Numerical and Experimental Study on Preheating Burner Characteristics for Peak Shaving
Abstract
:1. Introduction
2. Model Details
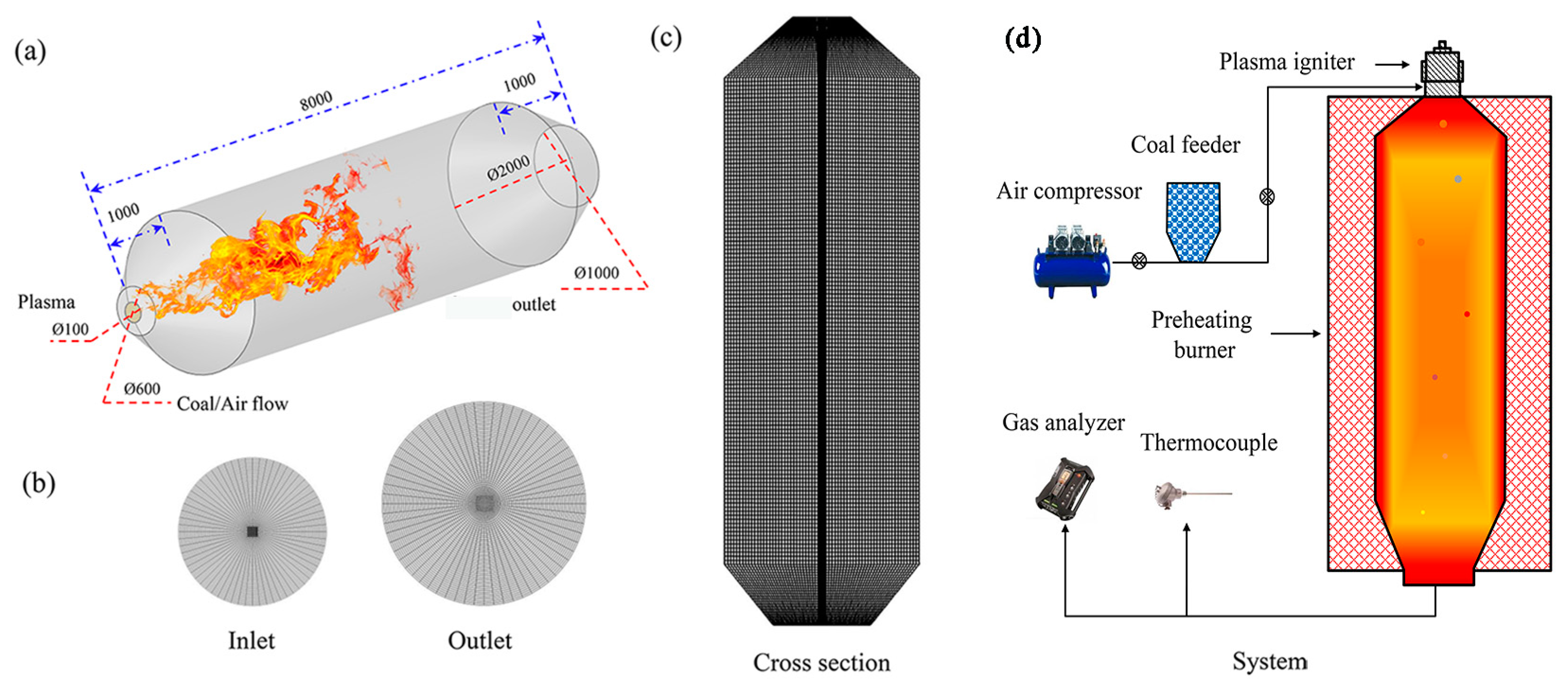
2.1. Physical Model
2.2. Mathematical Model
2.3. Chemical Mechanism
3. Experimental Steps and Case Conditions
3.1. Experimental Steps
3.2. Case Conditions
4. Results and Discussion
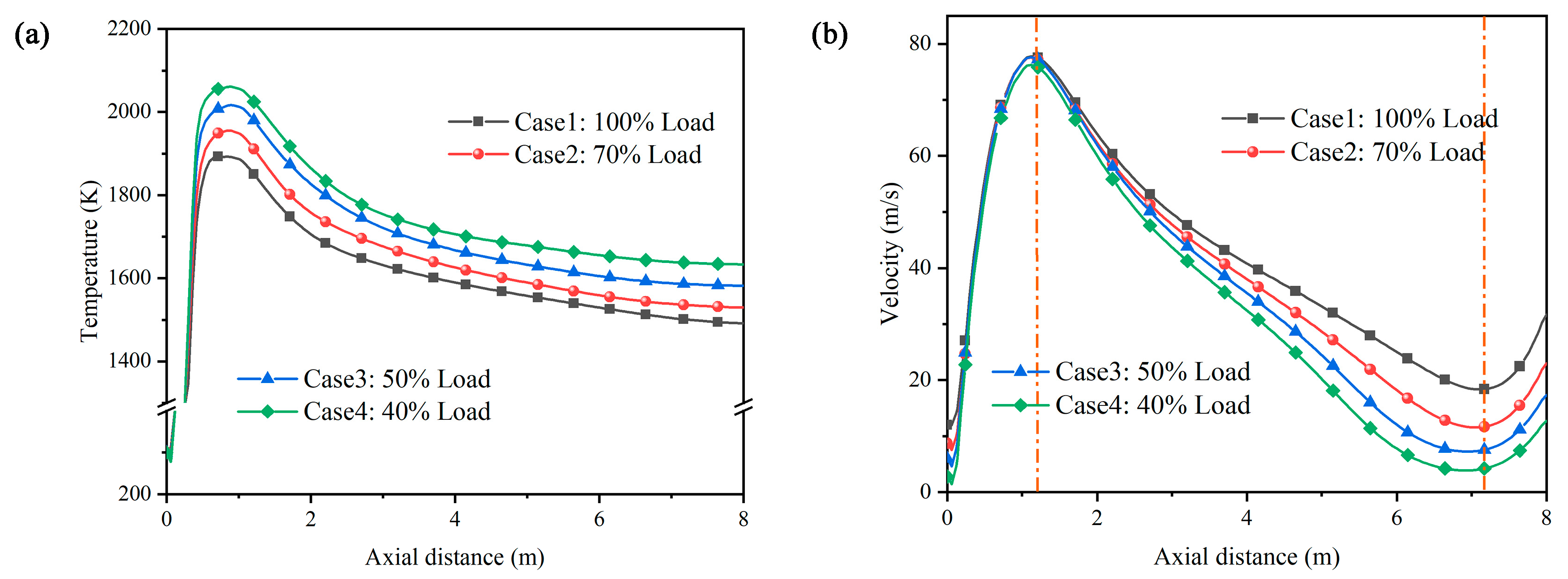
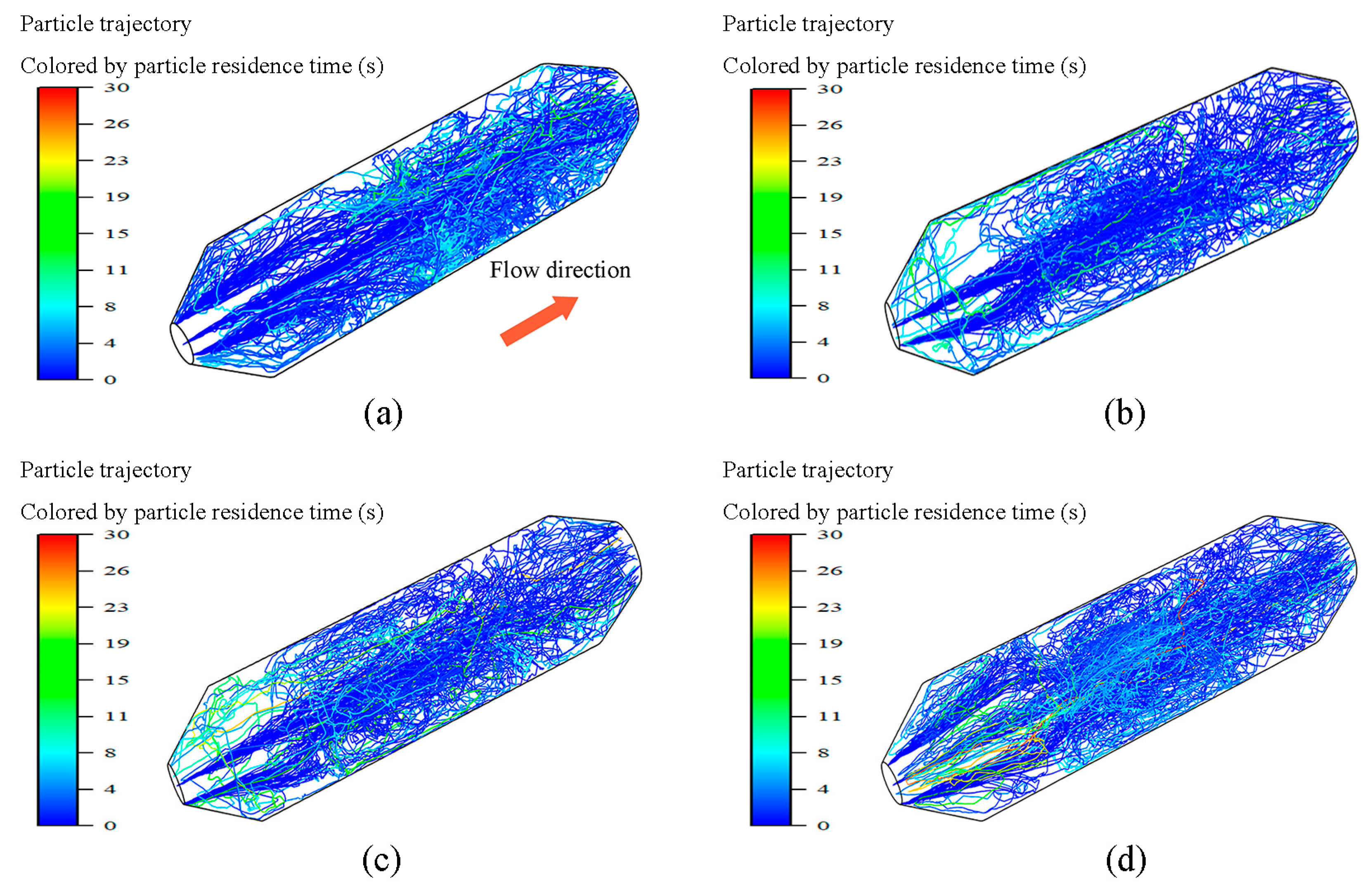
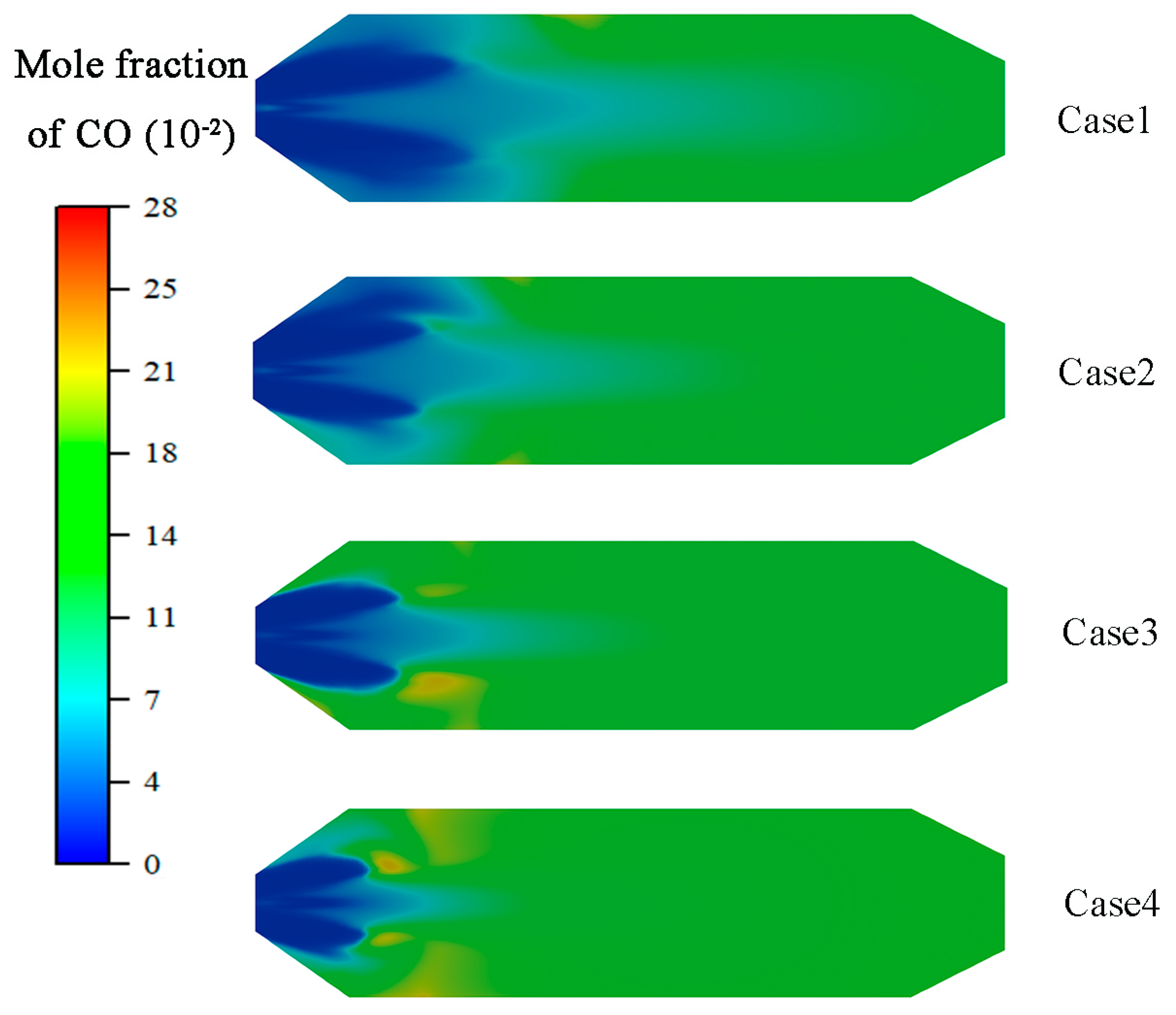
4.1. Flow Analysis
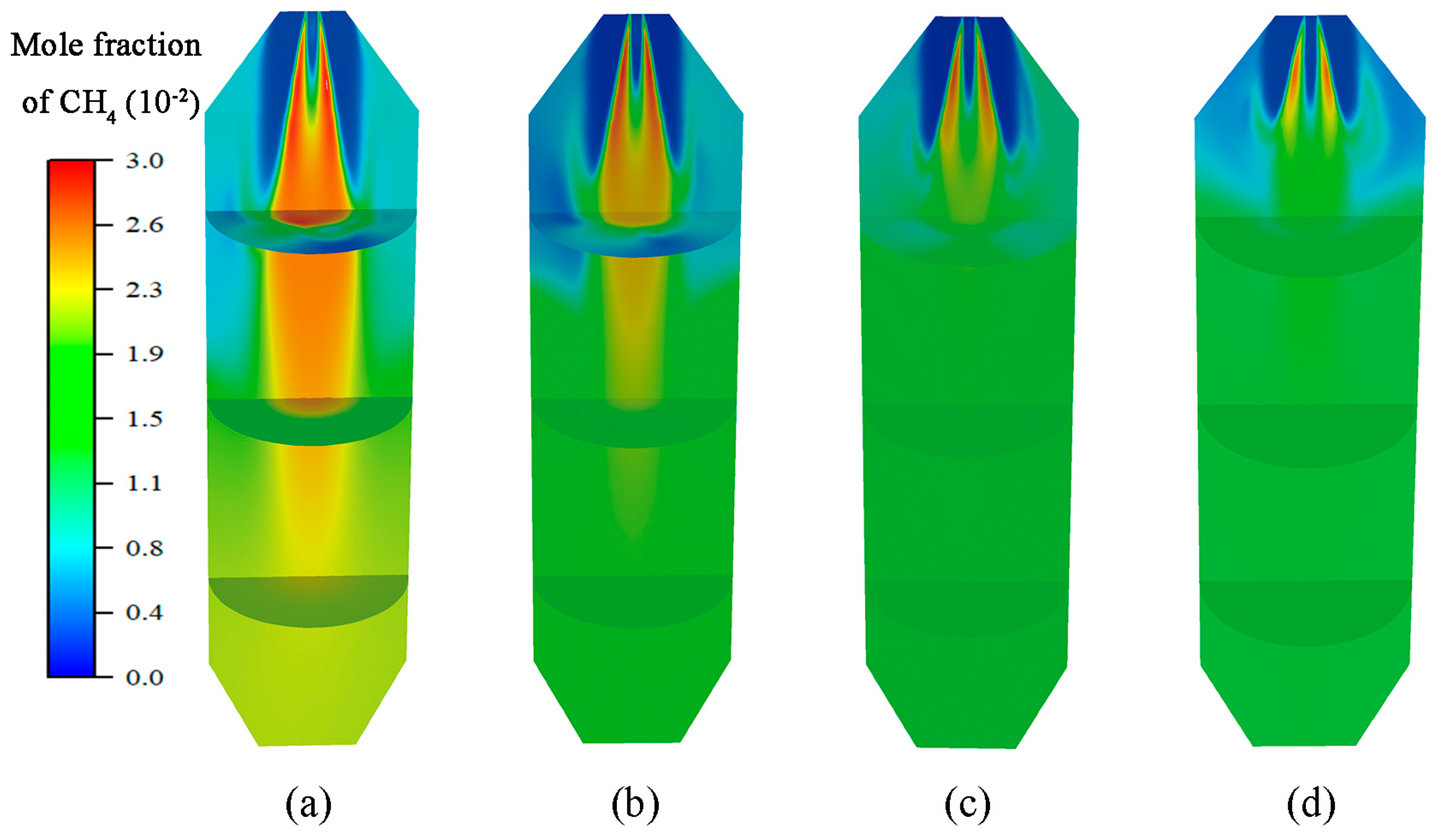
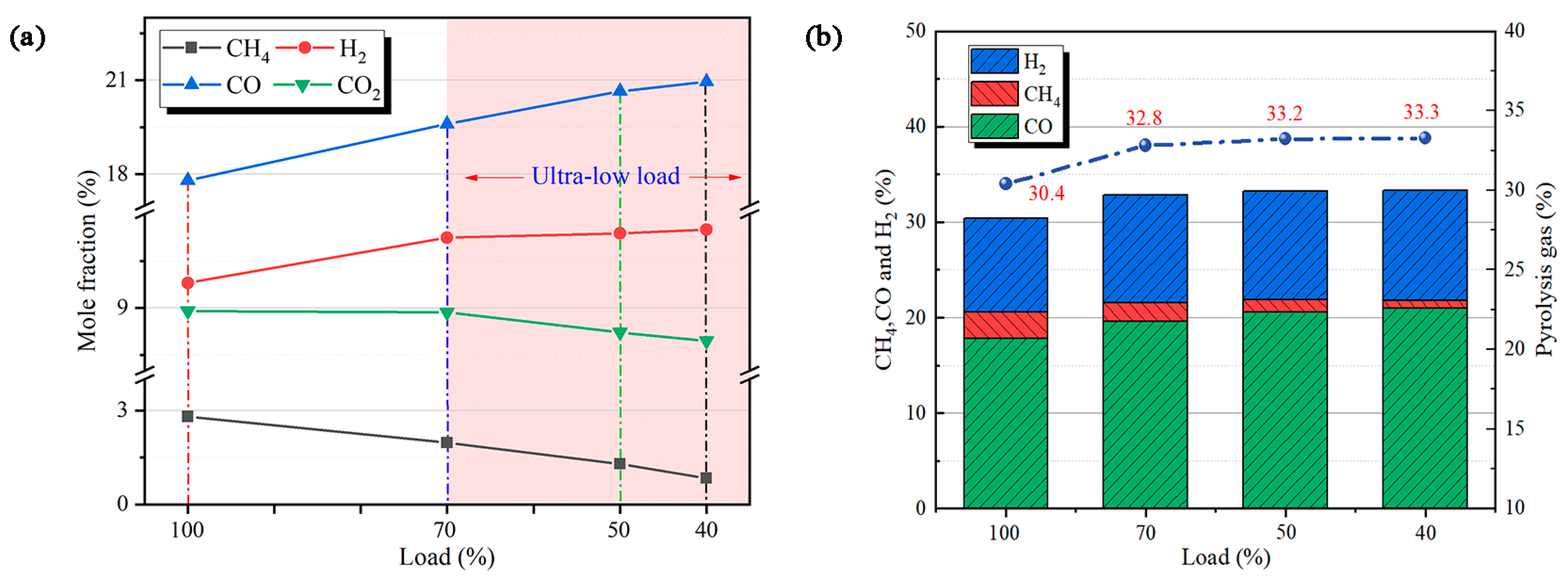
4.2. Pyrolysis Gas Analysis
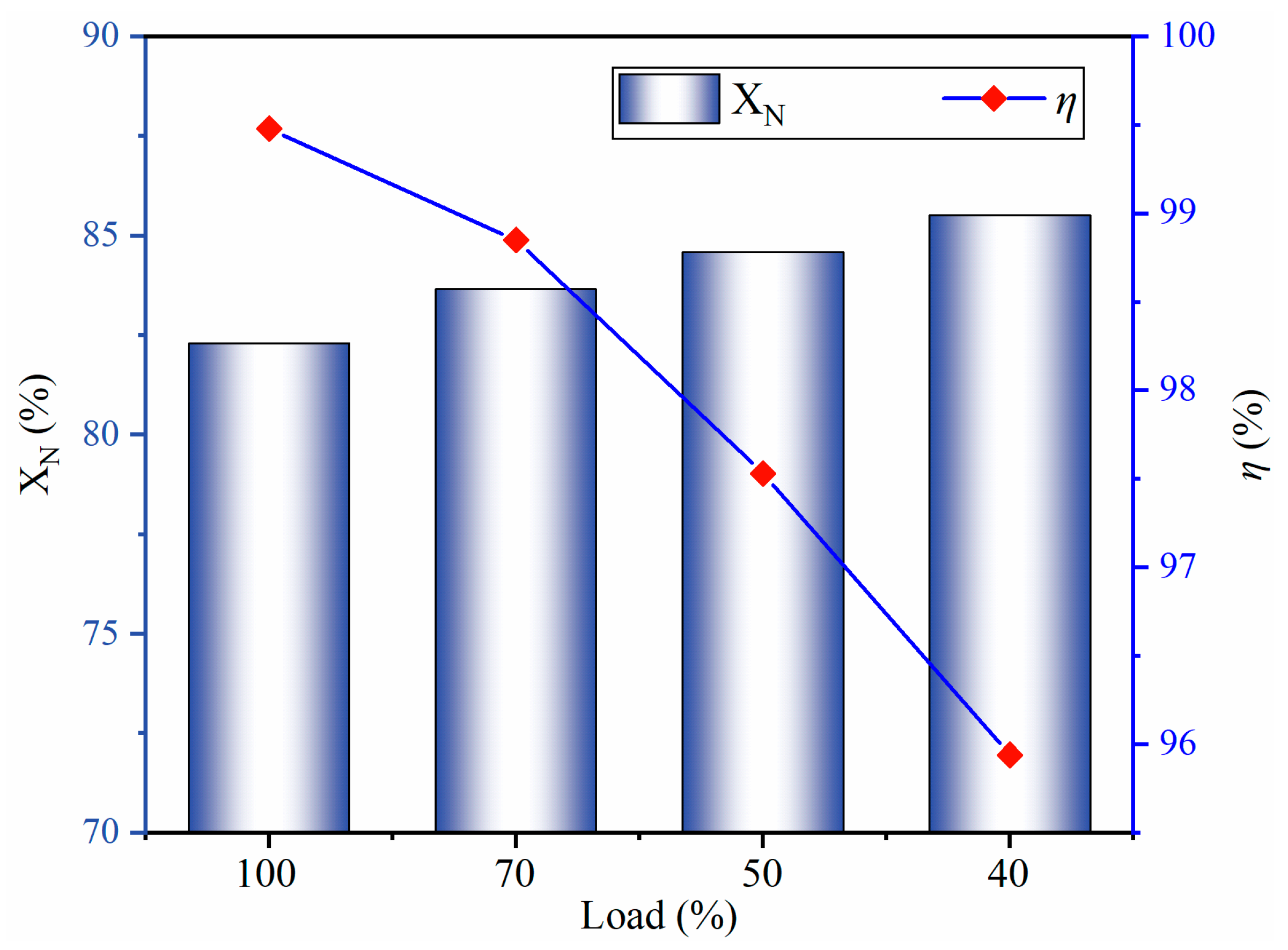
4.3. NOx Emissions Analysis
5. Conclusions
- Preheating is a peaking method with outstanding economy, combustion stability, and low NOx emissions. A preheating burner is capable of consistently producing high-temperature char and large amounts of pyrolysis gas (CH4, CO, and H2) at 40–100% load. With decreasing load, the burner outlet temperature increases and the airflow stiffness decreases. In addition, the particle residence time is extended, and the char conversion increases as the load decreases.
- CH4 hydrolysis results in a decrease in the CH4 mole fraction with decreasing load, and CO and H2 increase in the mole fraction due to volatile pyrolysis and char gasification. Xpyrolysis gas demonstrates little variation at 40–100% load, indicating that the preheating burner can produce pyrolysis gas stably below 50% load.
- The burner outlet NOx emissions are 26.82 mg/Nm3 at 100% load. As the load decreases, NOx emissions increase owing to high Fuel-N conversion and reduced hydrocarbons. NOx emissions are 91.53 mg/Nm3 at 40% load.
- The preheating method precipitates more than 80% of the Fuel-N from pulverized coal. The reduction efficiency decreases with a decrease in load. At 40% load, the reduction efficiency reaches 95.9%.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| SCR | Selective catalytic reduction |
| CCOFA | Close-coupled overfire air |
| SOFA | Separated overfire air |
| UDF | User-defined function |
| EDC | Eddy-dissipation concept |
| Fuel-N | Fuel nitrogen |
| Symbols | |
| Ap | Particle surface area (m2) |
| Ap,0 | Particle surface area at the initial state (m2) |
| x | Total carbon conversion of char particles |
| mp | Particle mass (kg) |
| mp,0 | Initial particle mass (kg) |
| mash | Mass of ash (kg) |
| r | Reaction order |
| mc,r | Mass of char particles consumed for reaction r (kg) |
| Yc | Mass fraction of char in the particles |
| Rr | Rate of particle surface species reaction per unit area (kg⋅m−2⋅s−1) |
| Xpyrolysis gas | Pyrolysis gas mole fraction (%) |
| CH4 | Mole fraction (%) |
| XCO | CO mole fraction (%) |
| H2 | Mole fraction (%) |
| XN | Fuel-N conversion (%) |
| a | Volatile conversion (%) |
| b | Char conversion (%) |
| XVolatile-N | Volatile-N percentage (%) |
| XChar-N | Char-N percentage (%) |
| mT-NO | Theoretical mass of fuel NO precipitated (kg/s) |
| mNO | Mass of outlet NO (kg/s) |
| m | Coal mass (kg) |
| n | Nitrogen share of coal |
| Greek Letters | |
| ψ | Pore structure parameter (dimensionless) |
| ηr | Effectiveness factor (dimensionless) |
| η | Reduction efficiency (%) |
References
- Flamant, G.; Grange, B.; Wheeldon, J.; Siros, F.; Valentin, B.; Bataille, F.; Zhang, H.; Deng, Y.; Baeyens, J. Opportunities and challenges in using particle circulation loops for concentrated solar power applications. Prog. Energy Combust. Sci. 2023, 94, 101056. [Google Scholar] [CrossRef]
- Thengane, S.K.; Kung, K.S.; Gomez-Barea, A.; Ghoniem, A.F. Advances in biomass torrefaction: Parameters, models, reactors, applications, deployment, and market. Prog. Energy Combust. Sci. 2022, 93, 101040. [Google Scholar] [CrossRef]
- Wang, Z.; Yao, G.; Xue, W.; Cao, S.; Xu, S.; Peng, X. A Data-Driven Approach for the Ultra-Supercritical Boiler Combustion Optimization Considering Ambient Temperature Variation: A Case Study in China. Processes 2023, 11, 2889. [Google Scholar] [CrossRef]
- Ali, H.M.; Rehman, T.-u.; Arıcı, M.; Said, Z.; Duraković, B.; Mohammed, H.I.; Kumar, R.; Rathod, M.K.; Buyukdagli, O.; Teggar, M. Advances in thermal energy storage: Fundamentals and applications. Prog. Energy Combust. Sci. 2024, 100, 101109. [Google Scholar] [CrossRef]
- Zhu, S.; Hui, J.; Lyu, Q.; Ouyang, Z.; Liu, J.; Zhu, J.; Zeng, X.; Zhang, X.; Ding, H.; Liu, Y. Experimental study on pulverized coal combustion preheated by a circulating fluidized bed: Preheating characteristics for peak shaving. Fuel 2022, 324, 124684. [Google Scholar] [CrossRef]
- Wayth, N.; Davenport, J. Statistical Review of World Energy; Energy Institute: London, UK, 2023. [Google Scholar]
- Zhang, X.; Cui, X.; Li, B.; Hidalgo-Gonzalez, P.; Kammen, D.M.; Zou, J.; Wang, K. Immediate actions on coal phaseout enable a just low-carbon transition in China’s power sector. Appl. Energy 2022, 308, 118401. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, L.; Xu, C.; Maréchal, F.; Yang, Y. Enhancing the operational flexibility of thermal power plants by coupling high-temperature power-to-gas. Appl. Energy 2020, 263, 114608. [Google Scholar] [CrossRef]
- Liang, Z.; Ma, X.; Lin, H.; Tang, Y. The energy consumption and environmental impacts of SCR technology in China. Appl. Energy 2011, 88, 1120–1129. [Google Scholar] [CrossRef]
- Zhu, S.; Hui, J.; Lyu, Q.; Ouyang, Z.; Zeng, X.; Zhu, J.; Liu, J.; Cao, X.; Zhang, X.; Ding, H.; et al. Experimental study on pulverized coal swirl-opposed combustion preheated by a circulating fluidized bed. Part A. Wide-load operation and low-NOx emission characteristics. Energy 2023, 284, 128573. [Google Scholar] [CrossRef]
- Jiang, Y.; Lee, B.-H.; Oh, D.-H.; Jeon, C.-H. Optimization of operating conditions to achieve combustion stability and reduce NOx emission at half-load for a 550-MW tangentially fired pulverized coal boiler. Fuel 2021, 306, 121727. [Google Scholar] [CrossRef]
- Zhao, S.; Fang, Q.; Yin, C.; Wei, T.; Wang, H.; Zhang, C.; Chen, G. New Fuel Air Control Strategy for Reducing NO x Emissions from Corner-Fired Utility Boilers at Medium–Low Loads. Energy Fuels 2017, 31, 6689–6699. [Google Scholar] [CrossRef]
- Chang, J.; Wang, X.; Zhou, Z.; Chen, H.; Niu, Y. CFD modeling of hydrodynamics, combustion and NOx emission in a tangentially fired pulverized-coal boiler at low load operating conditions. Adv. Powder Technol. 2021, 32, 290–303. [Google Scholar] [CrossRef]
- Gu, Y.; Xu, J.; Chen, D.; Wang, Z.; Li, Q. Overall review of peak shaving for coal-fired power units in China. Renew. Sustain. Energy Rev. 2016, 54, 723–731. [Google Scholar] [CrossRef]
- Xiao, P.; Zhang, Y.; Wang, Y.; Wang, J. Analysis of an improved economizer system for active control of the coal-fired boiler flue gas temperature. Energy 2019, 170, 185–198. [Google Scholar] [CrossRef]
- Rabovitser, J.; Bryan, B.; Knight, R.; Nester, S.; Wohadlo, S.; Tumanovsky, A.G.; Tolchinsky, E.N.; Verbovetsky, E.H.; Lisauskas, R.; Beittel, R. Development and testing of a novel coal preheating technology for NOx reduction from pulverized coal-fired boilers. Gas 2003, 1, 4. [Google Scholar]
- Rahimipetroudi, I.; Rashid, K.; Yang, J.B.; Dong, S.K. Development of environment-friendly dual fuel pulverized coal-natural gas combustion technology for the co-firing power plant boiler: Experimental and numerical analysis. Energy 2021, 228, 120550. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Zhu, J.; Ouyang, Z.; Man, C.; Zhu, S.; Zhang, Y.; Lyu, Q. Bituminous coal deep regulated ultra-low NOx flameless combustion with fluidized self-preheating fuel: A 2 MWth experimental study. Fuel 2021, 294, 120549. [Google Scholar] [CrossRef]
- Ouyang, Z.; Liu, W.; Man, C.; Zhu, J.; Liu, J. Experimental study on combustion, flame and NOX emission of pulverized coal preheated by a preheating burner. Fuel Process. Technol. 2018, 179, 197–202. [Google Scholar] [CrossRef]
- Lv, Z.; Xiong, X.; Ruan, R.; Wang, Y.; Tan, H. NO emission and burnout characteristics in co-combustion of coal and sewage sludge following high-temperature preheating. Fuel 2023, 331, 125887. [Google Scholar] [CrossRef]
- Yao, G.; Han, X.; Liu, Z.; Tang, H.; Zhou, Y.; Wang, Z. Low-NOx study of a 600 MW tangentially fired boiler based on pulverized coal preheating method. Case Stud. Therm. Eng. 2023, 48, 103156. [Google Scholar] [CrossRef]
- Yao, G.; Liu, Z.; Tang, H.; Meng, L. Prediction of reduction products in the preheating process. Therm. Sci. 2023, 27, 4021–4034. [Google Scholar] [CrossRef]
- Ibrahimoglu, B.; Yilmazoglu, M.Z. Numerical modeling of a downdraft plasma coal gasifier with plasma reactions. Int. J. Hydrogen Energy 2020, 45, 3532–3548. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, J.; Xu, R.; Zheng, A.; Zhu, J.; Li, T. Numerical investigation of hydrogen-rich gas and pulverized coal injection in the raceway of a blast furnace with lower carbon emissions. Fuel 2024, 356, 129462. [Google Scholar] [CrossRef]
- Wang, H.; Jin, D.; Liu, X.; Zhang, C. Analytical and numerical investigations on the high temperature upgrading solution of subcritical boilers. Appl. Therm. Eng. 2022, 200, 117628. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, C.; Liu, X. Heat transfer calculation methods in three-dimensional CFD model for pulverized coal-fired boilers. Appl. Therm. Eng. 2020, 166, 114633. [Google Scholar] [CrossRef]
- Alobaid, F.; Almohammed, N.; Farid, M.M.; May, J.; Rößger, P.; Richter, A.; Epple, B. Progress in CFD Simulations of Fluidized Beds for Chemical and Energy Process Engineering. Prog. Energy Combust. Sci. 2021, 91, 100930. [Google Scholar] [CrossRef]
- Benim, A.C.; Canal, C.D.; Boke, Y.E. Computational investigation of oxy-combustion of pulverized coal and biomass in a swirl burner. Energy 2022, 238, 121852. [Google Scholar] [CrossRef]
- Jin, D.; Yan, J.; Liu, X.; Zhang, C.; Wang, H. Prediction of tube temperature distribution of boiler platen superheater by a coupled combustion and hydrodynamic model. Energy 2023, 279, 128116. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, X.; Zhang, C.; Wang, H. Numerical investigations on the aerodynamics and flue gas recirculation of cyclone-fired coal boiler. Fuel 2022, 316, 123355. [Google Scholar] [CrossRef]
- Adamczyk, W.P.; Werle, S.; Ryfa, A. Application of the computational method for predicting NOx reduction within large scale coal-fired boiler. Appl. Therm. Eng. 2014, 73, 343–350. [Google Scholar] [CrossRef]
- Yan, J.; Jin, D.; Liu, X.; Zhang, C.; Wang, H. A coupled combustion and hydrodynamic model for the prediction of waterwall tube overheating of supercritical boiler. Fuel 2023, 334, 126589. [Google Scholar] [CrossRef]
- Iwaszenko, S.; Howaniec, N.; Smoliński, A. Determination of random pore model parameters for underground coal gasification simulation. Energy 2019, 166, 972–978. [Google Scholar] [CrossRef]
- Jeong, H.J.; Seo, D.K.; Hwang, J. CFD modeling for coal size effect on coal gasification in a two-stage commercial entrained-bed gasifier with an improved char gasification model. Appl. Energy 2014, 123, 29–36. [Google Scholar] [CrossRef]
- Ding, H.; Ouyang, Z.; Wang, W.; Zhang, X.; Zhu, S. Experimental study on the influence of O2/CO2 ratios on NO conversion and emission during combustion and gasification of high-temperature coal char. Fuel 2022, 310, 122311. [Google Scholar] [CrossRef]
- Fan, C.; Jin, H. A zero-dimensional model of porous char gasification in supercritical water: Experiments and mathematical modeling. Chem. Eng. J. 2022, 440, 135954. [Google Scholar] [CrossRef]
- Haugen, N.E.L.; Loong, B.K.Y.; Mitchell, R.E. Numerical approaches for thermochemical conversion of char. Prog. Energy Combust. Sci. 2022, 91, 100993. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, S.; Song, W.; Wang, X.; Zhu, J.; Chen, R.; Ding, H.; Hui, J.; Lyu, Q. Experimental study on conversion characteristics of anthracite and bituminous coal during preheating-gasification. Fuel 2022, 324, 124712. [Google Scholar] [CrossRef]
- Cui, K.; Liu, B.; Wu, Y.; Yang, H.; Lü, J.; Zhang, H. Numerical simulation of oxy-coal combustion for a swirl burner with EDC model. Chin. J. Chem. Eng. 2014, 22, 193–201. [Google Scholar] [CrossRef]
- Lupant, D.; Lybaert, P. Assessment of the EDC combustion model in MILD conditions with in-furnace experimental data. Appl. Therm. Eng. 2015, 75, 93–102. [Google Scholar] [CrossRef]
- Westbrook, C.K.; Dryer, F.L. Simplified reaction mechanisms for the oxidation of hydrocarbon fuels in flames. Combust. Sci. Technol. 1981, 27, 31–43. [Google Scholar] [CrossRef]
- Jones, W.; Lindstedt, R. Global reaction schemes for hydrocarbon combustion. Combust. Flame 1988, 73, 233–249. [Google Scholar] [CrossRef]
- Bustamante, F.; Enick, R.; Killmeyer, R.; Howard, B.; Rothenberger, K.; Cugini, A.; Morreale, B.; Ciocco, M. Uncatalyzed and wall-catalyzed forward water–gas shift reaction kinetics. AIChE J. 2005, 51, 1440–1454. [Google Scholar] [CrossRef]
- Ma, J.; Zitney, S.E. Computational fluid dynamic modeling of entrained-flow gasifiers with improved physical and chemical submodels. Energy Fuels 2012, 26, 7195–7219. [Google Scholar] [CrossRef]
- Kajitani, S.; Hara, S.; Matsuda, H. Gasification rate analysis of coal char with a pressurized drop tube furnace. Fuel 2002, 81, 539–546. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, Z.; Li, Z.; Lv, Z.; Cai, N. Optimizing in-situ char gasification kinetics in reduction zone of pulverized coal air-staged combustion. Combust. Flame 2018, 194, 52–71. [Google Scholar] [CrossRef]
- Hui, J.; Zhu, S.; Zhang, X.; Liu, Y.; Lin, J.; Ding, H.; Su, K.; Cao, X.; Lyu, Q. Experimental study of deep and flexible load adjustment on pulverized coal combustion preheated by a circulating fluidized bed. J. Clean. Prod. 2023, 418, 138040. [Google Scholar] [CrossRef]
- Lv, Z.; Xiong, X.; Yu, S.; Tan, H.; Xiang, B.; Huang, J.; Peng, J.; Li, P. Experimental investigation on NO emission of semi-coke under high temperature preheating combustion technology. Fuel 2021, 283, 119293. [Google Scholar] [CrossRef]
- Rahimipetroudi, I.; Rashid, K.; Yang, J.B.; Dong, S.K. Comprehensive study of the effect of a developed co-firing burner and its front-wall, opposed-wall, and tangential firing arrangements on the performance improvement and emissions reduction of coal-natural gas combustion in a boiler. Int. J. Therm. Sci. 2022, 173, 107379. [Google Scholar] [CrossRef]
- Ktistis, P.; Agathokleous, R.A.; Kalogirou, S.A. A design tool for a parabolic trough collector system for industrial process heat based on dynamic simulation. Renew. Energy 2022, 183, 502–514. [Google Scholar] [CrossRef]
- Alobaid, F.; Peters, J.; Epple, B. Experimental measurements for Polish lignite combustion in a 1 MWth circulating fluidized bed during load changes. Energy 2021, 228, 120585. [Google Scholar] [CrossRef]
- Zhu, J.; Ouyang, Z.; Lu, Q. An experimental study on NO x emissions in combustion of pulverized coal preheated in a circulating fluidized bed. Energy Fuels 2013, 27, 7724–7729. [Google Scholar] [CrossRef]
- Zhu, S.; Lyu, Q.; Zhu, J. Experimental investigation of NOx emissions during pulverized char combustion in oxygen-enriched air preheated with a circulating fluidized bed. J. Energy Inst. 2019, 92, 1388–1398. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Y.; Yi, G.; Nie, L.; Che, D. Effects of air staging conditions on the combustion and NO x emission characteristics in a 600 MW wall fired utility boiler using lean coal. Energy Fuels 2013, 27, 5831–5840. [Google Scholar] [CrossRef]
- Gonzalo-Tirado, C.; Jiménez, S.; Ballester, J. Kinetics of CO2 gasification for coals of different ranks under oxy-combustion conditions. Combust. Flame 2013, 160, 411–416. [Google Scholar] [CrossRef]
- Jayaraman, K.; Gokalp, I.; Bonifaci, E.; Merlo, N. Kinetics of steam and CO2 gasification of high ash coal–char produced under various heating rates. Fuel 2015, 154, 370–379. [Google Scholar] [CrossRef]
- Glarborg, P.; Miller, J.A.; Ruscic, B.; Klippenstein, S.J. Modeling nitrogen chemistry in combustion. Prog. Energy Combust. Sci. 2018, 67, 31–68. [Google Scholar] [CrossRef]
- Glarborg, P.; Jensen, A.D.; Johnsson, J.E. Fuel nitrogen conversion in solid fuel fired systems. Prog. Energy Combust. Sci. 2003, 29, 89–113. [Google Scholar] [CrossRef]
- Ding, H.; Ouyang, Z.; Zhang, X.; Zhu, S. The effects of particle size on flameless combustion characteristics and NOx emissions of semi-coke with coal preheating technology. Fuel 2021, 297, 120758. [Google Scholar] [CrossRef]
- Liu, X.; Tan, H.; Xiong, X.; Lv, Z.; Lu, X.; Wang, X. Mechanism study of nitric oxide reduction by light gases from typical Chinese coals. J. Energy Inst. 2020, 93, 1697–1704. [Google Scholar] [CrossRef]
- Yao, Y.; Zhu, J.; Lu, Q. Experimental study on nitrogen transformation in combustion of pulverized semi-coke preheated in a circulating fluidized bed. Energy Fuels 2015, 29, 3985–3991. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, J.; Tan, H.; Mo, Q.; Wang, X.; Wang, Y. Numerical and experimental study on co-firing of low volatile coal in a 330 MW tangentially fired boiler. J. Energy Inst. 2021, 96, 242–250. [Google Scholar] [CrossRef]
- Han, S.H.; Chang, D.; Yang, W. Numerical study on the reburning characteristics of biomass syngas in a 2MW pilot scale heavy oil furnace. Fuel 2016, 181, 277–285. [Google Scholar] [CrossRef]
- Wang, S.; Niu, Y.; Zhu, G.; Ding, Y.; Guo, X. NO formation and destruction during combustion of high temperature preheated pulverized coal. J. Energy Inst. 2021, 99, 82–87. [Google Scholar] [CrossRef]
- Frassoldati, A.; Faravelli, T.; Ranzi, E. Kinetic modeling of the interactions between NO and hydrocarbons at high temperature. Combust. Flame 2003, 135, 97–112. [Google Scholar] [CrossRef]




| Proximate Analysis, % | Ultimate Analysis, % | ||
|---|---|---|---|
| Moisture | 14.50 | C | 65.10 |
| Ash | 7.37 | H | 3.25 |
| Volatile | 28.67 | O | 8.08 |
| Fixed carbon | 49.46 | N | 0.66 |
| S | 0.71 | ||
| Item | Model | Parameters |
|---|---|---|
| Turbulence | Realizable k-ɛ model | |
| Radiation | Discrete ordinates model | Theta/Phi divisions 4 × 4 Theta/Phi pixels 2 × 2 |
| Absorption coefficient | WSGGM model | |
| Turbulence–chemistry interaction | EDC model | |
| Lagrangian stochastic tracking | Discrete random walk model | Number of tries: 10 |
| Particle radiation parameters | Emissivity: 0.9 Scattering factor: 0.6 | |
| Devolatilization | Single reaction rate model | A: 41,230 s−1 E: 3.76 × 107 J/kgmol |
| Char reaction model | Multiple surface reactions model | |
| Gas–particle coupling | Particle-source-in-cell method | |
| Thermal NOx model | Extended Zeldovich mechanism | |
| Fuel NOx model | De Soete mechanism | |
| NOx reduction by the char surface | AE: Area of the char surface PNO: Partial pressure of NO | |
| NOx reduction through reburning | k1 = 108; k2 = 1.4 × 106 e−550/T; k3 = 2 × 105 |
| Gas-Phase Reactions | Ar | Er (J/kmol) | m | a | b | c | Ref. | |
|---|---|---|---|---|---|---|---|---|
| R1 | Vol→x1CH4 + x2CO + x3H2 + x4SO2 + x5N2 | 1018 | 0 | 0 | 0 | 0 | 0 | [23] |
| R2 | CO + 0.5O2→CO2 | 2.24 × 1012 | 1.67 × 108 | 0 | 1 | 0.25 | 0.5[H2O] | [41] |
| R3 | H2 + 0.5O2→H2O | 6.8 × 1015 | 1.67 × 108 | −1 | 0.25 | 1.5 | 0 | [41] |
| R4 | CH4 + 2O2→CO2 + 2H2O | 2.12 × 1011 | 2.05 × 108 | 0 | 0.2 | 1.3 | 0 | [42] |
| R5 | CO + H2O→CO2 + H2 | 2.34 × 1010 | 2.88 × 108 | 0 | 0.5 | 1 | 0 | [43] |
| R6 | CO2 + H2→CO + H2O | 2.2 × 107 | 1.9 × 108 | 0 | 0.5 | 1 | 0 | [44] |
| R7 | CH4 + H2O→CO + 3H2 | 8.0 × 107 | 2.51 × 108 | 0 | 0.5 | 1 | 0 | [44] |
| Char Phase Reactions | Ar | Er (J/kmol) | n | Ref. | |
|---|---|---|---|---|---|
| R1 | C(s) + 0.5O2→CO | 113 | 1.3 × 108 | 0.68 | [45] |
| R2 | C(s) + CO2→2CO | 62.3 | 2.53 × 108 | 1 | [46] |
| R3 | C(s) + H2O→CO + H2 | 0.47 | 1.91 × 108 | 1 | [46] |
| Pulverized Coal (kg/s) | Primary Air (kg/s) | Excess Air Coefficient | Load (%) | |
|---|---|---|---|---|
| Case 1 a | 1.24 | 2.89 | 0.3 | 100 |
| Case 2 | 0.86 | 2.02 | 0.3 | 70 |
| Case 3 | 0.62 | 1.45 | 0.3 | 50 |
| Case 4 | 0.50 | 1.16 | 0.3 | 40 |
| Load (%) | Conversion of Particles (%) | Average Residue Time of Particles (s) | ||
|---|---|---|---|---|
| Volatile | Char | |||
| Case 1 | 100 | 100 | 50.97 | 1.87 |
| Case 2 | 70 | 100 | 54.74 | 2.02 |
| Case 3 | 50 | 100 | 57.34 | 2.24 |
| Case 4 | 40 | 100 | 59.89 | 2.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, G.; Han, X.; Tang, H.; Qu, J. Numerical and Experimental Study on Preheating Burner Characteristics for Peak Shaving. Processes 2024, 12, 346. https://doi.org/10.3390/pr12020346
Yao G, Han X, Tang H, Qu J. Numerical and Experimental Study on Preheating Burner Characteristics for Peak Shaving. Processes. 2024; 12(2):346. https://doi.org/10.3390/pr12020346
Chicago/Turabian StyleYao, Guojia, Xiaoju Han, Hong Tang, and Jianxin Qu. 2024. "Numerical and Experimental Study on Preheating Burner Characteristics for Peak Shaving" Processes 12, no. 2: 346. https://doi.org/10.3390/pr12020346





