A New Comprehensive Indicator for Monitoring Anaerobic Digestion: A Principal Component Analysis Approach
Abstract
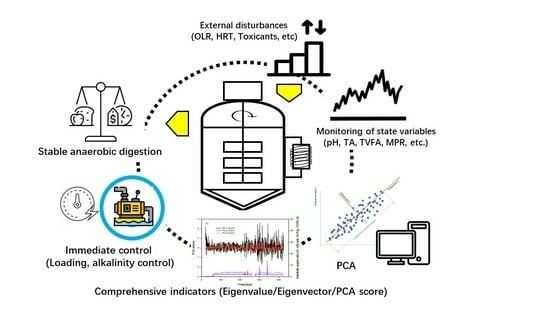
:1. Introduction
2. Materials and Methods
2.1. Setup and Operation of an Anaerobic Digester
2.2. Single and Coupled Indicators for the AD State
2.3. Principal Component Analysis for Comprehensive Indicators
3. Results and Discussion
3.1. Single Indicators for the State of Anaerobic Digestion
3.2. Coupled Indicators for the State of Anaerobic Digestion
3.3. Comprehensive Indicators Based on PCA
3.4. Implications
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jia, R.; Song, Y.C.; Piao, D.M.; Kim, K.; Lee, C.Y.; Park, J. Exploration of deep learning models for real-time monitoring of state and performance of anaerobic digestion with online sensors. Bioresour. Technol. 2022, 363, 127908. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Song, Y.C.; Yoo, K.; Kuppanan, N.; Subudhi, S.; Lal, B. Polarized electrode enhances biological direct interspecies electron transfer for methane production in upflow anaerobic bioelectrochemical reactor. Chemosphere 2018, 204, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Song, Y.C.; Kim, D.H.; Kim, M.S.; Kim, D.H. Influence of the temperature and hydraulic retention time in bioelectrochemical anaerobic digestion of sewage sludge. Int. J. Hydrog. Energy 2019, 44, 2170–2179. [Google Scholar] [CrossRef]
- Li, L.; He, Q.; Ma, Y.; Wang, X.; Peng, X. A mesophilic anaerobic digester for treating food waste: Process stability and microbial community analysis using pyrosequencing. Microb. Cell Factories 2016, 15, 65. [Google Scholar] [CrossRef] [PubMed]
- Vavilin, V.A.; Fernandez, B.; Palatsi, J.; Flotats, X. Hydrolysis kinetics in anaerobic degradation of particulate organic material: An overview. Waste Manag. 2008, 28, 939–951. [Google Scholar] [CrossRef]
- Yang, C.; Wu, H.; Cai, M.; Zhou, Y.; Guo, C.; Han, Y.; Zhang, L. Valorization of Biomass-Derived Polymers to Functional Biochar Materials for Supercapacitor Applications via Pyrolysis: Advances and Perspectives. Polymers 2023, 15, 2741. [Google Scholar] [CrossRef]
- Peces, M.; Astals, S.; Jensen, P.D.; Clarke, W.P. Deterministic mechanisms define the long-term anaerobic digestion microbiome and its functionality regardless of the initial microbial community. Water Res. 2018, 141, 366–376. [Google Scholar] [CrossRef]
- Jiang, M.; Qiao, W.; Wang, Y.; Zou, T.; Lin, M.; Dong, R. Balancing acidogenesis and methanogenesis metabolism in thermophilic anaerobic digestion of food waste under a high loading rate. Sci. Total Environ. 2022, 24, 153867. [Google Scholar] [CrossRef]
- Li, L.; Peng, X.; Wang, X.; Wu, D. Anaerobic digestion of food waste: A review focusing on process stability. Bioresour. Technol. 2018, 48, 20–28. [Google Scholar] [CrossRef]
- Li, L.; He, Q.; Ma, Y.; Wang, X.; Peng, X. Dynamics of microbial community in a mesophilic anaerobic digester treating food waste: Relationship between community structure and process stability. Bioresour. Technol. 2015, 189, 113–120. [Google Scholar] [CrossRef]
- Ajayi-Banji, A.; Rahman, S. A review of process parameters influence in solid-state anaerobic digestion: Focus on performance stability thresholds. Renew. Sustain. Energy Rev. 2022, 67, 112756. [Google Scholar] [CrossRef]
- Li, D.; Ran, Y.; Chen, L.; Cao, Q.; Li, Z.; Liu, X. Instability diagnosis and syntrophic acetate oxidation during thermophilic digestion of vegetable waste. Water Res. 2018, 139, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Wang, C.; He, Z.; Qin, Y.; Li, Y.Y. Biogas production performance and system stability monitoring in thermophilic anaerobic co-digestion of lipids and food waste. Bioresour. Technol. 2022, 358, 127432. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Li, L.; Peng, Y.; Yang, P.; Peng, X.; Sun, Y.; Wang, X. State indicators of anaerobic digestion: A critical review on process monitoring and diagnosis. Renew. Sustain. Energy Rev. 2021, 148, 111260. [Google Scholar] [CrossRef]
- Dong, F.; Zhao, Q.B.; Li, W.W.; Sheng, G.P.; Zhao, J.B.; Tang, Y.; Yu, H.Q.; Kubota, K.; Li, Y.Y.; Harada, H. Novel online monitoring and alert system for anaerobic digestion reactors. Environ. Sci. Technol. 2011, 45, 9093–9100. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Ni, P.; Angelidaki, I.; Dong, R.; Wu, S. Exploring stability indicators for efficient monitoring of anaerobic digestion of pig manure under perturbations. Waste Manag. 2019, 91, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Li, L.; Zhen, F.; Liu, H.; Xiao, F.; Sun, Y.; Peng, X.; Li, Y.; Wang, X. Thermodynamics of volatile fatty acid degradation during anaerobic digestion under organic overload stress: The potential to better identify process stability. Water Res. 2022, 214, 118187. [Google Scholar] [CrossRef]
- Awhangbo, L.; Bendoula, R.; Roger, J.M.; Béline, F. Detection of early imbalances in semi-continuous anaerobic co-digestion process based on instantaneous biogas production rate. Water Res. 2020, 171, 115444. [Google Scholar] [CrossRef]
- Jia, R.; Song, Y.-C.; An, Z.; Kim, K.; Oa, S.-W. Unraveling Anaerobic Digestion Instability: A Simple Index Based on the Kinetic Balance of Biochemical Reactions. Processes 2023, 11, 2852. [Google Scholar] [CrossRef]
- Yu, J. Local and global principal component analysis for process monitoring. J. Process Control 2012, 22, 1358–1373. [Google Scholar] [CrossRef]
- Kim, M.; Chul, P.; Kim, W.; Cui, F. Application of data smoothing and principal component analysis to develop a parameter ranking system for the anaerobic digestion process. Chemosphere 2022, 299, 134444. [Google Scholar] [CrossRef] [PubMed]
- Castura, J.C.; Varela, P.; Næs, T. Investigating paired comparisons after principal component analysis. Food Qual. Prefer. 2023, 106, 104814. [Google Scholar] [CrossRef]
- Anderson, G.K.; Yang, G. Determination of bicarbonate and total volatile acid concentration in anaerobic digesters using a simple titration. Water Environ. Res. 1992, 64, 53–59. [Google Scholar] [CrossRef]
- Nguyen, D.; Gadhamshetty, V.; Nitayavardhana, S.; Khanal, S.K. Automatic process control in anaerobic digestion technology: A critical review. Bioresour. Technol. 2015, 193, 513–522. [Google Scholar] [CrossRef] [PubMed]
- De la Rubia, M.A.; Riau, V.; Raposo, F.; Borja, R. Thermophilic anaerobic digestion of sewage sludge: Focus on the influence of the start-up. A review. Crit. Rev. Biotechnol. 2013, 33, 448–460. [Google Scholar] [CrossRef] [PubMed]
- Jantsch, T.G.; Mattiasson, B. An automated spectrophotometric system for monitoring buffer capacity in anaerobic digestion processes. Water Res. 2004, 38, 3645–3650. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Peng, X.; Li, L.; Yang, P.; Peng, Y.; Liu, H.; Wang, X. Commercial biogas plants: Review on operational parameters and guide for performance optimization. Fuel 2021, 303, 121282. [Google Scholar] [CrossRef]
- Steinberg, L.M.; Regan, J.M. Phylogenetic comparison of the methanogenic communities from an acidic, oligotrophic fen and an anaerobic digester treating municipal wastewater sludge. Appl. Environ. Microbiol. 2008, 74, 6663–6671. [Google Scholar] [CrossRef]
- Kim, I.S.; Hwang, M.H.; Jang, N.J.; Hyun, S.H.; Lee, S.T. Effect of low pH on the activity of hydrogen utilizing methanogen in bio-hydrogen process. Int. J. Hydrogen Energy 2004, 29, 1133–1140. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Wang, J.; Meng, L. Effects of volatile fatty acid concentrations on methane yield and methanogenic bacteria. Biomass Bioenergy 2009, 33, 848–853. [Google Scholar] [CrossRef]
- Guo, X.; Wang, C.; Sun, F.; Zhu, W.; Wu, W. A comparison of microbial characteristics between the thermophilic and mesophilic anaerobic digesters exposed to elevated food waste loadings. Bioresour. Technol. 2014, 152, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.N.; Wang, Q.; Li, Y.; Wang, H.; Gao, Y.; Sun, Y.; Wang, B.; Bian, R.; Li, W.; Zhan, M. Impact of incineration slag co-disposed with municipal solid waste on methane production and methanogens ecology in landfills. Bioresour. Technol. 2023, 377, 128978. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; He, Q.; Wei, Y.; He, Q.; Peng, X. Early warning indicators for monitoring the process failure of anaerobic digestion system of food waste. Bioresour. Technol. 2014, 171, 491–494. [Google Scholar] [CrossRef] [PubMed]
- Abd Nasir, M.A.; Jahim, J.M.; Abdul, P.M.; Silvamany, H.; Maaroff, R.M.; Yunus, M.F.M. The use of acidified palm oil mill effluent for thermophilic biomethane production by changing the hydraulic retention time in anaerobic sequencing batch reactor. Int. J. Hydrogen Energy 2019, 44, 3373–3381. [Google Scholar] [CrossRef]
- Aromolaran, A.; Sartaj, M.; Abdallah, M. Supplemental Sewage Scum and Organic Municipal Solid Waste Addition to the Anaerobic Digestion of Thickened Waste Activated Sludge: Biomethane Potential and Microbiome Analysis. Fermentation 2023, 9, 237. [Google Scholar] [CrossRef]
- Li, W.; Khalid, H.; Zhu, Z.; Zhang, R.; Liu, G.; Chen, C.; Thorin, E. Methane production through anaerobic digestion: Participation and digestion characteristics of cellulose, hemicellulose and lignin. Appl. Energy 2018, 226, 1219–1228. [Google Scholar] [CrossRef]
- Kafle, G.K.; Kim, S.H. Sludge exchange process on two serial CSTRs anaerobic digestions: Process failure and recovery. Bioresour. Technol. 2011, 102, 6815–6822. [Google Scholar] [CrossRef]
- Zou, J.; Nie, E.; Lü, F.; Peng, W.; Zhang, H.; He, P. Screening of early warning indicators for full-scale dry anaerobic digestion of household kitchen waste. Environ. Res. 2022, 214, 114136. [Google Scholar] [CrossRef]
- Li, D.; Chen, L.; Liu, X.; Mei, Z.; Ren, H.; Cao, Q.; Yan, Z. Instability mechanisms and early warning indicators for mesophilic anaerobic digestion of vegetable waste. Bioresour. Technol. 2017, 245, 90–97. [Google Scholar] [CrossRef]
- Wu, D.; Li, L.; Zhao, X.; Peng, Y.; Yang, P.; Peng, X. Anaerobic digestion: A review on process monitoring. Renew. Sustain. Energy Rev. 2019, 103, 1–12. [Google Scholar] [CrossRef]
- Maspolim, Y.; Zhou, Y.; Guo, C.; Xiao, K.; Ng, W.J. Determination of the archaeal and bacterial communities in two-phase and single-stage anaerobic systems by 454 pyrosequencing. J. Environ. Sci. 2015, 36, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Liu, R.; van Loosdrecht, M.C.; Cao, D. Batch influences of exogenous hydrogen on both acidogenesis and methanogenesis of excess sludge. Chem. Eng. J. 2017, 317, 544–550. [Google Scholar] [CrossRef]
- Dupla, M.; Conte, T.; Bouvier, J.C.; Bernet, N.; Steyer, J.P. Dynamic evaluation of a fixed bed anaerobic digestion process in response to organic overloads and toxicant shock loads. Water Sci. Technol. 2004, 49, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Qin, Z.; Zhang, P.; Li, X.; Feng, L. Polycyclic aromatic hydrocarbons stimulate acidogenesis, acetogenesis and methanogenesis during anaerobic co-digestion of waste activated sludge and food waste. Bioresour. Technol. 2022, 360, 127567. [Google Scholar] [CrossRef]
- Meegoda, J.N.; Li, B.; Patel, K.; Wang, L.B. A review of the processes, parameters, and optimization of anaerobic digestion. Int. J. Environ. Res. Public Health 2018, 15, 2224. [Google Scholar] [CrossRef]
- Paranjpe, A.; Saxena, S.; Jain, P. A Review on Performance Improvement of Anaerobic Digestion Using Co-Digestion of Food Waste and Sewage Sludge. J. Environ. Manag. 2023, 338, 117733. [Google Scholar] [CrossRef]
- Lü, F.; Chen, W.; Duan, H.; Zhang, H.; Shao, L.; He, P. Monitor process state of batch anaerobic digestion in reliance on volatile and semi-volatile metabolome. Bioresour. Technol. 2022, 351, 126953. [Google Scholar] [CrossRef]
- McCormick, M.; Villa, A.E. LSTM and 1-D convolutional neural networks for predictive monitoring of the anaerobic digestion process. In Artificial Neural Networks and Machine Learning–ICANN 2019: Workshop and Special Session; ICAN, Lecture Notes in Computer Science; Springer: Cham, Switzerland, 2019; Volume 11731, pp. 725–736. [Google Scholar] [CrossRef]





| Operational Modes | Single Indicators | Comprehensive Indicators | |||
|---|---|---|---|---|---|
| Eigenvalues | PC Score Range | State | |||
| Range /Average | Trends | ||||
| Start-up | Decreases in vs. and COD; low SCOD, TVFA; increases in MPR and CH4 (%) | 0.01~3.55 /0.47 | Decrease | −0.62~2.17 | Slightly disturbed |
| Steady-state | Stable for all single indicators | 0.02~0.52 /0.15 | Stable | −0.65~0.73 | Stable |
| Impulse OLRs (<200%) | Small fluctuations in SCOD, TVFA, MPR, and TA | 0.07~2.47 /0.44 | Single peak | −0.90~1.38 | Slightly disturbed |
| Interruption of substrate supply | Small fluctuations in all single indicators | 0.06~1.28 /0.40 | Repeated small peaks | −0.57~0.93 | Slightly disturbed |
| Resumption of substrate supply | Increases in VS, COD, TVFA, SOD, pH, and TA; decreases in MPR, CH4 (%) | 0.03~4.39 /1.37 | Repeated large peaks | −1.40~2.42 | Disturbed |
| 300% impulse OLR (short-term) | Increases in TVFA, TA, pH, VS, and COD; large fluctuations in CH4 (%) | 0.34~5.81 /2.18 | Repeated large peaks | −1.70~2.58 | Disturbed |
| 300% impulse OLR (long-term) | Increase in MPR; decreases in TVFA | 0.06~1.53 /0.60 | Repeated small peaks | −0.89~1.11 | Slightly disturbed |
| 500% impulse OLR (short-term) | Increases in COD, VS, TVFA, and SCOD; decreases in MPR and CH4 (%) | 0.09~4.70 /0.80 | Repeated large peaks | −1.32~2.50 | Severely disturbed |
| 500% impulse OLR (long-term) | Decrease in TVFA; high variability of MPR and CH4 (%) | 0.06~3.17 /0.77 | Repeated large peaks | −1.35~1.96 | Severely disturbed |
| Eigenvalue Maximum | Eigenvalue Average | Fluctuation Pattern | State |
|---|---|---|---|
| <1.0 | <0.2 | Stable | Stable |
| <4.0 | <0.6 | Decreasing | Slight perturbed |
| <3.0 | <0.6 | Single or repeated small peaks | Slight perturbed |
| >3.0 | >0.6 | Repeated peaks in short-term | Perturbed |
| >3.0 | >0.6 | Repeated peaks in long-term | Severely perturbed |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, R.; Song, Y.-C.; An, Z.; Kim, K.; Lee, C.-Y.; Bae, B.-U. A New Comprehensive Indicator for Monitoring Anaerobic Digestion: A Principal Component Analysis Approach. Processes 2024, 12, 59. https://doi.org/10.3390/pr12010059
Jia R, Song Y-C, An Z, Kim K, Lee C-Y, Bae B-U. A New Comprehensive Indicator for Monitoring Anaerobic Digestion: A Principal Component Analysis Approach. Processes. 2024; 12(1):59. https://doi.org/10.3390/pr12010059
Chicago/Turabian StyleJia, Ru, Young-Chae Song, Zhengkai An, Keugtae Kim, Chae-Young Lee, and Byung-Uk Bae. 2024. "A New Comprehensive Indicator for Monitoring Anaerobic Digestion: A Principal Component Analysis Approach" Processes 12, no. 1: 59. https://doi.org/10.3390/pr12010059