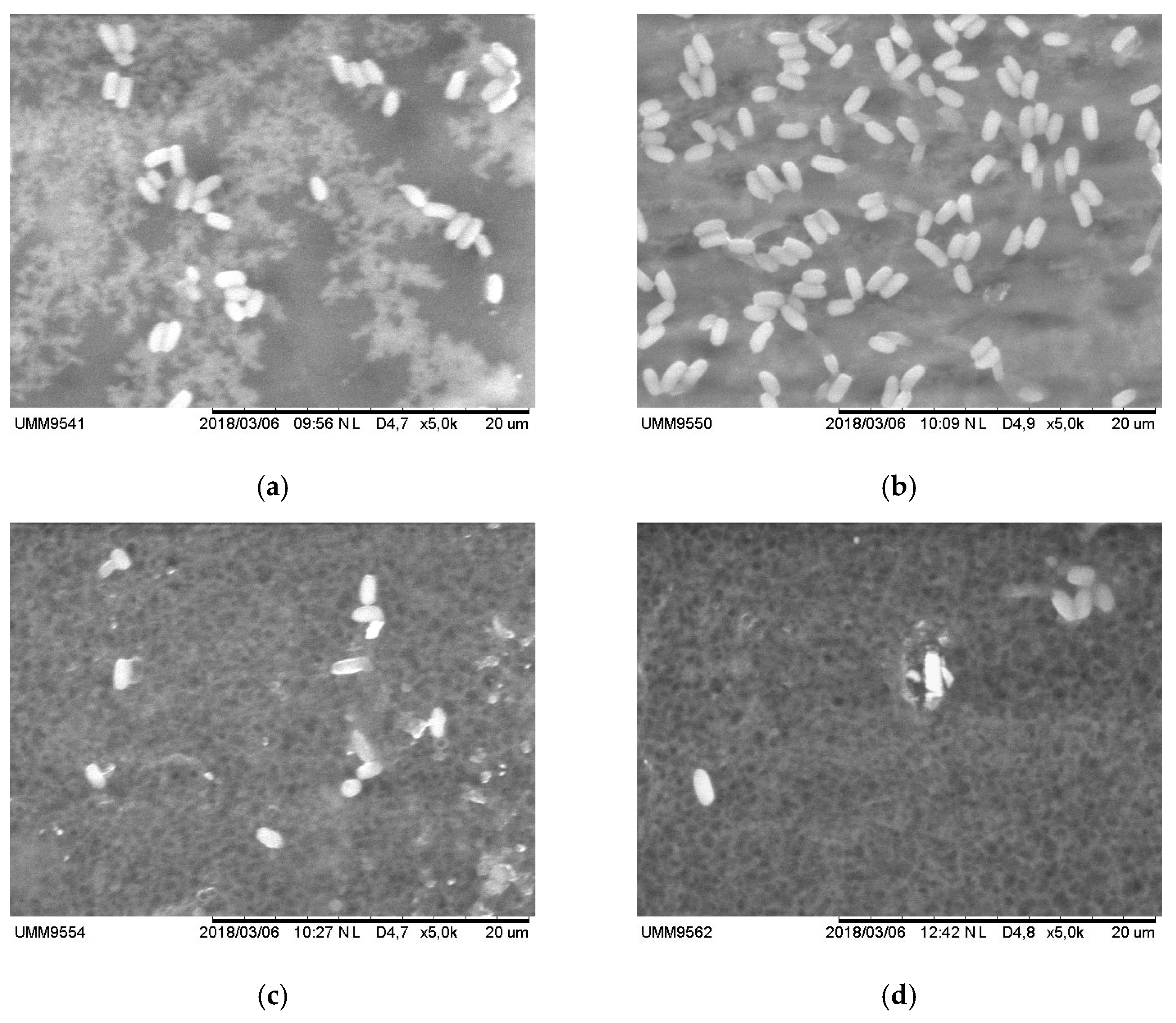
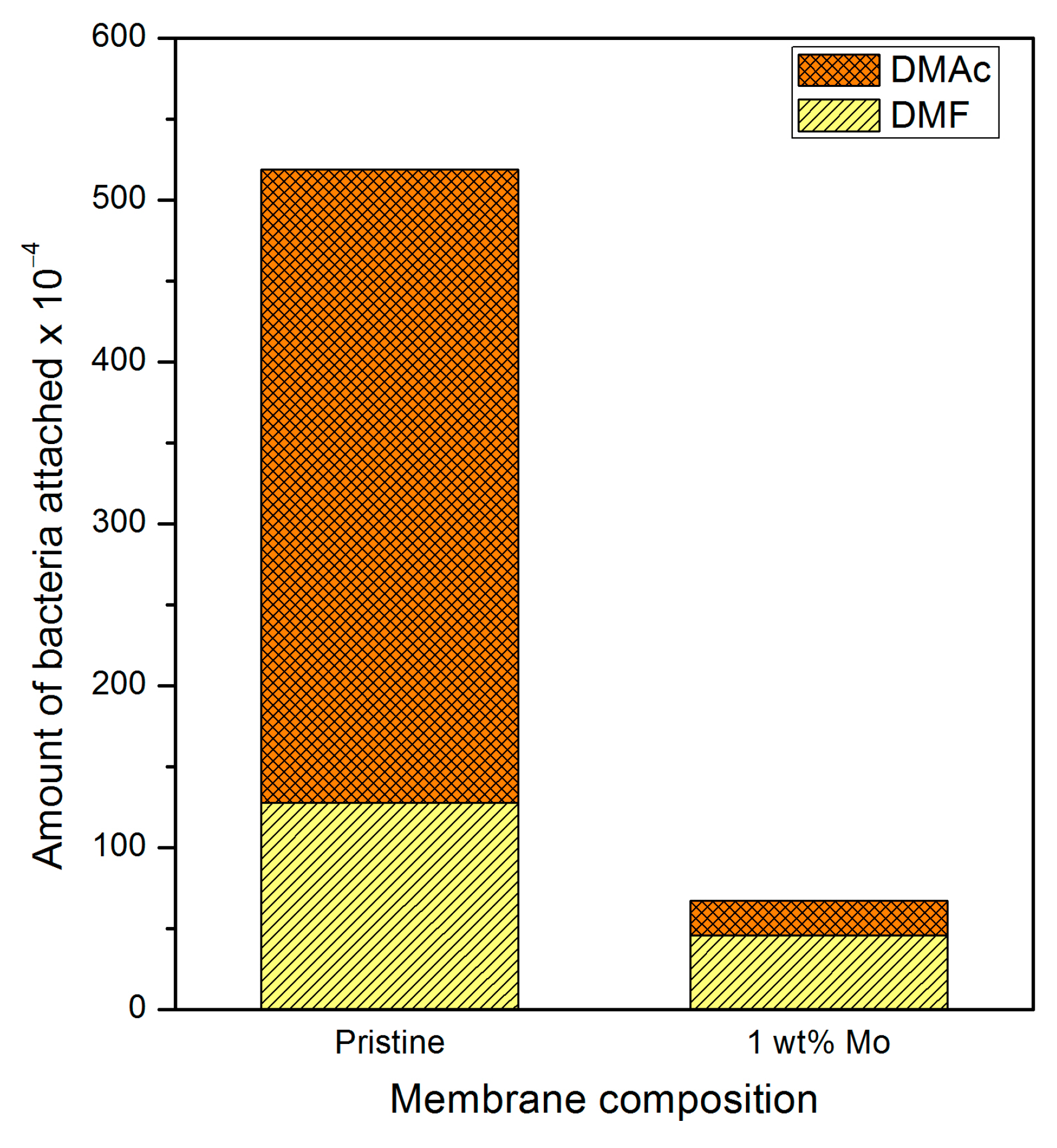
3.5. Effect of Adding M. oleifera on Bacterial Activity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wibisono, Y. Two-Phase Flow for Fouling Control in Membranes. Ph.D. Thesis, University of Twente, Enschede, The Netherlands, 2014; p. 250. [Google Scholar]
- Capannelli, G.; Bottino, A.; Munari, S.; Ballarino, G.; Mirzaian, H.; Rispoli, G.; Lister, G.; Maschio, G. Ultrafiltration of fresh orange and lemon juices. Lebensmittel-Wissenschaft Technologie 1992, 25, 518–522. [Google Scholar]
- De Barros, S.T.D.; Andrade, C.M.G.; Mendes, E.S.; Peres, L. Study of fouling mechanism in pineapple juice clarification by ultrafiltration. J. Membr. Sci. 2003, 215, 213–224. [Google Scholar] [CrossRef]
- Mondal, S.; Cassano, A.; Tasseli, F.; Sirshendu, D. A generalized model for clarification of fruit juice during ultrafiltration under total recycle and batch mode. J. Membr. Sci. 2011, 366, 295–303. [Google Scholar] [CrossRef]
- Wibisono, Y.; Cornelissen, E.R.; Kemperman, A.J.B.; Nijmeijer, D.C.; Van der Meer, W.G.J. Influence of feed spacer geometries on air/water cleaning in spiral wound membrane elements. Procedia Eng. 2012, 44, 613–617. [Google Scholar] [CrossRef]
- Wibisono, Y.; El Obied, K.E.; Cornelissen, E.R.; Kemperman, A.J.B.; Nijmeijer, K. Biofouling removal in spiral wound nanofiltration elements using two-phase flow cleaning. J. Membr. Sci. 2015, 475, 131–146. [Google Scholar] [CrossRef]
- Wibisono, Y.; Yandi, W.; Golabi, M.; Nugraha, R.; Cornelissen, E.R.; Kemperman, A.J.B.; Ederth, T.; Nijmeijer, K. Hydrogel-coated feed spacers in two-phase flow cleaning in spiral wound membrane elements: A novel platform for eco-friendly biofouling mitigation. Water Res. 2015, 71, 171–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, J.; Burgal, J.D.; Szekely, G.; Davies, R.P.; Braddock, D.C.; Livingston, A. Hybrid polymer/MOF membranes for Organic Solvent Nanofiltration (OSN): Chemical modification and the quest for perfection. J. Membr. Sci. 2016, 503, 166–176. [Google Scholar] [CrossRef] [Green Version]
- Fei, F.; Cseri, L.; Szekely, G.; Blanford, C.F. Robust covalently cross-linked polybenzimidazole/graphene oxide membranes for high-flux organic solvent nanofiltration. ACS Appl. Mater. Interfaces 2018, 10, 16140–16147. [Google Scholar] [CrossRef]
- Pal, A.; Dey, T.K.; Debnath, A.K.; Bhusan, B.; Sahu, A.K.; Bindal, R.C.; Kar, S. Mixed-matrix membranes with enhancing antifouling activity: Probing the surface-tailoring potential of Tiron and chromotropic acid for nano-TiO2. R. Soc. Open Sci. 2017, 4, 170368. [Google Scholar] [CrossRef]
- Vatanpour, V.; Shockravi, A.; Zarrabi, H.; Nikjavan, Z.; Javandi, A. Fabrication and characterization of anti-fouling and anti-bacterial Ag-loaded graphene oxide/polyethersulfone mixed matrix membrane. J. Ind. Eng. Chem. 2015, 30, 342–352. [Google Scholar] [CrossRef]
- Lim, M.Y.; Choi, Y.S.; Shin, H.; Kim, K.; Shin, D.M.; Lee, J.C. Cross-linked graphene oxide membrane functionalized with self-cross-linkable and bactericidal cardanol for oil/water separation. ACS Appl. Nano Mater. 2018, 1, 2600–2608. [Google Scholar] [CrossRef]
- Viera, G.H.F.; Mourao, J.A.; Angelo, A.M.; Costa, R.A.; Fernandes, R.H.S. Antibacterial effect (in vitro) of Moringa oleifera and Annona muricata against Gram positive and Gram negative bacteria. Rev. Inst. Med. Trop. Sao Paulo 2010, 52, 129–132. [Google Scholar] [CrossRef]
- Broin, M.; Santaella, C.; Cuine, S.; Kokou, K.; Peltier, G.; Joet, T. Flocculent activity of a recombinant protein from Moringa oleifera Lam seeds. Appl. Microbial. Biotechnol. 2002, 60, 114–119. [Google Scholar] [CrossRef]
- Sun, X.; Lu, C.; Zhang, W.; Tian, D.; Zhang, X. Acetone-soluble cellulose acetate extracted from waste blended fabrics via ionic liquid catalyzed acetylation. Carbohyd. Polym. 2013, 98, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Qinfeng, Z.; Tianhao, W.; Liping, Z. Effects of GO and MOF@GO on the permeation and antifouling properties of cellulose acetate ultrafiltration membrane. J. Membr. Sci. 2019, 569, 48–59. [Google Scholar] [CrossRef]
- Abdellah, M.H.; Perez-Manriquez, L.; Puspasari, T.; Scholes, C.A.; Kentish, S.K.; Peinemann, K.V. A catechin/cellulose composite membrane for organic solvent nanofiltration. J. Membr. Sci. 2018, 567, 139–145. [Google Scholar] [CrossRef]
- Colburn, A.; Wanninayake, N.; Kim, D.Y.; Bhattacharyya, D. Cellulose-graphene quantum dot composite membranes using ionic liquid. J. Membr. Sci. 2018, 556, 293–302. [Google Scholar] [CrossRef]
- Ramirez, J.A.L.; Coello, O.M.D.; Quiroga, A.J.M. Comparative studies of reverse osmosis membranes for wastewater reclamation. Desalination 2006, 191, 137–147. [Google Scholar] [CrossRef]
- Zularisam, A.W.; Ismail, A.F.; Salim, M.R.; Mimi, S.; Ozaki, H. The effects of natural organic matter (NOM) fractions on fouling characteristics and flux recovery of ultrafiltration membranes. Desalination 2007, 212, 191–208. [Google Scholar] [CrossRef]
- Boricha, A.G.; Murthy, Z.V.P. Preparation of N,O-carboxymethyl chitosan/cellulose acetate blend nanofiltration membrane and testing its performance in treating industrial wastewater. Chem. Eng. J. 2010, 157, 393–400. [Google Scholar] [CrossRef]
- Saranya, R.G.A.; Ismail, A.F.; Dionysios, D.D.; Paul, D. Zero-valent iron impregnated cellulose acetate mixed matrix membranes for the treatment of textile industry effluent. RSC Adv. 2015, 5, 62486–62497. [Google Scholar] [CrossRef]
- Dima, L.R.H. Uji aktivitas antibakteri ekstrak daun kelor (Moringa oleifera L.) terhadap bakteri Escherichia coli dan Staphylococcus aureus (in Bahasa Indonesia). Pharmacon 2016, 5, 282–289. [Google Scholar]
- McLandsborough, L. Food Microbiology Laboratory; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Wibisono, Y.; Faradilla, A.; Utoro, P.A.; Sukoyo, A.; Izza, N.; Dewi, S.R. Natural anti-biofoulant Moringa oleifera impregnated cellulose acetate mixed matrix membrane for juice clarification. J. Chem. Eng. Env. 2018, 13, 100–109. [Google Scholar]
- Pratiwi, M.K.; Masyrifah, L.; Hawa, L.C.; Dewi, S.R.; Izza, N.; Argo, B.D.; Sucipto, S.; Wibisono, Y. Enhanced antibiofouling properties of chitosan-based membranes by coating and blending of Moringa oleifera L. extract. IOP Conf. Ser. Mater. Sci. Eng. 2018, 434, 012191. [Google Scholar] [CrossRef]
- Camacho, F.P.; Bongiovani, M.C.; Silva, M.O.; Coldebella, P.F.; Amorim, M.T.; Bergamasco, R. Coagulation/flocculation/flotation/nanofiltration processes using Moringa oleifera as coagulant of eutrophized river. Chem. Eng. Trans. 2015, 43, 1123–1128. [Google Scholar] [CrossRef]
- Singh, R.G.; Negi, P.S.; Radha, C. Phenolic composition, antioxidant and antimicrobial activities of free and bound phenolic extracts of Moringa oleifera seed flour. J. Funct. Foods 2013, 5, 1883–1891. [Google Scholar] [CrossRef]
- Brandup. Handbook Polymer, 2nd ed.; John W & Son: New York, NY, USA, 1975. [Google Scholar]
- Mulder, M. Basic Principles of Membrane Technology; Kluwer Academic Publisher: Dordrecht, The Netherlands, 1996. [Google Scholar]
- Xi, J.; Li, Z.; Yu, L.; Yin, B.; Wang, L.; Liu, L.; Qiu, X.; Chen, L. Effect of degree of sulfanation and casting solvent on sulfanated poly (ether ether ketone) membrane for vanadium redox flow battery. J. Power Sources 2015, 285, 195–204. [Google Scholar] [CrossRef]
- Chakrabarty, B.; Ghshal, A.K.; Purkait, M.K. Effect of molecular weight of PEG on membrane morphology and transport properties. J. Membr. Sci. 2008, 309, 209–221. [Google Scholar] [CrossRef]
- Liu, H.; Tang, C. Electrospinning of cellulose acetate in solvent mixture N,N-Dimethylacetamide (DMAc)/Acetone. Polym. J. 2007, 30, 65–72. [Google Scholar] [CrossRef]
- Ahmad, A.; Waheed, S.; Khan, S.M.; Shafiq, M.; Farooq, M.; Sanaullah, K.; Jamil, T. Effect of silica on the properties of cellulose acetate/polyethylene glycol membranes for reverse osmosis. Desalination 2015, 355, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Chunyu, L.; Alejandro, S. Cohesive energy density and solubility parameter evolution during the curing of thermoset. Polymer 2018, 135, 162–170. [Google Scholar] [CrossRef]
- Hildebrand, J.H.; Scott, R.L. The Solubility of Non-Electrolytes; Reinhold: New York, NY, USA, 1936. [Google Scholar]
- Razali, M.; Didaskalou, C.; Kim, J.F.; Babaei, M.; Drioli, E.; Lee, Y.M.; Szekely, G. Exploring and exploiting the effect of solvent treatment in membrane separation. ACS Appl. Mater. Interfaces 2017, 9, 11279–11289. [Google Scholar] [CrossRef]
- David, D.J.; Sincock, T.F. Estimation of miscibility of polymer blends using the solubility parameter concept. Polymer 1992, 33, 4505–4514. [Google Scholar] [CrossRef]
- Sivakumar, M.; Mohan, D.R.; Rangarajan, R. Studies on cellulose acetate-polysulfone ultrafiltration membranes II. Effect of additive concentration. J. Membr. Sci. 2006, 268, 208–219. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Roque, E.B.; Da Fonseca, F.V.; Borges, C.P. High flux microfiltration membranes with silver nanoparticles for water disinfection. Desalin. Water Treat. 2015, 56, 3590–3598. [Google Scholar] [CrossRef]
- Ariono, D.; Aryanti, P.T.P.; Subagjo, S.; Wenten, I.G. The effect of polymer concentration on flux stability of polysulfone membrane. AIP Conf. Proc. 2017, 1788, 030048. [Google Scholar] [CrossRef]
- Miller, D.J.; Kasemset, S.; Paul, D.R.; Freeman, B.D. Comparison of membrane fouling at constant flux and constant transmembrane pressure conditions. J. Membr. Sci. 2013, 454, 505–515. [Google Scholar] [CrossRef]
- Karim, Z.; Mathew, A.J.; Kokol, V.; Wei, J.; Grahn, M. High-flux affinity membranes based on cellulose nanocomposites for removal of heavy metal ions from industrial effluents. RSC Adv. 2016, 6, 20644–20653. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, R.S.; Napoleao, T.H.; Santos, A.F.S.; Sa, R.A.; Carneiro-da-Cunha, M.G.; Morais, M.M.C.; Silva-Lucca, R.A.; Oliva, M.L.V.; Coelho, L.C.B.B.; Paiva, P.M.G. Coagulant and antibacterial activties of the water-soluble seed lectin from Moringa oeifera. Lett. Appl. Microbiol. 2011, 53, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Didaskalou, C.; Kupai, J.; Cseri, L.; Barabas, J.; Vass, E.; Holtzl, T.; Szekely, G. Membrane-grafted asymmetric organocatalyst for an integrated synthesis-separation platform. ACS Catal. 2018, 8, 7430–7438. [Google Scholar] [CrossRef]
- Valtcheva, I.B.; Marchetti, P.; Livingston, A.G. Crosslinked polybenzimidazole membranes for organic solvent nanofiltration (OSN): Analysis of crosslinking reaction mechanism and effect of reaction parameters. J. Membr. Sci. 2015, 493, 568–579. [Google Scholar] [CrossRef]
- Wibisono, Y.; Ahmad, F.; Cornelissen, E.R.; Kemperman, A.J.B.; Nijmeijer, K. Dominant factors controlling the efficiency of two-phase flow cleaning in spiral-wound membrane elements. Desalin. Water. Treat. 2016, 57, 17625–17636. [Google Scholar] [CrossRef]



| Treatment | CA (g) | Mo (g) | CA + Mo (g) | DMF (mL) | DMAc (mL) |
|---|---|---|---|---|---|
| CA + DMF (Pristine DMF) | 4 | - | 4 | 20 | - |
| CA + 1 wt% Mo + DMF | 3.96 | 0.04 | 4 | 20 | - |
| CA + 2 wt% Mo + DMF | 3.92 | 0.08 | 4 | 20 | - |
| CA + 3 wt% Mo + DMF | 3.88 | 0.12 | 4 | 20 | - |
| CA + DMAc (Pristine DMAc) | 4 | - | 4 | - | 20 |
| CA + 1 wt% Mo + DMAc | 3.96 | 0.04 | 4 | - | 20 |
| CA + 2 wt% Mo + DMAc | 3.92 | 0.08 | 4 | - | 20 |
| CA + 3 wt% Mo + DMAc | 3.88 | 0.12 | 4 | - | 20 |
| Peak (cm−1) | Functional Group | Name |
|---|---|---|
| 986.32 | C=C | Alkene |
| 1057.68 | C–O | Primary Alcohol |
| 1237.05 | C–O | Alkyl Aryl Ester |
| 1269.84 | C–O | Aromatic ester |
| 1379.77 | O–H | Phenol |
| 1746.22 | C=O | Ester |
| 2689.35 | C–H | Aldehyd |
| 2924.65 | C–H/N–H | Alkene/Amine Salt |
| 3321.96 | O–H | Alcohol |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Utoro, P.A.R.; Sukoyo, A.; Sandra, S.; Izza, N.; Dewi, S.R.; Wibisono, Y. High-Throughput Microfiltration Membranes with Natural Biofouling Reducer Agent for Food Processing. Processes 2019, 7, 1. https://doi.org/10.3390/pr7010001
Utoro PAR, Sukoyo A, Sandra S, Izza N, Dewi SR, Wibisono Y. High-Throughput Microfiltration Membranes with Natural Biofouling Reducer Agent for Food Processing. Processes. 2019; 7(1):1. https://doi.org/10.3390/pr7010001
Chicago/Turabian StyleUtoro, Panggulu Ahmad R., Agung Sukoyo, Sandra Sandra, Nimatul Izza, Shinta Rosalia Dewi, and Yusuf Wibisono. 2019. "High-Throughput Microfiltration Membranes with Natural Biofouling Reducer Agent for Food Processing" Processes 7, no. 1: 1. https://doi.org/10.3390/pr7010001






