Abstract
Application of titanium dioxide (TiO2) as a photocatalyst has presented a promising avenue for the safe photocatalytic degradation of pollutants. Increasing levels of the release of pharmaceuticals in the environment and formation of the intermediates during their degradation may impose health and environmental risks and therefore require more attention. Photocatalytic degradation of acetylsalicylic acid (aspirin) was carried out in the presence of the TiO2-filled polymeric film as a photocatalyst under solar light irradiation. The polymeric film incorporates TiO2 in the matrix, which acts as a photocatalyst under solar illumination and degrades the acetyl salicylic acid (ASA) into a range of organic compounds before complete demineralization (formation of carbon dioxide and water as final products). Among the intermediates, acetic acid was found to be present in a larger amount compared to other organic acids. The qualitative/quantitative analyses of the intermediates resulted in the determination of the most probable reaction’s mechanism in the degradation process. The mechanism of degradation of acetylsalicylic acid and its reaction pathway were developed from liquid chromatography/mass spectroscopy (LC/MS), Fourier Transform Infra Red (FTIR) and UV spectrophotometric analysis. It was found that hydroxyl groups were dominant in the degradation process compared to electrons and holes generated by TiO2. The total organic carbon (TOC) analysis was also carried out to analyze the organic carbon content of the intermediates formed during the course of degradation.
1. Introduction
The increasing release of pharmaceutical organic compounds into ground water and the toxic nature of these drugs and the intermediates resulting from their degradation may cause health and environmental issues. These compounds are usually resistant to conventional biological treatments and may require new technologies such as advanced oxidation processes. Aspirin, known as acetylsalicylic acid (ASA), is a commonly used non-steroidal anti-inflammatory drug (NSAIDs) for relieving minor aches and pains. It is also used as an antipyretic to reduce fever, as an anti-inflammatory medication, and for reduction of death risk due to heart attack. The main undesirable side effects of aspirin are gastrointestinal ulcers, stomach bleeding, and tinnitus, especially in higher doses [,]. Aspirin degrades in an aqueous medium into several toxic intermediates causing environmental pollution, which affects human health. Removal of these organic compounds by traditional techniques (adsorption, ozonization, etc.) is challenging and costly. These methods usually generate concentrated effluent streams and gaseous emissions, which are harmful to the environment and require additional treatment before being discharged into a landfill or nearby river [,]. Recent advanced technologies in photocatalytic oxidation of organic materials can be safely employed in the treatment of organic wastes as the final products are mostly carbon dioxide and water. The electrochemical method was successfully employed for the degradation of wastewater with a high concentration of aspirin using new electrodes, which provided a high mass transfer and mineralization current efficiency, and lower energy consumption []. Hydrogen peroxide was found to be effective in accelerating aspirin degradation using the conventional electrochemical method. A degradation mechanism was also proposed based on the formation of a hydroxyl group involving hydrogen peroxide []. In another attempt, complete mineralization of aspirin was obtained through anodic oxidation over a boron-doped diamond electrode following pseudo-first-order kinetics []. A nickel-doped PbO2 electrode (1 wt.% Nickel) was shown to substantially enhance the electrochemical degradation of aspirin in aqueous solutions due to an increase in the utilization rate of the hydroxyl group and lower energy requirements []. Carlsson et al. introduced negative surface charges onto cellulose nanofibrils and showed that the aspirin degradation rate had increased significantly by electrochemical degradation []. The application of titanium dioxide (TiO2) in the photocatalytic oxidation of organic compounds has been extensively studied in recent years. TiO2 is also capable of producing a hydroxyl radical (.OH) when exposed to ultraviolet/solar light. Upon illumination of TiO2 by UV light, electrons are promoted from the valence band to the conduction band to provide electron-hole pairs. The holes in TiO2 react with water molecules or hydroxide ions and produce hydroxyl radicals. The interaction of the positive holes or the negative electrons with the adsorbed organic pollutants provides unstable intermediates, which are further attacked by hydroxyl or peroxyl species, occasioning a carbon-carbon bond rupture (e.g., disintegration of the molecules and aromatic ring opening) with concomitant release of low molecular weight products, which may in turn be further oxidized to CO2 and water. Contradictory results have been reported for the contribution of TiO2 in the degradation of organic materials. It was shown that at low pH, the positive holes are considered the main oxidizing species, whereas hydroxyl radicals are considered the predominant species at neutral or basic pH levels []. Interestingly, it was claimed in another report that holes are the major oxidizing species at pH = 3, while below and above this pH the hydroxyl radicals are the major degrading agents []. Such a discrepancy is most likely due to the nature of the organic compounds and operating conditions. Aspirin undergoes the following three general reactions when exposed to the photocatalyst illuminated by ultraviolet /solar light:
[Aspirin+ hvb+ → Oxidation products]
[Aspirin + ecb− → Reduction products]
[Aspirin + OH → Oxidation products]
Several mechanisms have been proposed to account for the initial steps of semiconductor-mediated photodegradation of aliphatic and aromatic organics. The heterogeneous reaction mechanisms proposed are similar to their homogeneous counterparts [,,,]. These mechanisms can be summarized as: (i) direct charge transfer from the semiconductor to the dissolved molecule; (ii) generation of radicals from water decomposition, which then attack the organic molecules. Most of the studies on photocatalytic degradation of several drugs include a detailed examination of the so-called primary processes under different operating conditions, while little information is available on the reaction mechanisms involved in the degradation process. To the best of our knowledge, there is no published study regarding the degradation mechanism of aspirin in the presence of a TiO2 photocatalyst. In this study, the photocatalytic degradation of aspirin exposed to TiO2 under solar light is studied. The most probable reactions and the mechanism were suggested based on the qualitative and quantitative measurements of the intermediates and final products of degradation.
2. Experimental Setup
2.1. Materials and Methods
The catalyst was prepared by mixing 24.3% w/w polyvinyl alcohol (PVA) and 11% w/w gelatin in distilled water. Next, 21.6% w/w polyvinyl pyrolidone dissolved in the solution of ethyl alcohol and water, which was mixed with PVA-gelatin solution. The solution was mixed and reacted properly at 50 °C, followed by dispersion of 43% w/w of TiO2 Degussa P25 powder in the mixture. Thereafter, the polymeric-TiO2 solution was cross-linked by physical cross-linking method while storing the solution at −2 °C.
2.2. High Performance Liquid Chromatography
A number of analytical techniques such as high performance liquid chromatography (HPLC), FTIR were employed for determination of several organic intermediates for degradation of several dyes [,]. Aspirin molecules are non-volatile and highly soluble in hot water and cannot be analyzed by Gas Chromatography/Mass Spectroscopy GC/MS. Therefore, liquid chromatography technique was employed for analysis of the mixtures of aspirin photodegradation and identification of the intermediates. HPLC (Agilent, Santa Clara, CA, USA) was used to analyze the intermediates. A capillary column (C-18, 5 μm, 100 mm × 4.6 mm length) (Agilent, Santa Clara, CA, USA) was used with eluent acetonitrile and water (70:30 wt.%) with flow rate of 0.1 mL/min. UV detection was at 298 nm. Peaks were observed within 15 min of retention time. The present study is focused on degradation of acetylsalicylic acid in the presence of solar light and TiO2/polymeric photocatalyst, and to identify degradation products of aspirin by LC/MS and FTIR (Thermo Scientific, Toronto, ON, Canada) to propose the most probable mechanism for aspirin photocatalytic degradation.
2.3. Photodegradation and LC/MS Analysis
The photodegradation of 2 ppm aspirin solution was carried out in a batch reactor (250 mL) at pH 3.5, under solar light of intensity −77 mW/cm2. Samples taken at various time intervals were analyzed by LC/MS (Shimadzu LCMS-2010 EV Liquid Chromatography Mass spectrometer, Kyoto, Japan) to identify the intermediate compounds. The mobile phase was a mixture of acetonitrile-water (70/30, v/v) with 0.04% glacial acetic acid to maintain acidic pH. The flow rate of elute was 0.1 mL·min−1 and the injection volume was 20 μL. The eluent from the chromatographic column successively entered the UV-Vis diode array detector, the Electrospray Ionization (ESI) interface and the quadruple ion trap mass analyzer. MS analysis in the negative ions mode was performed on a mass spectrometer equipped with an ESI ion source. The ESI probe tip and capillary potentials were set at 2.5 kV and 25 V, respectively. The mass range was 50–750 m/z. The heated capillary was set to 200 °C.
2.4. Fourier Transform Infrared (FTIR)
Fourier transform infrared analysis of the aspirin solution and its degraded products were carried out in Bruker FTIR (Vector 22) to analyze the intermediates formed at different time intervals. The analysis of one broad banded pass of radiation through the sample gives rise to a complete IR spectrum. The radiation containing all IR wavelengths ranges from 400 to 5000 cm−1. Results of 32 scans were combined to average out random absorption artifacts, and excellent spectra from very little amount of samples were obtained.
3. Results and Discussion
3.1. Results
The samples taken at different time intervals were subjected to HPLC for determination of intermediates. The rate of information of each intermediate was also obtained by measuring the concentration and the degradation time. Products formed during the photodegradation process were analyzed by HPLC which reported the formation of six new intermediates at different intervals as shown in Figure 1.

Figure 1.
Liquid chromatography results.
Most of the peaks were observed within the first 15 min of the process. The concentrations of the intermediates were calculated from the area of the peaks. However, it was not possible to detect the compounds directly; therefore, intermediates were further analyzed by LC/MS. The components eluted with different retention times were subjected to mass spectrometry and identified by interpretation of their fragment ions in the mass spectra. The chromatographic and Total Organic Carbon (TOC) data suggested that all the components disappeared after 5 h of photocatalytic degradation.
Figure 2 shows typical mass spectra obtained in the experiments. The mass spectra of degraded products showed species at 59 m/z which was acetic acid and 137 m/z which was salicylic acid. Further, hydroxylation produced products at 110 m/z, which again formed species upon hydroxylation, showing fragments at 116 m/z (maleic acid) and at 90 m/z (oxalic acid). Further hydroxylation gave species at 104 m/z (malonic acid), which on hydroxylation formed products and showed fragments at 59 m/z. Mass spectroscopy and the chromatograms showed the presence of only eight intermediates. A few more compounds which were assumed to have formed during the degradation were not detected by the instrumental analysis. The absence of these compounds may be due to their unstable nature, which made them degrade too fast to be detected. Benzoquinone, oxalo-acetic acid, and formic acid were not detected in LC/MS.
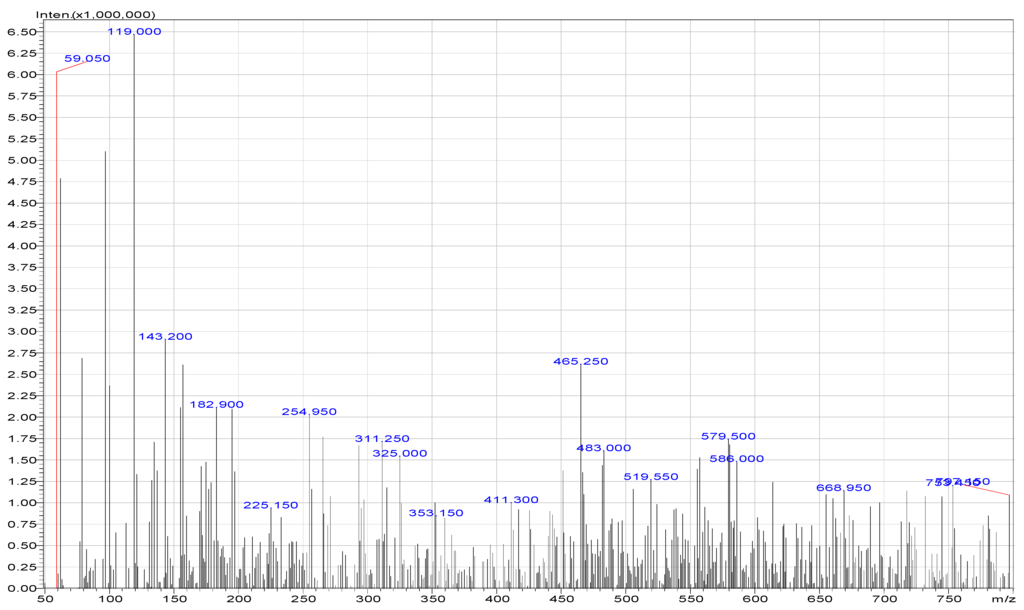
Figure 2.
Mass spectra for aspirin degradation.
Table 1 shows the obtained data for aspirin and the six intermediates formed during the degradation process.

Table 1.
Identification of intermediates.
Among hvb+, ecb− and OH, based on the observed reactions pathway, it seems that the hydroxyl radical is the most dominant factor in the degradation of aspirin in an aqueous TiO2 suspension []. On the basis of chemical structures and concentration profiles of identified intermediates, possible reaction pathways during the photocatalytic degradation have been proposed.
3.2. Mechanism of Photodegradation of ASA
Initially, aspirin got hydrolyzed in water, forming salicylic acid and acetic acid. The main products identified were salicylic acid, acetic acid, fumaric acid or maleic acid, malic acid and malonic acid. Salicylic acid got oxidized to 1,2-dihydroxybenzene, which further oxidized to form hydroquinone. While a part of the salicylic acid also got oxidized to 2,3-dihydroxybenzoic acid, it was less prominent than the 1,2-dihydroxybenzene formation reaction []. Hydroquinone hydroxylated to benzoquinone, which, being unstable in aqueous solution, underwent ring-opening followed by further oxidation to muconic acid []. The addition of a hydroxyl radical to the double bond of muconic acid yielded maleic acid and oxalic acid. Maleic/fumaric acid then got oxidized to malic acid, which is a precursor of malonic acid. Malonic acid further produces acetic acid and carbon dioxide []. Oxalic acid got oxidized to CO2, through the formation of formic acid. Acetic acid was observed to form both by the hydrolysis of aspirin at the initial stage and also by the oxidation of malonic acid. It was then oxidized to form CO2 through the formation of CH4, C2H6 and H2O. The pathway of conversion of acetic acid to carbon dioxide [,,] fit well in the degradation reaction pathway. The adsorbed CH3COOH dissociated to CH3COO− species, which reacted with photogenerated holes to form CH3 radicals and CO2 (known as the photo-Kolbe reaction) []. The active CH3 radicals then reacted with H2O to release CH4, or they reacted with another CH3 radical to produce C2H6. In consequence, CH3 radicals reacted with OH radicals to generate CH3OH. Next, the photogenerated holes attacked the produced CH3OH to form HCHO, which was further oxidized by both OH radicals and photogenerated holes to produce HCOOH []. At the last step, the HCOOH was decarboxylated by the photo-Kolbe reaction to release CO2. Decarboxylation of the COOH group forms CO2 using the photo-Kolbe reaction []. Based on the analyses of the data and the nature of the identified intermediate, the following reaction scheme is proposed as the most probable reaction mechanism for the photodegradation of aspirin:
ASA (aspirin) + H2O → SA (salicylic acid) + Ac (acetic acid)
SA (salicylic acid) → HQ (hydroquinone)
HQ + OH → BQ (benzoquinone) + H2O
BQ + OH → Muconic acid
Mc (Muconic acid) + OH → MA (Maleic acid) + Oxalic acid
MA + OH → Malic acid
MA + OH → Oxaloacetic acid
ML + OH → Ac + CO2
CH3COO− + hvb+ → CH3 + CO2
CH3 + OH → CH3OH
CH3 + H2O → CH4 + OH
CH3 + CH3 → C2H6
CH3 + OH → CH3OH
CH3OH + hvb+ → + H + CH2OH
CH2OH +hvb+ → HCHO
HCHO + OH + hvb+ → HCOOH
Dai et al. [,] proposed the reaction mechanism for the electrochemical degradation of aspirin and, despite some similarities between the proposed mechanism in this study and that reported mechanism, there are differences which are most likely due to the inherently different methodologies employed.
3.3. Reaction Pathways of Acetyl Salicylic Acid
Generally the sites near double bonds are attacked first in the degradation process. Aromatic intermediates were identified at the initial stages of degradation. The intermediates formed were most probably aromatic acids and aliphatic acids. Therefore, the following reaction pathway may be proposed:
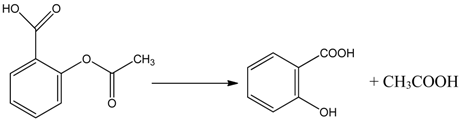
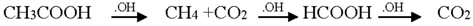

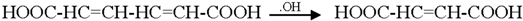
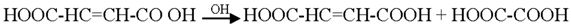
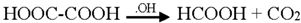
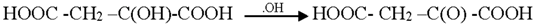

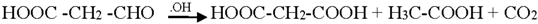




Among the intermediates, acetic acid was detected almost in larger amounts compared to other aromatic acids. The formation of CO2 is generally related to the degree of mineralization that occurred during the photodegradation. In general, at low reactant levels, reactants disappear exponentially, but at higher reactant levels, mineralization is slower than the degradation of the parent compound [].
Table 2 shows the total organic carbon analysis for the aspirin solution.

Table 2.
Total organic carbon content of aspirin solution.
TOC values decreased with the increase of the irradiation time. This can be explained by the fact that, with time, the acetylsalicylic acid gets hydrolyzed and oxidized to lower molecular weight compounds so the TOC content was observed to decrease with time. Though the TOC value does not reach zero at the end, it becomes negligible as compared to the initial value of the solution before degradation. Towards the end of the reaction, the parent compound forms acetic acid, which gradually forms carbon dioxide through the formation of CH4 and C2H6. It can be assumed that some of these CH3 radicals were unable to form carbon dioxide.
4. Conclusions
The degradation of acetylsalicylic acid by a solar stimulator in the presence of a TiO2-polymeric film catalyst was studied. The intermediates formed during the degradation process were found to be more toxic than aspirin. The qualitative/quantitative analyses of the intermediates resulted in the determination of the most probable reaction’s mechanism which governs the degradation process. Although some of the intermediates were not identified due to their instability and fast degradation, they were determined through the reaction’s pathways. The resulting mechanism showed that salicylic acid, acetic acid, and muconic acid were the dominant components in the reactions. Major aliphatic acids such as muconic, malic and malonic acids were formed by the photocatalytic oxidation process, which explains the opening of the aromatic ring. The TOC results, combined with LC-MS and FTIR, concluded that the products formed during the reactions decomposed into carbon dioxide and water.
Acknowledgments
The authors are grateful for financial support of the Natural Sciences and Engineering Research Council of Canada (NSERC) throughout the project.
Author Contributions
A.K.R. and S.B. conceived and design the experiments, D.M. (first author) did most of the experiments and partially contributed to some of the analysis. A.K.R. and S.B. analyzed the experimental data, A.K.R. contributed reagents/material/analysis tools and other financial supports, D.M., A.K.R. and S.B. wrote the paper, A.K.R. and S.B. contributed to the publishing process.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| TiO2 | Titatium Dioxide |
| FTIR | Fourier Transform Infra Red |
| LC/MS | Liquid Chromatography Mass Spectroscopy |
| TOC | Total Organic Carbon content |
| Ppm | Parts per million |
References
- Terry, L.A. Water Pollution. Environ. Law Pract. 1996, 4, 19–29. [Google Scholar]
- Calza, P.; Sakkas, V.A.; Medana, C.; Baiocchi, C.; Dimou, A.; Pelizzetti, E.; Albanis, T. Photocatalytic degradation study of diclofenac over aqueous TiO2 suspensions. Appl. Catal. B Environ. 2006, 67, 197–205. [Google Scholar] [CrossRef]
- Chemseddine, A.; Boehm, H.P. A study of the primary step in the photochemical degradation of acetic acid and chloroacetic acids on a TiO2 photocatalyst. J. Mol. Catal. 1990, 60, 295–311. [Google Scholar] [CrossRef]
- Muggli, S.D.; Falconer, J.L. Parallel pathways for photocatalytic decomposition of acetic acid on TiO2. J. Catal. 1999, 187, 230–237. [Google Scholar] [CrossRef]
- Dai, Q.; Xia, Y.; Chen, J. Mechanism of enhanced electrochemical degradation of highly concentrated aspirin wastewater using a rare earth La-Y co-doped PbO2 electrode. Electrochim. Acta 2016, 188, 871–881. [Google Scholar] [CrossRef]
- Dai, Q.; Xia, Y.; Jiang, L.; Li, W.; Wang, J.; Chen, J. Enhanced degradation of aspirin by electrochemical oxidation with modified PbO2 electrode and hydrogen peroxide. Int. J. Electrochem. Sci. 2012, 7, 12895–12906. [Google Scholar]
- He, Y.; Huang, W.; Chen, R.; Zhang, W.; Lin, H.; Li, H. Anodic oxidation of aspirin on PbO2, BDD and porous Ti/BDD electrodes: Mechanism, kinetics and utilization rate. Sep. Purif. Technol. 2015, 156, 124–131. [Google Scholar] [CrossRef]
- Xia, Y.; Dai, Q.; Chen, J. Electrochemical degradation of aspirin using a Ni doped PbO2 electrode. J. Electroanal. Chem. 2015, 744, 117–125. [Google Scholar] [CrossRef]
- Carlsson, D.O.; Hua, K.; Forsgren, J.; Mihranyan, A. Aspirin degradation in surface-charged TEMPO-oxidized mesoporous crystalline nanocellulose. Int. J. Pharm. 2014, 461, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.Z.; Wei, L.F.; Zhang, Z.H.; Jiang, Q.J.; Wei, Y.J.; Xie, B.; Wei, M.B. Research on photocatalytic H2 production from acetic acid solution by Pt/TiO2 nanoparticles under UV irradiation. Int. J. Hydr. Energy 2009, 34, 9033–9041. [Google Scholar] [CrossRef]
- Méndez-Arriaga, F.; Esplugas, S.; Giménez, J. Photocatalytic degradation of non-steroidal anti-inflammatory drugs with TiO2 and simulated solar irradiation. Water Res. 2008, 42, 585–594. [Google Scholar]
- Uges, D.R.A.; Bloemhof, H.; Juul Christensen, E.K. An HPLC method for the determination of salicylic acid, phenacetin and paracetamol in serum, with indications; two case-reports of intoxication. Pharm. World Sci. 1981, 3, 1309–1315. [Google Scholar] [CrossRef]
- Li, D.; Cheng, X.; Yu, X.; Xing, Z. Preparation and characterization of TiO2-based nanosheets for photocatalytic degradation of acetylsalicylic acid: Influence of calcination temperature. Chem. Eng. J. 2015, 279, 994–1003. [Google Scholar] [CrossRef]
- Christoph, K.; Scheck, F.F. Degradation of phenol and salicylic acid by ultraviolet radiation/hydrogen peroxide/oxygen. Water Res. 1995, 29, 2346–2352. [Google Scholar]
- Masende, Z.P.G.; Kuster, B.F.M.; Ptasinski, K.J.; Janssen, F.J.J.G.; Katima, J.H.Y.; Schouten, J.C. Kinetics of malonic acid degradation in aqueous phase over Pt/graphite catalyst. Appl. Catal. B Environ. 2004, 56, 189–199. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).