Operator Training Simulator for an Industrial Bioethanol Plant
Abstract
:1. Introduction
- Time-efficient training of operators within a safe virtual environment
- Rapid evaluation of operating concepts and procedures
- Introduction of new operators to operational procedures
- Reduction in time for start-up and shut-down due to better-trained operators
- Increased operator skills in identifying faults and adjusting process parameters
- Training of troubleshooting without compromising the real process.
2. Materials and Methods
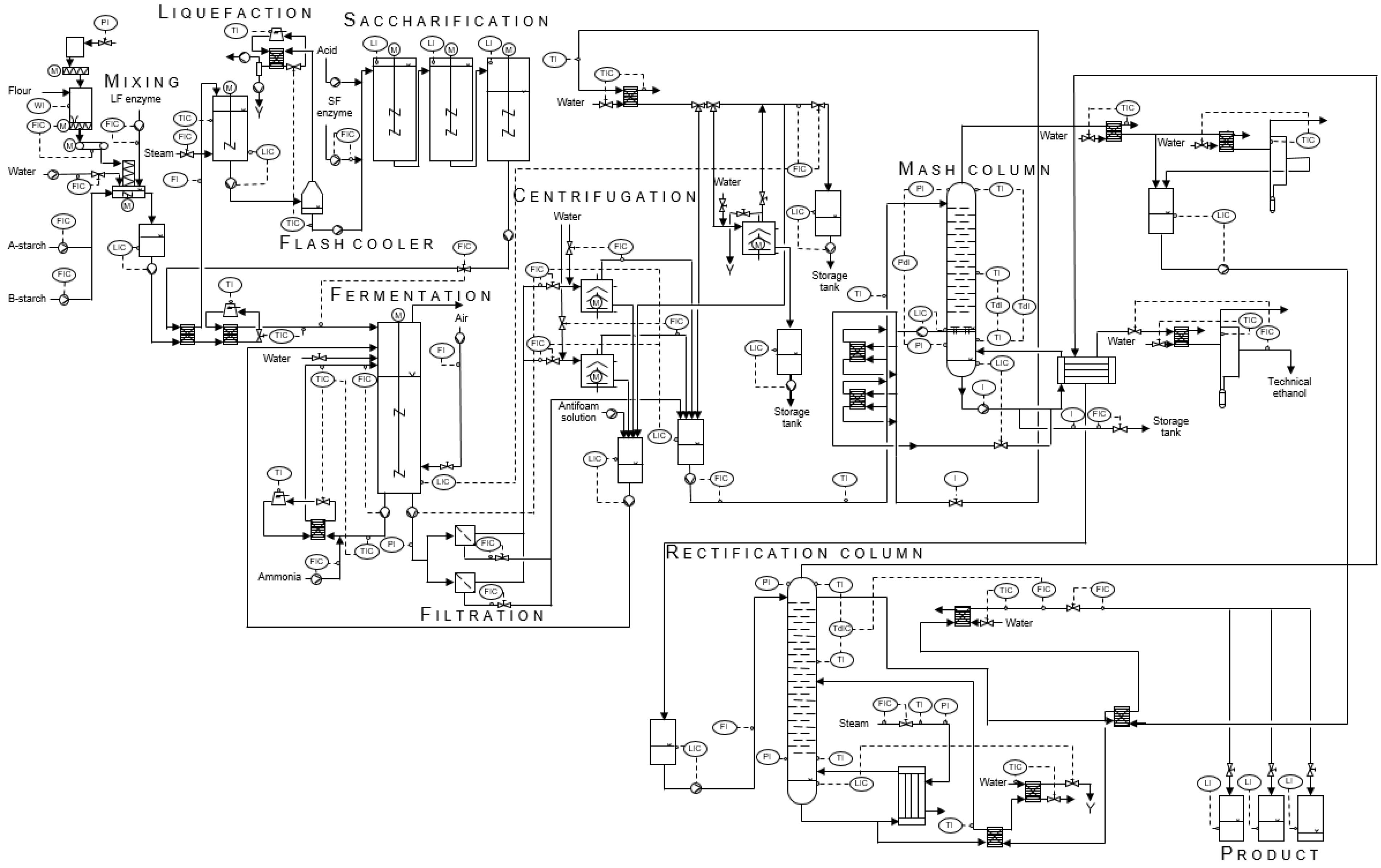
2.1. The Bioethanol Plant
- A pretreatment section, for mixing of the cereal raw materials
- A hydrolysis section, for liquefaction and saccharification
- A fermentation section, for conversion of glucose to ethanol by a commercial yeast strain
- A separation section, using filtration and centrifugation
- A distillation section, including one mash and one rectification column.
2.2. Simulation Software
3. Results and Discussion
3.1. Strategy for Development of OTS
- Support existing training procedures at the plant for increasing training efficiency and decrease training time
- Exploit the possibility of accelerating real process time in the simulation to enhance time-efficient training
- Allow training of existing standard operating procedures (SOPs) at the plant
- Allow training of start-up and shut-down procedures without interfering with the real process
- Allow training of typical troubleshooting events and incident situations occurring at the plant
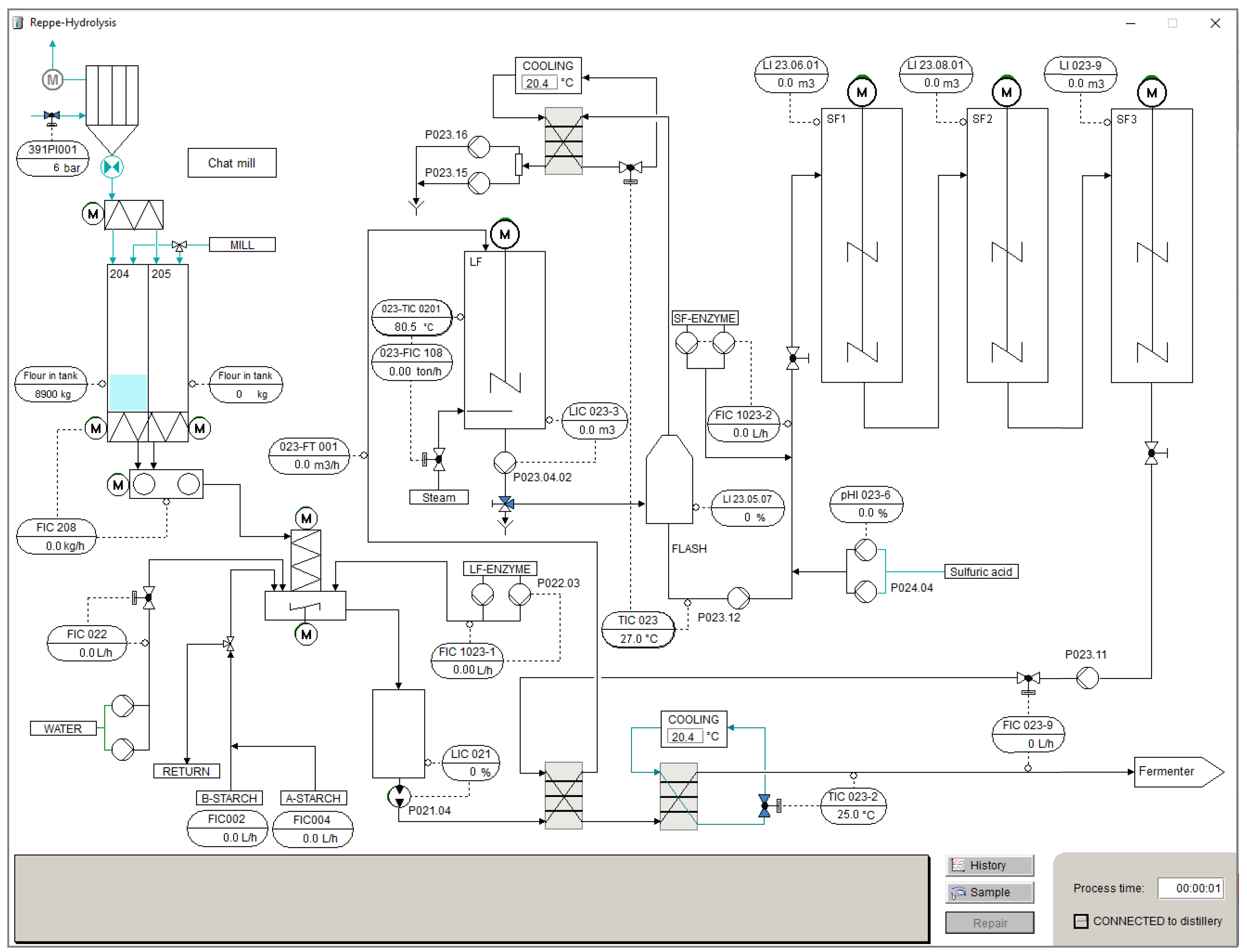
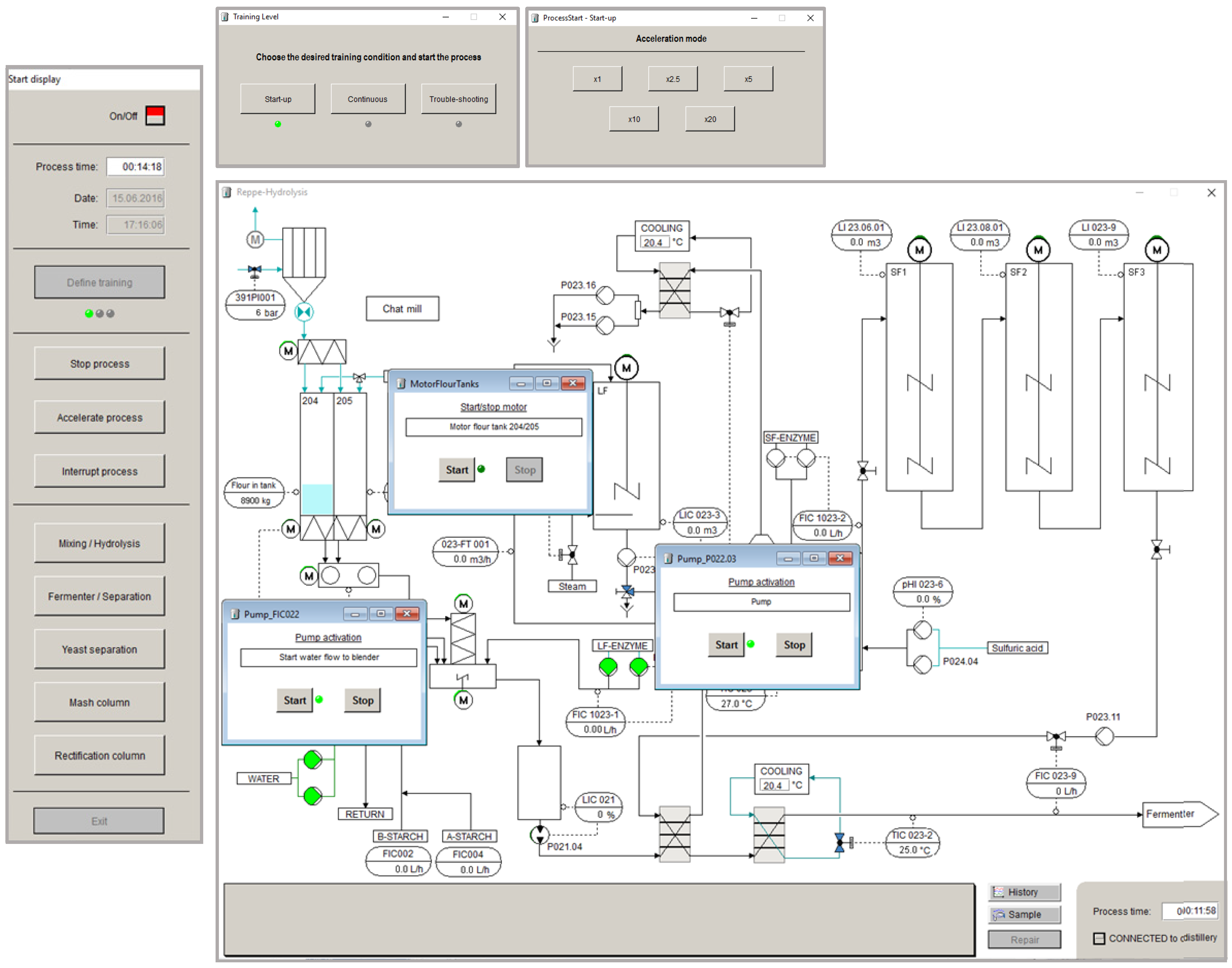
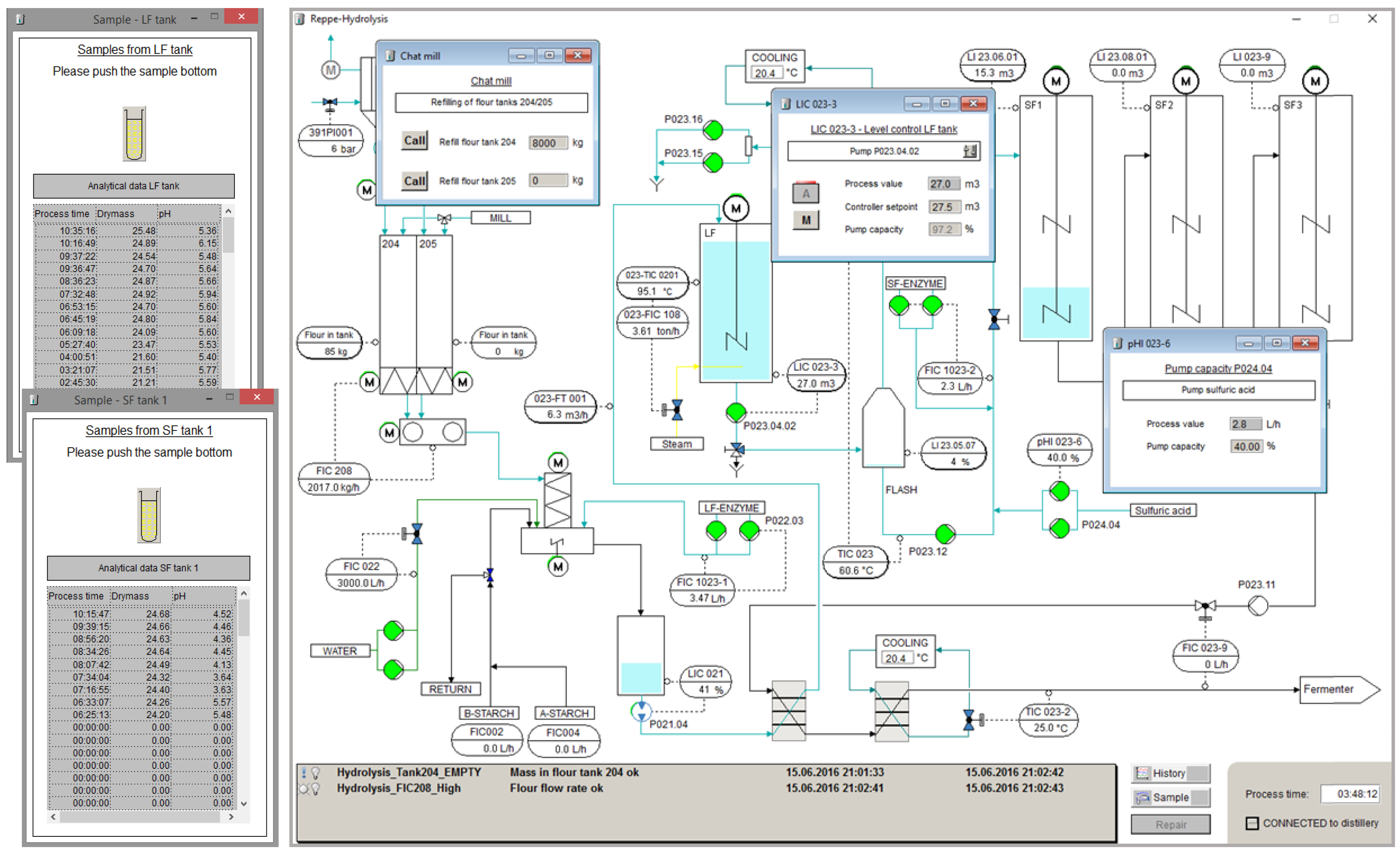
3.2. Graphical User Interfaces
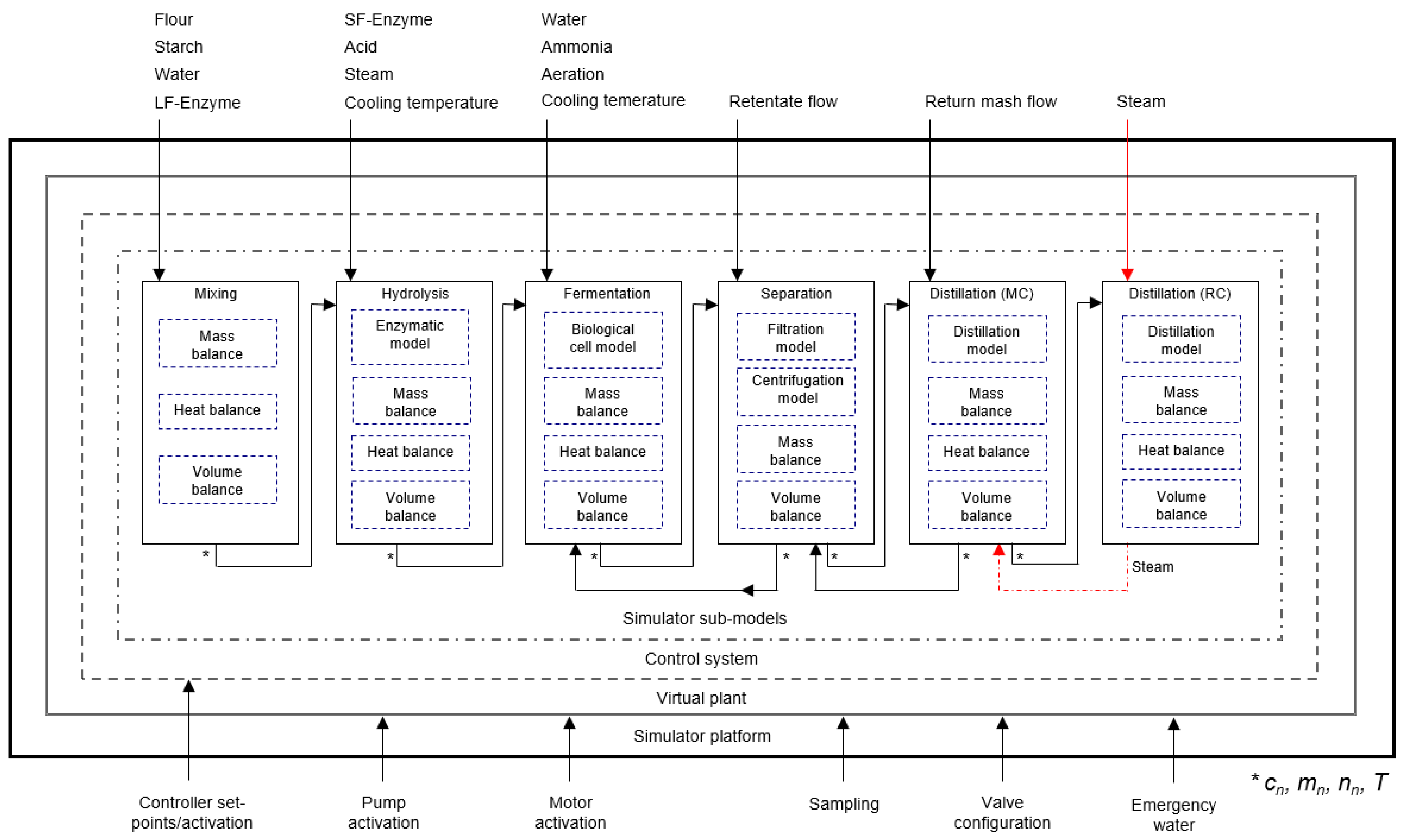
3.3. Modelling Framework of the Simulator
- Pre-treatment (general tank model for mixing of components)
- Hydrolysis of starch (including liquefaction, saccharification, flash cooler and heat exchangers)
- Fermentation of glucose to ethanol by yeast cells (including heat exchanger)
- Separation of yeast and return mash (including filtration and centrifugation as well as intermediate tanks)
- Distillation of the broth in the mash column to approximately 40% (v/v) ethanol (including heat exchangers and intermediate tanks)
- Distillation of the distillate in the rectification column to approximately 96% (v/v) ethanol (including heat exchangers as well as intermediate and product tanks).
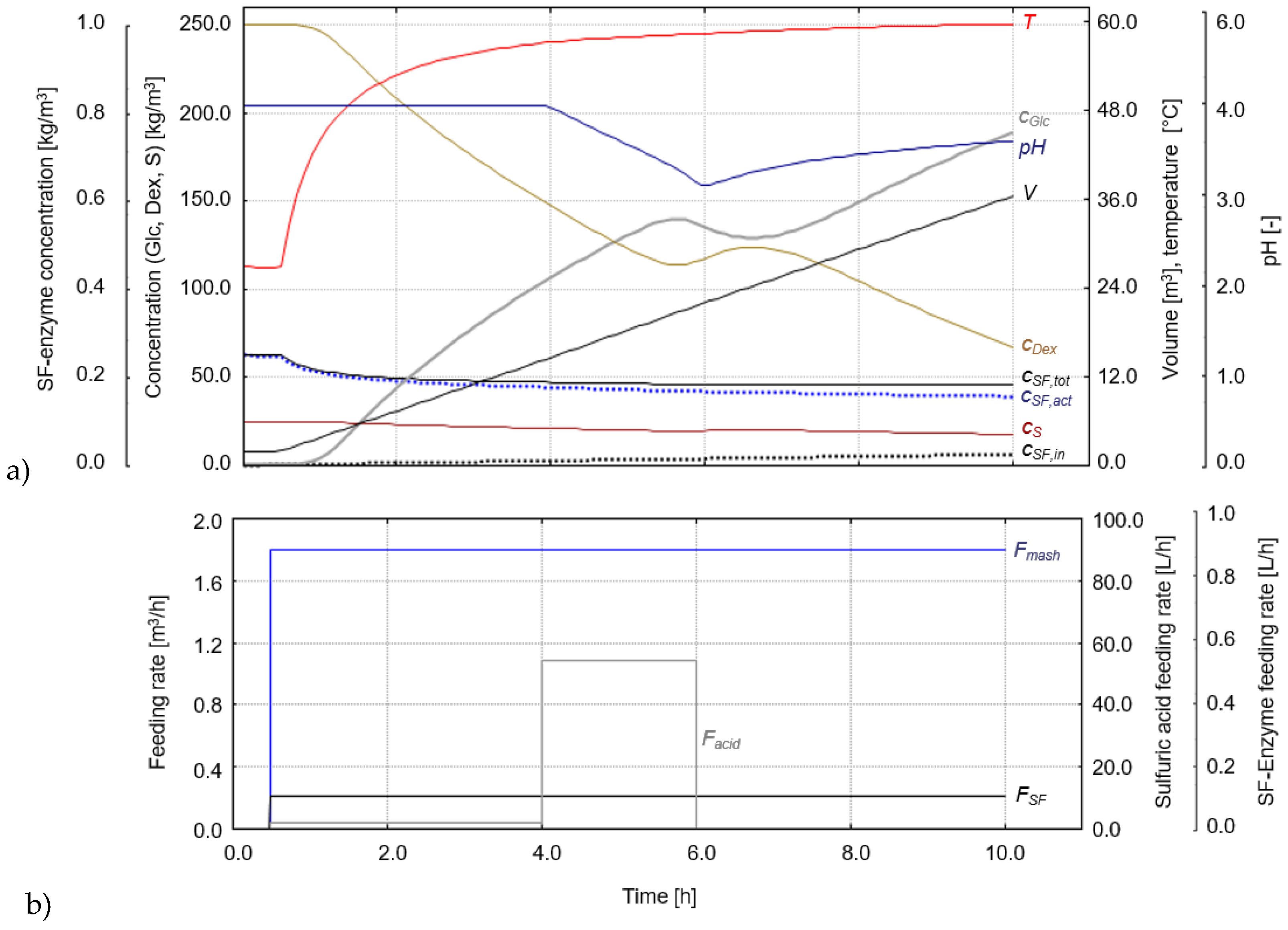
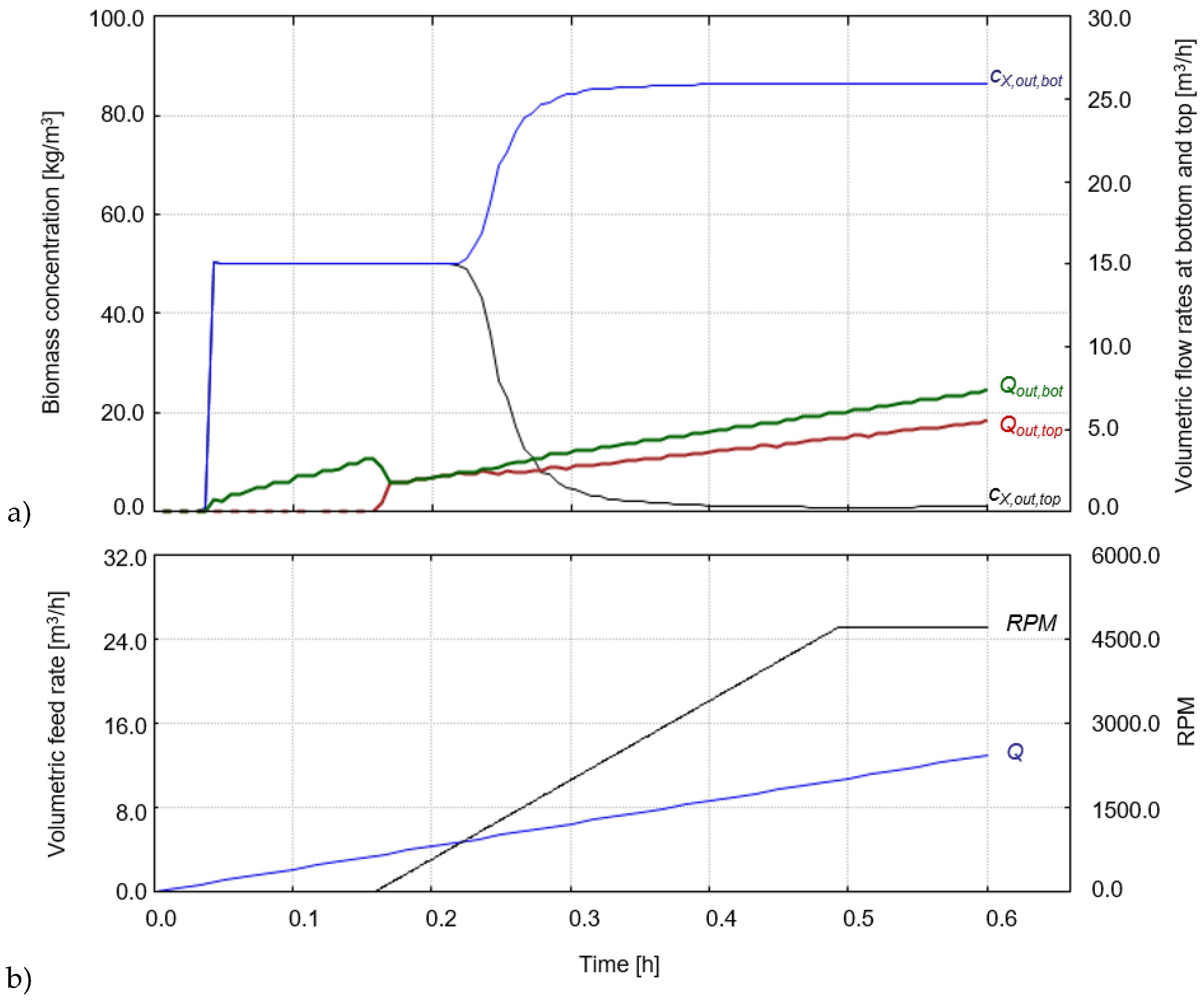
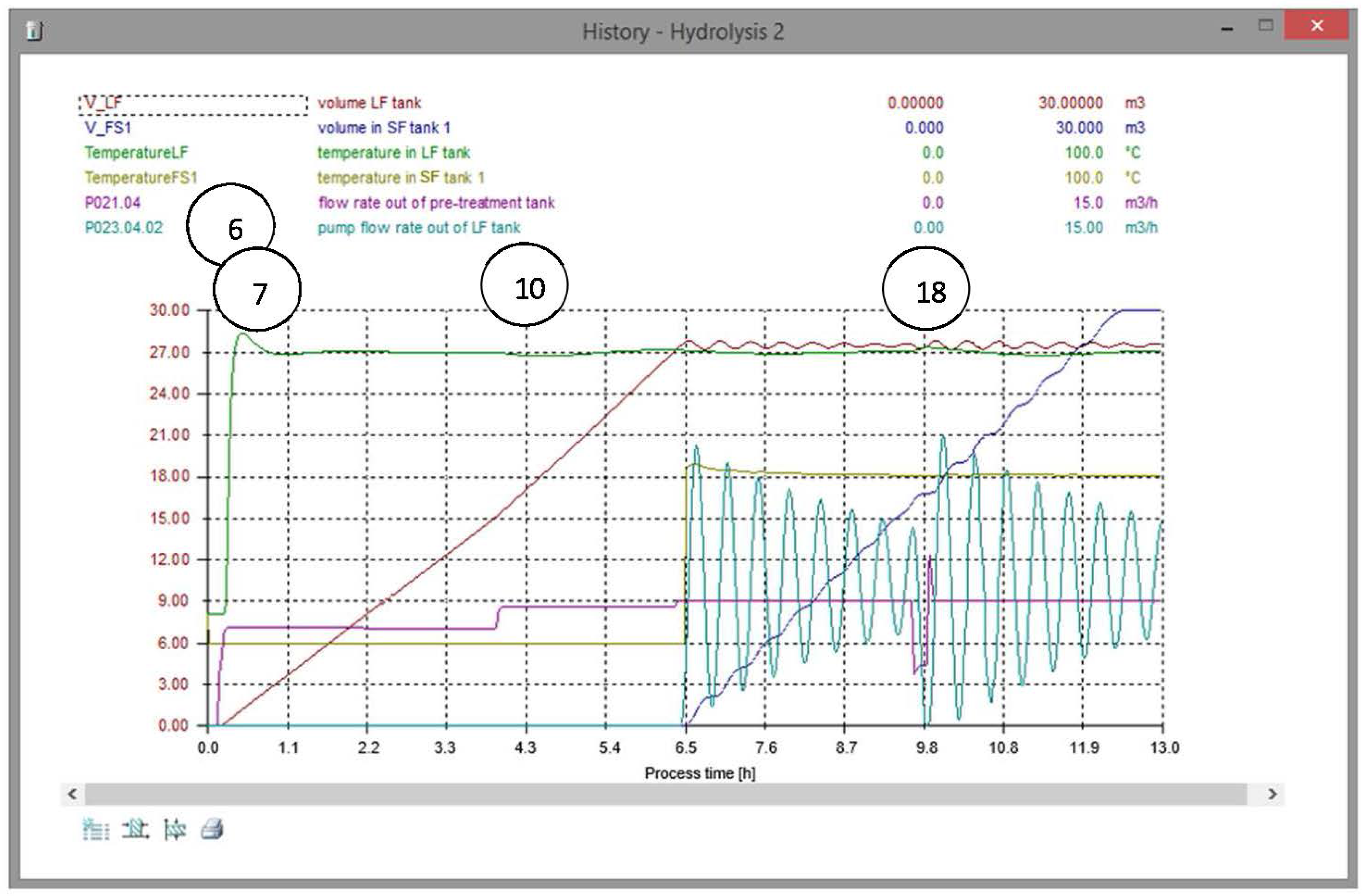
3.3.1. Hydrolysis
3.3.2. Fermentation
3.3.3. Filtration
3.3.4. Centrifugation
3.3.5. Distillation
3.4. Experiences of Training with the OTS
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mani, S.; Shoor, S.K.; Pedersen, H.S. Experience with a simulator for training ammonia plant operators. Plant Oper. Prog. 1990, 9, 6–10. [Google Scholar] [CrossRef]
- Bell, H.H.; Waag, W.L. Evaluating the effectiveness of flight simulators for training combat skills: A review. Int. J. Aviat. Psychol. 1998, 8, 223–242. [Google Scholar] [CrossRef]
- Taylor, H.L.; Lintern, G.; Hulin, C.L. Transfer of training effectiveness of a personal computer aviation training device. Int. J. Aviat. Psychol. 1999, 9, 319–335. [Google Scholar] [CrossRef]
- Young, B.R.; Mahoney, D.P.; Svrcek, W.Y. Simulation workshops for the process control education of undergraduate chemical engineers. Comp. App. Eng. Educ. 2001, 9, 57–62. [Google Scholar] [CrossRef]
- Kneebone, R. Simulation in surgical training: Educational issues and practical implications. Med. Educ. 2003, 37, 267–277. [Google Scholar] [PubMed]
- Lee, A.T. Flight Simulators: Virtual Environment in Aviation; Ashgate Publishing Ltd.: London, UK, 2005. [Google Scholar]
- Diaz, M.; Garrido, D.; Romero, S.; Rubio, B.; Soler, E.; Troya, J.M. Experiences with component-oriented technologies in nuclear power plant simulators. Softw. Pract. Exp. 2006, 36, 1489–1512. [Google Scholar] [CrossRef]
- Cosman, P.; Hemli, J.M.; Ellis, A.M.; Hugh, T.J. Learning the surgical craft: A review of skills training options. ANZ J. Surg. 2007, 77, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Fletscher, J.D. Education and training technology in the military. Science 2009, 323, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Brambilla, S.; Manca, D. Recommended features of an industrial accident simulator for training of operators. J. Loss Prev. Process Ind. 2011, 24, 344–355. [Google Scholar] [CrossRef]
- Murai, K.; Okazaki, T.; Hayashi, Y. A few comments on visual systems of a ship handling simulator for sea pilot training: Training for entering a port. Electron. Commun. (Jpn.) 2011, 94, 10–17. [Google Scholar] [CrossRef]
- Andersson, H.; Herzog, E.; Ölvander, J. Experience from model and software reuse in aircraft simulator product line engineering. Inf. Softw. Technol. 2013, 55, 595–606. [Google Scholar] [CrossRef]
- Balaton, M.G.; Nagy, L.; Szeifert, F. Operator training simulator process model implementation of a batch processing unit in a packaged simulation software. Comput. Chem. Eng. 2013, 48, 335–344. [Google Scholar] [CrossRef]
- Patle, D.S.; Ahmad, Z.; Rangaiah, G.P. Operator training simulators in the chemical industry: Review, issues, and future directions. Rev. Chem. Eng. 2014, 30, 199–216. [Google Scholar] [CrossRef]
- Abel, J. Aging HPI workforce drives need for operator training systems. Hydrocarb. Process. 2011, 90, 11–16. [Google Scholar]
- Szafnicki, K.; Narce, C.; Bourgois, J. Towards an integrated tool for control, supervision and operator training: Application to industrial wastewater detoxication plants. Control Eng. Pract. 2005, 13, 729–738. [Google Scholar] [CrossRef]
- Nazir, S.; Colombo, S.; Manca, D. Testing and analyzing different training methods for industrial operators: An experimental approach. Comp. Aided Chem. Eng. 2013, 32, 667–672. [Google Scholar]
- Nazir, S.; Colombo, S.; Manca, D. Minimizing the risk in the process industry by using a plant simulator: A novel approach. Chem. Eng. Trans. 2013, 32, 109–114. [Google Scholar]
- Nazir, S.; Manca, D. How a plant simulator can improve industrial safety. Process Safety Prog. 2015, 34, 237–243. [Google Scholar] [CrossRef]
- Nazir, S.; Colombo, S.; Manca, D. Impact of training methods on distributed awareness of industrial operators. Saf. Sci. 2015, 73, 135–145. [Google Scholar] [CrossRef]
- Kluge, A.; Nazir, S.; Manca, D. Advanced applications in process control and training needs of field and control room operators. IIE Trans. Occup. Ergon. Hum. Factors 2014, 2, 121–136. [Google Scholar] [CrossRef]
- Blesgen, A.; Hass, V.C. Efficient biogas production through process simulation. Energy Fuels 2010, 24, 4721–4727. [Google Scholar] [CrossRef]
- Hass, V.C.; Kutzsch, S.; Gerlach, I.; Kühn, K.; Winterhalter, M. Towards the development of a training simulator for biorefineries. Chem. Eng. Trans. 2012, 29, 247–252. [Google Scholar]
- Gerlach, I.; Brüning, S.; Hass, V.C.; Mandenius, C.F. Virtual bioreactor cultivation for operator training and simulation: Application to ethanol and protein production. J. Chem. Biotechnol. 2013, 88, 2159–2168. [Google Scholar] [CrossRef]
- Gerlach, I.; Brüning, S.; Gustavsson, R.; Mandenius, C.F.; Hass, V.C. Operator training in recombinant protein production using a structured simulator model. J. Biotechnol. 2014, 177, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Gerlach, I.; Mandenius, C.F.; Hass, V.C. Operator training simulation for integrating cultivation and homogenisation in protein production. Biotechnol. Rep. 2015, 6, 91–99. [Google Scholar] [CrossRef]
- Kuntzsch, S. Energy Efficiency Investigations with a New Operator Training Simulator for Biorefineries. [dissertation, Ph.D. Biochemical Engineering], Jacobs University Bremen, Bremen, Germany, 24 February 2014. [Google Scholar]
- Hariri, S.; Srivastava, S.; Youngblood, P.; Ladd, A. Evaluation of a surgical simulator for learning clinical anatomy. Med. Educ. 2004, 38, 896–902. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Liu, B.S. Inflight workload assessment: Comparison of subjective and physiological measurements. Aviat. Space Environ. Med. 2003, 74, 1078–1084. [Google Scholar] [PubMed]
- Vaden, E.A.; Hall, S. The effect of simulator platform motion on pilot training transfer: A meta-analysis. Int. J. Aviat. Psychol. 2005, 15, 375–393. [Google Scholar] [CrossRef]
- Matsumoto, E.D.; Pace, K.T.; D’a Honey, R.J. Virtual reality ureteroscopy simulator as valid tool for assessing endourological skills. Int. J. Urol. 2006, 13, 896–901. [Google Scholar] [CrossRef] [PubMed]
- Gerlach, I.; Hass, V.C.; Mandenius, C.F. Conceptual design of an operator simulator for a bio-ethanol plant. Processes 2015, 3, 664–683. [Google Scholar] [CrossRef]
- Chematur Engineering. Available online: www.chematur.se (accessed on 9 August 2016).
- Ingenieurbüro Dr.-Ing. Schoop. Available online: www.schoop.de/en/softeware/winers (accessed on 9 August 2016).
- Cox, R.K.; Smith, J.F.; Dimitratos, Y. Can simulation technology enable a paradigm shift in process control? Modeling for the rest of us. Comp. Chem. Eng. 2006, 30, 1542–1552. [Google Scholar] [CrossRef]
- Seymour, N.E.; Gallagher, A.G.; Roman, S.A.; O’Brien, M.K.; Bansal, V.K.; Andersen, D.K.; Satava, R.M. Virtual reality training improves operating room performance results of a randomized, double-blinded study. Ann. Surg. 2002, 236, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Perry, R.H.; Green, D.W. Perry's Chemical Engineering Handbook, 8th ed.; McGraw-Hill Co.: New York, NY, USA, 2008. [Google Scholar]
- Atkinson, B.; Manituva, F. Biochemical Engineering and Biotechnology Handbook, 2nd ed.; Stockton Press: New York, NY, USA, 1991. [Google Scholar]
- Laganier, F. Dynamic process simulation trends and perspectives in an industrial context. Comput. Chem. Eng. 1996, 20 (Suppl. S1), 1595–1600. [Google Scholar] [CrossRef]
- Reinig, G.; Winter, P.; Linge, V.; Nägler, K. Training simulators. Engineering and use. Chem. Eng. Technol. 1998, 21, 711–716. [Google Scholar] [CrossRef]
- Zhiyun, Z.; Baoyu, C.; Xinjun, G.; Chenguang, C. The development of a novel type chemical process simulator. Comput. Aided Chem. 2003, 15, 1447–1452. [Google Scholar]
- Brandam, C.; Meyer, X.M.; Proth, J.; Strehaiano, P.; Pingaud, H. An original kinetic model for the enzymatic hydrolysis of starch during mashing. Biochem. Eng. J. 2003, 13, 43–52. [Google Scholar] [CrossRef]
- Kroumov, A.D.; Módenes, A.N.; de Araujo Tait, M.C. Development of new unstructured model for simultaneous saccharification and fermentation of starch to ethanol by recombinant strain. Biochem. Eng. J. 2006, 28, 243–255. [Google Scholar] [CrossRef]
- Richardson, J.F.; Harker, J.H.; Backhurst, J.R. Coulson and Richardson’s Chemical Engineering. Vol. 2—Particle Technology and Separation Processes, 5th ed.; Butterworth-Heinemann, Elsevier Ltd: Oxford, UK, 2002; Chapters 17 and 18. [Google Scholar]
- Harrison, R.G.; Todd, P.W.; Rudge, S.R.; Petrides, D.P. Bioseparation Science and Engineering, 2nd ed.; Oxford University Press: New York, NY, USA, 2015. [Google Scholar]
- Ambler, C.M. The evaluation of centrifuge performance. Chem. Eng. Prog. 1952, 48, 150–158. [Google Scholar]
- Kuntzsch, S.; Hass, V.C.; Winterhalter, M. Untersuchung des Energiebedarfs der batch-Rektifikation durch einen neuen Trainingssimulator. Chem. Ing. Technol. 2014, 86, 714–724. [Google Scholar] [CrossRef]
- Ferney, M.J. Process simulators for safety. Plant Oper. Prog. 1991, 10, 133–135. [Google Scholar] [CrossRef]

| Training Events/Activity/Task | Actions and Observations | Effect of Training |
|---|---|---|
| Training of the start-up sequence of plant section. Steps in the procedure to be sequentially initiated by operator. | Start of pumps. | OTS prototype animates with high fidelity cause-and-effect relationships during the start-up of the section. The trained operator becomes immediately aware of mistakes in actions. |
| Start of cooling circuits to units. | ||
| Start of return flows. | ||
| Start of sub-flows at units. | ||
| Overflow failure occurs in a tank. The operator is responsible for observing the failure and acting correctly upon it. | Typically, the overflow is caused by return flow from slurry centrifuge operating at too high of a rate. The operator shall decrease the flow rate. | OTS prototype animates the overflow failure event and the effects. The operator actions are directly shown on the GUI. This allows further interactive actions by the operator. The operator becomes aware of the cause-and-effect relationship of the failure. |
| The fibre sieve of the filtration system is clogging. The operator is responsible for observing the failure and responding adequately. | Filtration system between fermenter and yeast centrifuges clogs if the motor is not started by operator. The operator shall be able to observe this and start motor. | OTS prototype animates the clogging event of the fibre and records the action of the operator. Effect of operator action is directly shown in the GUI. The operator becomes aware of the cause-and-effect relationship of not starting the filter motor on time. |
| Training Effects | Benefits | Simulator Contribution |
|---|---|---|
| Operator awareness | Number of faulty actions during plant operation reduced. | The high fidelity of the OTS to animate repeatedly. |
| Operator initiative and independency | The complexity of the bio-plant requires operators with a high degree of ability to take own initiatives. This ability must be trained. | By regular simulator training the operator constantly increased the ability to take initiative. |
| Operator understanding of cause and effect | During operation of such a complex process as described here, the variability of failure and its occurrences are significant. A deeper understanding by operators are therefore necessary for correct actions. | The transparency of the simulator and the possibility to repeat training in much shorter time cycles than in real situations create efficient process understanding. |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerlach, I.; Tholin, S.; Hass, V.C.; Mandenius, C.-F. Operator Training Simulator for an Industrial Bioethanol Plant. Processes 2016, 4, 34. https://doi.org/10.3390/pr4040034
Gerlach I, Tholin S, Hass VC, Mandenius C-F. Operator Training Simulator for an Industrial Bioethanol Plant. Processes. 2016; 4(4):34. https://doi.org/10.3390/pr4040034
Chicago/Turabian StyleGerlach, Inga, Sören Tholin, Volker C. Hass, and Carl-Fredrik Mandenius. 2016. "Operator Training Simulator for an Industrial Bioethanol Plant" Processes 4, no. 4: 34. https://doi.org/10.3390/pr4040034





