1. Introduction
As an effective method to solve bottom-hole fluid accumulation, foam-drainage agents have become an economically efficient and commonly used auxiliary recovery method, and most studies have verified their economic and effective characteristics. In order to develop a high-performance foam-dispersant system, different types of surfactants were compounded [
1,
2,
3,
4,
5,
6,
7,
8,
9]. In the process of natural gas development, there is not only the problem of liquid accumulation, but also the problem of hydrate and pipeline corrosion in an environment of low temperature and high pressure. For research into an integrated agent that has the simultaneous functions of foam drainage, corrosion inhibition and hydrate anti-polymerization, the combination of any two commonly used agents may produce antagonism [
10,
11,
12,
13,
14,
15,
16,
17]. For example, methanol is often added for the purpose of hydrate anti-polymerization to alleviate the accumulation of hydrate, and methanol has an obvious effect as an anti-polymerization agent, However, methanol plays a defoaming role in foam dispersants, while commonly used corrosion inhibitors lack the performance of hydrate anti-aggregation.
Cage hydrates are ice-like compounds composed of small gas molecules encapsulated in water molecules. The formation of natural gas hydrates in oil and gas pipelines may lead to the failure of natural gas flow and serious safety and environmental issues. The use of anti-condensates is a promising method to reduce the risks of natural gas hydrates in actual production [
18]. Therefore, effectively suppressing the generation and blockage of hydrates is a very important and urgent problem for all oil and gas production. One of the most effective methods to improve natural gas extraction is to suppress the generation of natural gas hydrates. Due to the fact that the use of anti-polymerization agents is not limited by temperature (supercooling) conditions and the concentration of use is below 3%, the price of anti-polymerization agents is relatively high [
19]. Considering the economic benefits, in practical production applications, different types of inhibitors are often mixed and reused to reduce costs. This can significantly improve both economic composition and the inhibition effect. At present, this method is the most widely used in the development and application of natural gas in domestic and foreign gas fields to suppress hydrate generation and accumulation [
20,
21].
To address the issues of high water content in gas wells, hydrate formation under low temperature and high pressure, and wellbore-corrosion susceptibility, a foam-drainage hydrate anti-aggregation corrosion-inhibitor system has been developed. By utilizing experimental and theoretical research, we evaluated the foaming and stabilizing properties of the foam-drainage hydrate anti-aggregation corrosion-inhibitor system, and revealed the relationship between the structure of the foaming agent and the foaming performance [
22,
23,
24,
25,
26]. We tested the phase-change point of hydrate formation caused by the system, and explored the corrosion-inhibition performance of the system for injection and production systems such as wellbore [
27,
28,
29,
30]. Therefore, based on these three points, a foam-agent system with the multiple performances of foaming, polymerization prevention and corrosion inhibition has been developed.
2. Experimental
2.1. Materials
AOST was purchased from Lusen Chemical Co., Ltd. (Linyi, China). BS-12 was purchased from Huajun New Materials Co., Ltd. (Huainan, China). Methanol and petroleum ether were purchased from Fuyu Fine Chemical Co., Ltd. (Tianjin, China). All products were used as received without further purification.
2.2. Surface-Tension Measurement
The surface tension of each solution was measured by the hanging-ring method at room temperature. Before the measurements, the tensiometer (Kruss K 100, Hamburg, Germany) was used to test the surface tension of a distilled water sample to confirm the accuracy of the instrument; the surface tension of distilled water measured in this manner was 72.65 mN/m. Each measurement was repeated at least three times and results reported as the average.
2.3. Foaming-Capacity Evaluation
A high-speed agitation method was used to generate foams using a high-speed mixer (GJ-3S, Qingdao Haitongda Special Instrument Co., Ltd., Qingdao, China). In each test, 100 mL surfactant solution was agitated at 7000 r/min for 3 min at ambient conditions. After the foam preparation, the foam was transferred into a graduated cylinder immediately. The volume and half-life time (the time that 50 mL of free-water phase accumulated at the bottom of the cylinder) of the foam were recorded. Each test was repeated in triplicate. All measurements were performed at 25 °C and atmospheric pressure.
2.4. Salt-Resistance Evaluation
Generally, the salinity of formation water has a strong adverse effect on the generation of foam. To study the effect of concentration and species of inorganic ions on the foaming ability and related foaming stability of the surfactants, surfactant solutions with different salt concentrations (NaCl, KCl, MgCl2, CaCl2) were prepared.
2.5. Temperature Resistance
The temperature in a well has a significant effect on the performance of foaming agents. Therefore, the Ross–Miles method was used to measure the foaming capacity and stability of the formula at temperatures ranging from 30 to 70 °C. Each test was repeated three times.
2.6. Methanol Effect Evaluation
During gas production, methanol was usually used to prevent the formation of gas hydrate. However, the presence of methanol may also retard the performance of foaming agents. Therefore, it was necessary to check the methanol’s influence on the foaming ability of the optimized foaming agents. In this section, the foaming performance of optimized surfactant solutions with 0, 5, 10 and 15% methanol was tested in a temperature range of 40 to 70 °C using the Ross–Miles method.
2.7. Liquid-Carrying-Capacity Test
According to the performance requirements for the foam-discharge agent in the Changqing Gas Field, the liquid-carrying performance of the integrated agent required evaluation. After aging 200 mL of the integrated agent at 65 °C for 30 min, 3.0 L/min of N
2 was introduced into the dedicated foam tube. After 15 min, the volume vs. of the remaining liquid in the foam tube was recorded. The formula for calculating the liquid-carrying rate is:
where W is liquid-carrying rate (%), Vs is the remaining liquid in the foam tube.
2.8. Adsorption Experiment
By scanning the wavelength of a UV spectrophotometer, the adsorption capacity of different concentrations of 4DF-4 as a whole agent on iron powder was tested, and the adsorption relationship between 4DF-4 as a foaming agent and metals was evaluated using a standard curve.
2.9. Surface Micromorphology of Steel Sheet
A DSX-500 (OLYMPUS, Tokyo, Japen) fully automatic three-dimensional imaging microscope was used to scan steel sheets in bright field (BF) mode, the scanning area of the Q235 sample was 277 μm × 277 μm, in order to obtain 2D and 3D images as well as height maps.
2.10. Micro-Morphology of Hydrates
The assembled hydrate-growth observation instrument, consisting of temperature control system, low-temperature reaction system, and image processing system, was used to observe the micro-morphology of hydrate growth of 20% tetrahydrofuran aqueous solution and 20% tetrahydrofuran integrated agent solution at different times.
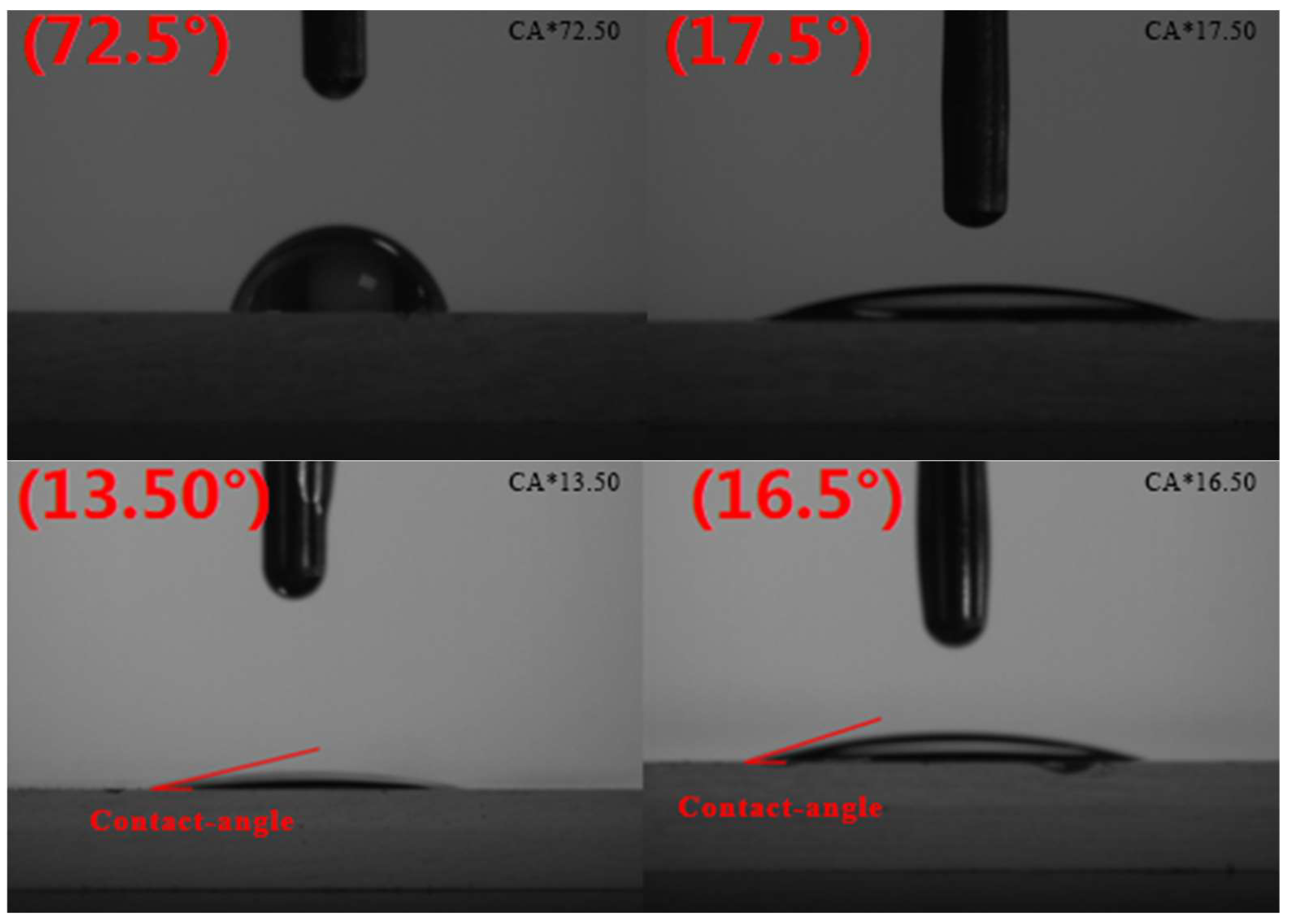
2.11. Contact-Angle Experiment
The contact angle of water and oil droplets on the surface of glass treated with reagents was measured using a contact-angle measuring instrument.
2.12. Thermodynamic Analysis of Hydrates
The phase-transition point of hydrate with 20% tetrahydrofuran aqueous solution and 20% tetrahydrofuran surfactant solution was measured by DSC.
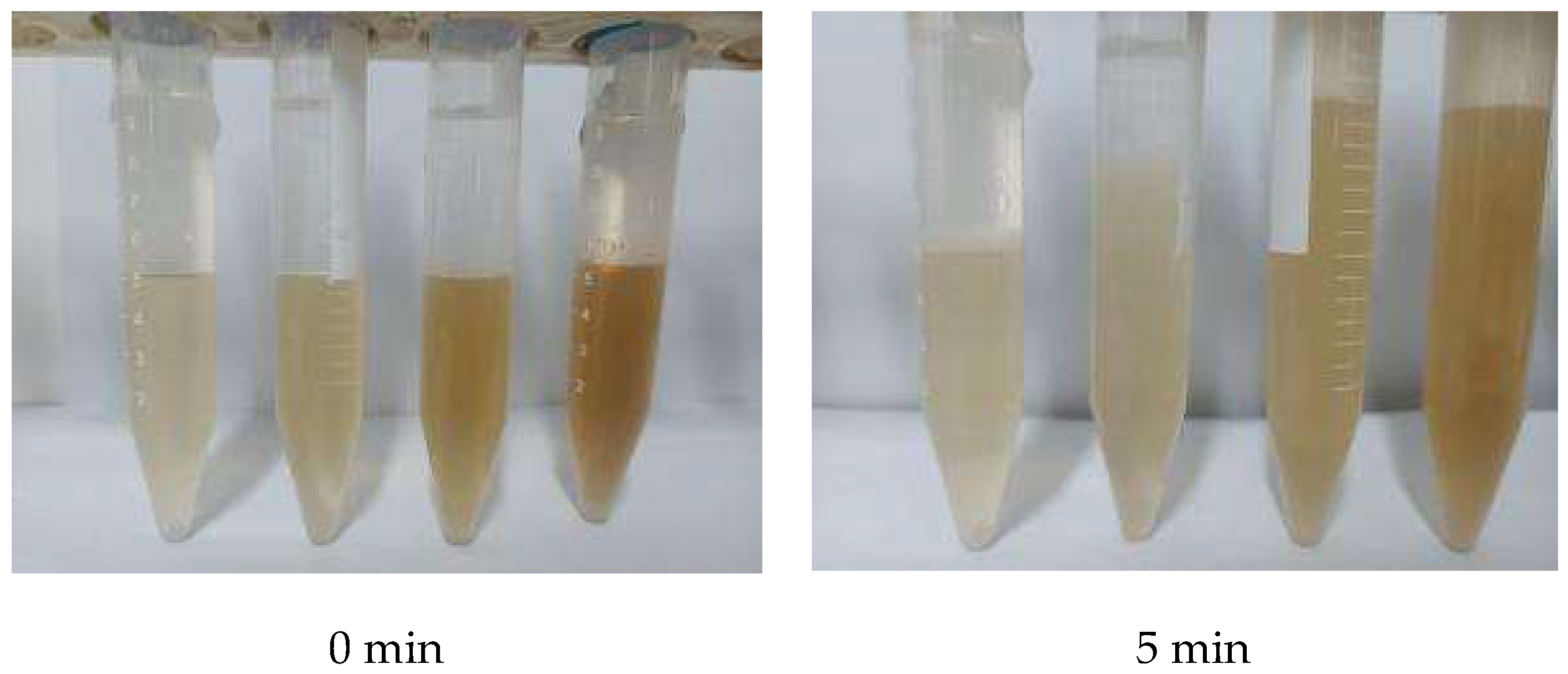
2.13. Emulsification Experiment and Microstructure Analysis after Emulsification
Samples of 10 mL of different concentration multiples (times 0.5, 1, 1.5, 2) were taken of the integrated agent and condensate oil in a 1:1 ratio and placed in a centrifuge tube. They were placed in a 45 °C water bath at a constant temperature for 10 min and then taken out. They were shaken in the left and right hands 50 times each to form an emulsion, then placed in a water bath and the time and amount of water released was recorded. The precipitation rate was calculated:
where V
w is precipitation rate (%), V
T is the volume of water that separates water (mL), V
0 is the total volume of water (mL).
Samples of 100 mL of different concentration multiples (times 0.5, 1, 1.5, 2) were taken of the integrated agent and crude oil in a 1:1 ratio and placed in a high stirring cup. After stirring according to the high-stirring evaluation method, the emulsion was placed under a polarizing microscope to observe the micro-morphology of the emulsion of the integrated agent and oil at different concentrations.
4. Conclusions
In this study, the final integrated formulation was determined: 0.1% sodium alpha-olefin sulfonate (AOST) + 0.3% dodecyl dimethyl betaine (BS-12) + 0.3% sodium lignosulfonate + 0.5% hydrazine hydrate. With this formulation, the performances of foaming, polymerization prevention and corrosion inhibition were improved. After adding this integrated agent, the growth rate of hydrates was slow and the maximum corrosion depth decreased to 5.24 µm. The good performance of this anti-aggregation agent is largely because of its oil-in-water type emulsion, preventing the dispersed water droplets in the oil phase from coalescing in one place. These excellent abilities to produce foam and inhibit corrosion indicate that they can be used in the oil and petrochemical industry for foam drainage and to inhibit corrosion, which have a certain practical uses.