A Review of Treatment Technologies for Perfluorooctane Sulfonate (PFOS) and Perfluorooctanoic Acid (PFOA) in Water
Abstract
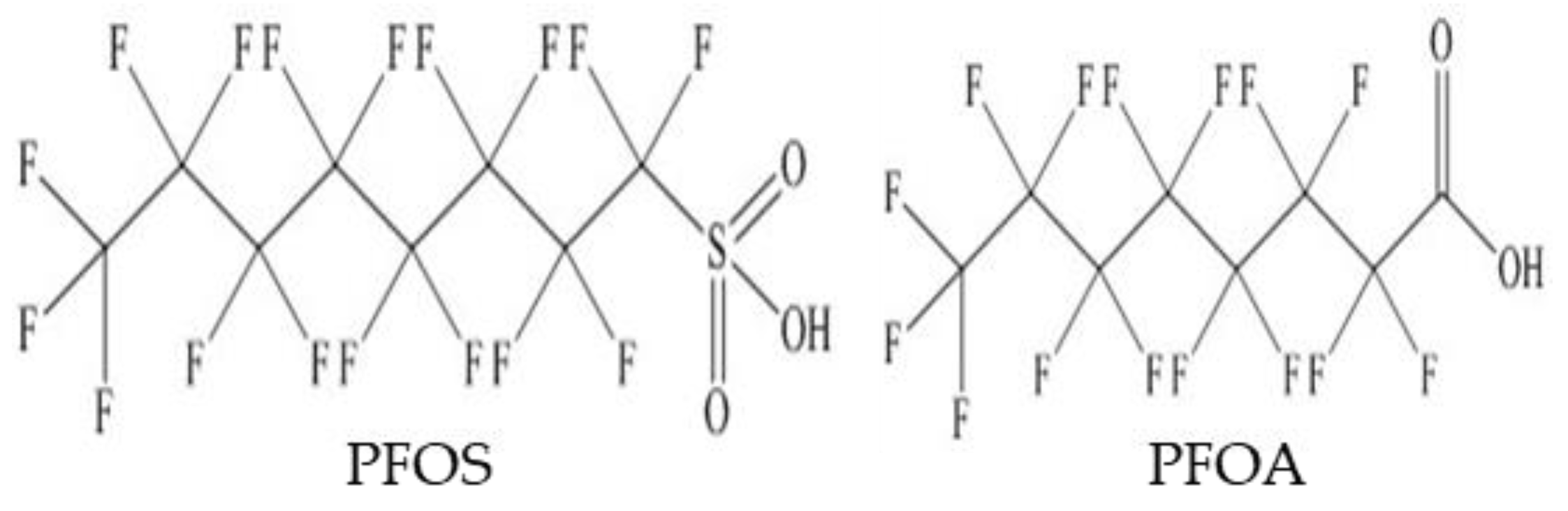
:1. Introduction
2. Existing Technologies
2.1. Adsorption
2.1.1. Activated Carbon (AC)
2.1.2. Mineral Materials
2.1.3. Anion Exchange (AE) Resin
2.1.4. Carbonaceous Nanomaterials (CNMs)
2.2. Advanced Oxidation Processes (AOP)
2.2.1. Electrochemical Oxidation (EO)
2.2.2. Fenton Oxidation
2.2.3. Photochemical Oxidation
2.2.4. Ultrasonic Oxidation
2.3. Microbial Treatment
2.4. Membrane Separation
3. Discussion and Perspectives
- Up to now, most of the studies on removal technologies for industrial wastewater have only observed the changes and removal rates of PDCs throughout the process. However, in practical applications, not only the removal of other pollutants from wastewater by the process should be studied, but also the impact of treatment technology on wastewater quality should be fully considered.
- Considering the advantages and disadvantages of different technologies, multiple technologies can be considered for coupling in the future to achieve the effect of improving processing efficiency or reducing side effects.
- Due to the late start of research on PFCs in China, the research on PFCs removal by Chinese scholars is relatively limited. Most of the removal research is also limited to common PFCs such as PFOS and PFOA. Next, we can increase the research on the removal of other types of PFCs.
- With the development of industry, the environmental pollution situation is becoming increasingly serious, and groundwater has been polluted to varying degrees. However, currently, various types of research on PFOS and PFOA are concentrated in laboratory-simulated water bodies or surface water bodies, with little research on groundwater. It is recommended to increase the detection of PFCs in groundwater, establish simple and economical PFCs detection methods, and provide research data for the treatment of PFCs in groundwater.
- Currently, there is a huge demand for PFCs substitutes in many fields, and increasing research on PFCs substitutes can alleviate the current environmental pollution caused by PFCs.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cheng, X.; Wei, Y.; Zhang, Z.; Wang, F.; He, J.; Wang, R.; Xu, Y.; Keerman, M.; Zhang, S.; Zhang, Y. Plasma PFOA and PFOS levels, DNA methylation, and blood lipid levels: A pilot study. Environ. Sci. Technol. 2022, 56, 17039–17051. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Yang, B.; Peng, L.; Wu, J.; Hao, H.; Lou, S. Target-triggered double fluorescent biosensors for rapid and sensitive detection of long-chain perfluorinated compounds using DNA probe and lysozyme fiber. Sci. Total Environ. 2023, 860, 160496. [Google Scholar] [CrossRef] [PubMed]
- Na, S.; Hai, R.; Wang, X.; Li, N. Trends and levels of perfluorinated compounds in soil and sediment surrounding a cluster of metal plating industries. Soil Sediment Contam. Int. J. 2021, 30, 423–435. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, H.; Zhu, L.; Hu, W.; Lin, Z. Simultaneous determination of 11 volatile perfluorinated compound precursors in textiles using gas chromatography-triple quadrupole mass spectrometry. Chin. J. Chromatogr. 2021, 39, 1239–1246. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.H.; Gai, N.; Zhang, P.Y.; Piao, H.T.; Chen, S.; Wang, X.C.; Jiao, X.C.; Yin, X.C.; Tan, K.Y.; Yang, Y.L. Perfluoroalkyl acids in surface waters and tapwater in the Qiantang River watershed—Influences from paper, textile, and leather industries. Chemosphere 2017, 185, 610–617. [Google Scholar] [CrossRef]
- Fang, C.; Megharaj, M.; Naidu, R. Electrochemical studies on self-assembled monolayer (SAM) upon exposure to anionic surfactants: PFOA, PFOS, SDS and SDBS. Electroanalysis 2017, 29, 2155–2160. [Google Scholar]
- Sajid, M.; Ilyas, M. PTFE-coated non-stick cookware and toxicity concerns: A perspective. Environ. Sci. Pollut. Res. 2017, 24, 23436–23440. [Google Scholar] [CrossRef]
- Costanza, J.; Arshadi, M.; Abriola, L.M.; Pennell, K.D. Accumulation of PFOA and PFOS at the air–water interface. Environ. Sci. Technol. Lett. 2019, 6, 487–491. [Google Scholar] [CrossRef]
- Wang, Z.; Cousins, L.T.; Scheringer, M.; Hungerbühler, K. Fluorinated alternatives to long-chain perfluoroalkyl carboxylic acids (PFCAs), perfluoroalkane sulfonic acids (PFSAs) and their potential precursors. Environ. Int. 2013, 60, 242–248. [Google Scholar] [CrossRef]
- Leung, S.C.E.; Shukla, P.; Chen, D.; Eftekhari, E.; An, H.; Zare, F.; Ghasemi, N.; Zhang, D.; Nguyen, N.T.; Li, Q. Emerging technologies for PFOS/PFOA degradation and removal: A review. Sci. Total Environ. 2022, 827, 153669. [Google Scholar] [CrossRef]
- Zhou, J.; Li, S.; Liang, X.; Feng, X.; Wang, T.; Li, Z.; Zhu, L. First report on the sources, vertical distribution and human health risks of legacy and novel per- and polyfluoroalkyl substances in groundwater from the Loess Plateau, China. J. Hazard. Mater. 2021, 404, 124134. [Google Scholar] [CrossRef]
- Death, C.; Bell, C.; Champness, D.; Milne, C.; Reichman, S.; Hagen, T. Per- and polyfluoroalkyl substances (PFAS) in livestock and game species: A review. Sci. Total Environ. 2021, 774, 144795. [Google Scholar]
- Ghisi, R.; Vamerali, T.; Manzetti, S. Accumulation of perfluorinated alkyl substances (PFAS) in agricultural plants: A review. Environ. Res. 2019, 169, 326–341. [Google Scholar] [CrossRef]
- Jian, J.M.; Chen, D.; Han, F.J.; Guo, Y.; Zeng, L.; Lu, X.; Wang, F. A short review on human exposure to and tissue distribution of per- and polyfluoroalkyl substances (PFASs). Sci. Total Environ. 2018, 636, 1058–1069. [Google Scholar] [CrossRef] [PubMed]
- Loewen, M.; Halldorson, T.; Wang, F.; Tom, G. Fluorotelomer carboxylic acids and PFOS in rainwater from an urban center in Canada. Environ. Sci. Technol. 2005, 39, 2944–2951. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, E.; Kannan, K. Mass loading and fate of perfluoroalkyl surfactants in wastewater treatment plants. Environ. Sci. Technol. 2006, 40, 1408–1414. [Google Scholar] [CrossRef] [PubMed]
- Exner, M.; Färber, H. Perfluorinated surfactants in surface and drinking waters (9 pp). Environ. Sci. Pollut. Res. 2006, 13, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Saito, N.; Sasaki, K.; Nakatome, K.; Harada, K.; Yoshinaga, T.; Koizumi, A. Perfluorooctane sulfonate concentrations in surface water in Japan. Arch. Environ. Contam. Toxicol. 2003, 45, 149–158. [Google Scholar] [CrossRef]
- Hu, Y.; Guo, M.; Zhang, S.; Jiang, W.; Xiu, T.; Yang, S.; Kang, M.; Dongye, Z.; Li, Z.; Wang, L. Microwave synthesis of metal-organic frameworks absorbents (DUT-5-2) for the removal of PFOS and PFOA from aqueous solutions. Microporous Mesoporous Mater. 2022, 333, 111740. [Google Scholar] [CrossRef]
- Rodea-Palomares, I.; Leganés, F.; Rosal, R.; Fernández-Piñas, F. Toxicological interactions of perfluorooctane sulfonic acid (PFOS) and perfluorooctanoic acid (PFOA) with selected pollutants. J. Hazard. Mater. 2012, 201, 209–218. [Google Scholar] [CrossRef]
- UNEP (United Nations Environment Programme). Report of the Conference of the Parties of the Stockholm Convention on Persistent Organic Pollutants on the Work of its Fourth Meeting. In United Nations Environment Programme: Stockholm Convention on Persistent Organic Pollutants; UNEP: Geneva, Switzerland, 2009. [Google Scholar]
- UNEP (United Nations Environment Programme). Report of the Conference of the Parties to the Stockholm Convention on Persistent Organic Pollutants on the Work of Its Ninth Meeting. In United Nations Environment Programme: Stockholm Convention on Persistent Organic Pollutants; UNEP: Geneva, Switzerland, 2019. [Google Scholar]
- Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L. Risk to human health related to the presence of perfluorooctane sulfonic acid and perfluorooctanoic acid in food. EFSA J. 2018, 16, e05194. [Google Scholar]
- Xie, S.; Wang, T.; Liu, S.; Jones, K.C.; Sweetman, A.J.; Lu, Y. Industrial source identification and emission estimation of perfluorooctane sulfonate in China. Environ. Int. 2013, 52, 1–8. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Shi, G.; Liu, C.; Hao, Q.; Wu, L. Occurrence and risk assessment of perfluorooctanoate (PFOA) and perfluorooctane sulfonate (PFOS) in surface water, groundwater and sediments of the Jin River Basin, Southeastern China. Bull. Environ. Contam. Toxicol. 2022, 108, 1026–1032. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, K.; Zheng, P.; Yin, S.; Jin, H.; Bai, X.; Li, Y.; Zheng, J.; Dai, Y.; Zhao, M. Prenatal exposure and transplacental transfer of perfluoroalkyl substance isomers in participants from the upper and lower reaches of the Yangtze River. Environ. Pollut. 2021, 270, 116202. [Google Scholar] [CrossRef]
- Meng, J.; Wang, T.; Wang, P.; Giesy, J.P.; Lu, Y. Perfluoroalkyl substances and organochlorine pesticides in sediments from Huaihe watershed in China. J. Environ. Sci. 2014, 26, 2198–2206. [Google Scholar] [CrossRef]
- Zhao, Z.; Tang, J.; Mi, L.; Tian, C.; Zhong, G.; Zhang, G.; Wang, S.; Li, Q.; Ebinghaus, R.; Xie, Z. Perfluoroalkyl and polyfluoroalkyl substances in the lower atmosphere and surface waters of the Chinese Bohai Sea, Yellow Sea, and Yangtze River estuary. Sci. Total Environ. 2017, 599, 114–123. [Google Scholar] [CrossRef]
- Xiao, Y.; Xiao, Q. Identifying key areas of ecosystem services potential to improve ecological management in Chongqing City, southwest China. Environ. Monit. Assess. 2018, 190, 258. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Guo, C.; Liang, S.; Zhang, Y.; Xu, J. Partitioning behavior, source identification, and risk assessment of perfluorinated compounds in an industry-influenced river. Environ. Sci. Eur. 2019, 31, 55. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Wang, P.; Hu, B.; Wang, C.; Li, D. Perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS) in surface water of China: National exposure distributions and probabilistic risk assessment. Arch. Environ. Contam. Toxicol. 2021, 81, 470–481. [Google Scholar] [PubMed]
- Cao, L.Y.; Zhang, X.Y.; Xu, Y.; Xiang, W.; Wang, R.; Ding, F.J.; Hong, P.Z.; Gao, B. Straw and wood based biochar for CO2 capture: Adsorption performance and governing mechanisms. Sep. Purif. Technol. 2022, 287, 120592. [Google Scholar] [CrossRef]
- Punyapalakul, P.; Suksomboon, K.; Prarat, P.; Khaodhiar, S. Effects of surface functional groups and porous structures on adsorption and recovery of perfluorinated compounds by inorganic porous silicas. Sep. Sci. Technol. 2013, 48, 775–788. [Google Scholar] [CrossRef]
- Deng, S.; Yu, Q.; Huang, J.; Yu, G. Removal of perfluorooctane sulfonate from wastewater by anion exchange resins: Effects of resin properties and solution chemistry. Water Res. 2010, 44, 5188–5195. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Zhang, R.; Deng, S.; Huang, J.; Yu, G. Sorption of perfluorooctane sulfonate and perfluorooctanoate on activated carbons and resin: Kinetic and isotherm study. Water Res. 2009, 43, 1150–1158. [Google Scholar] [CrossRef]
- Kuvayskaya, A.; Lotsi, B.; Mohseni, R.; Vasiliev, A. Mesoporous adsorbents for perfluorinated compounds. Microporous Mesoporous Mater. 2020, 305, 110374. [Google Scholar] [CrossRef]
- Yea, Y.; Kim, G.; Wang, D.; Kim, S.; Yoon, Y.; Elanchezhiyan, S.S.; Park, C.M. Selective sequestration of perfluorinated compounds using polyaniline decorated activated biochar. Chem. Eng. J. 2022, 430, 132837. [Google Scholar] [CrossRef]
- Meng, P.; Fang, X.; Maimaiti, A.; Yu, G.; Deng, S. Efficient removal of perfluorinated compounds from water using a regenerable magnetic activated carbon. Chemosphere 2019, 224, 187–194. [Google Scholar] [CrossRef]
- Du, Z.; Deng, S.; Chen, Y.; Wang, B.; Huang, J.; Wang, Y.; Yu, G. Removal of perfluorinated carboxylates from washing wastewater of perfluorooctanesulfonyl fluoride using activated carbons and resins. J. Hazard. Mater. 2015, 286, 136–143. [Google Scholar] [CrossRef]
- Tang, J.; Liu, Y.; Su, P.; Quan, J.; Hu, Y.; Wang, W.; Zhang, C. Removal of COD, NH(4)-N, and perfluorinated compounds from wastewater treatment plant effluent using ZnO-coated activated carbon. Water Sci. Technol. 2020, 81, 2459–2470. [Google Scholar]
- Wang, Y.; Guo, J.Q.; Sumita; Shi, C.J.; Zhu, Q.J.; Li, C.; Pang, W.H. A review of recent advances in detection and treatment technology for perfluorinated compounds. Water 2022, 14, 3919. [Google Scholar] [CrossRef]
- Zhao, L.; Bian, J.; Zhang, Y.; Zhu, L.; Liu, Z. Comparison of the sorption behaviors and mechanisms of perfluorosulfonates and perfluorocarboxylic acids on three kinds of clay minerals. Chemosphere 2014, 114, 51–58. [Google Scholar] [CrossRef]
- Ordonez, D.; Podder, A.; Valencia, A.; Sadmani, A.H.M.A.; Reinhart, D.; Chang, N.B. Continuous fixed-bed column adsorption of perfluorooctane sulfonic acid (PFOS) and perfluorooctanoic acid (PFOA) from canal water using zero-valent Iron-based filtration media. Sep. Purif. Technol. 2022, 299, 121800. [Google Scholar] [CrossRef]
- Fagbayigbo, B.O.; Opeolu, B.O.; Fatoki, O.S.; Olatunji, O.S.; Akharame, M.O.; Human, I.S. Sorption and partitioning of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) onto sediments of Diep and Plankenburg river systems Western Cape, South Africa. Environ. Technol. Innov. 2022, 25, 102110. [Google Scholar] [CrossRef]
- Kothawala, D.N.; Kohler, S.J.; Ostlund, A.; Wiberg, K.; Ahrens, L. Influence of dissolved organic matter concentration and composition on the removal efficiency of perfluoroalkyl substances (PFASs) during drinking water treatment. Water Res. 2017, 121, 320–328. [Google Scholar] [CrossRef]
- Gagliano, E.; Sgroi, M.; Falciglia, P.P.; Vagliasindi, F.G.A.; Roccaro, P. Removal of poly- and perfluoroalkyl substances (PFAS) from water by adsorption: Role of PFAS chain length, effect of organic matter and challenges in adsorbent regeneration. Water Res. 2020, 171, 115381. [Google Scholar] [CrossRef]
- Maimaiti, A.; Deng, S.; Meng, P.; Wang, W.; Wang, B.; Huang, J.; Wang, Y.; Yu, G. Competitive adsorption of perfluoroalkyl substances on anion exchange resins in simulated AFFF-impacted groundwater. Chem. Eng. J. 2018, 348, 494–502. [Google Scholar] [CrossRef]
- Dixit, F.; Dutta, R.; Barbeau, B.; Berube, P.; Mohseni, M. PFAS removal by ion exchange resins: A review. Chemosphere 2021, 272, 129777. [Google Scholar] [CrossRef]
- Dixit, F.; Barbeau, B.; Mostafavi, S.G.; Mohseni, M. PFOA and PFOS removal by ion exchange for water reuse and drinking applications: Role of organic matter characteristics. Environ. Sci. Water Res. Technol. 2019, 5, 1782–1795. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Y.; Gao, B.; Ji, R.; Li, C.; Wang, S. Removal of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) from water by carbonaceous nanomaterials: A review. Crit. Rev. Environ. Sci. Technol. 2020, 50, 2379–2414. [Google Scholar] [CrossRef]
- Kumar, S.; Rani, R.; Dilbaghi, N.; Tankeshwar, K.; Kim, K.H. Carbon nanotubes: A novel material for multifaceted applications in human healthcare. Chem. Soc. Rev. 2017, 46, 158–196. [Google Scholar] [PubMed]
- Deng, S.; Niu, L.; Bei, Y.; Wang, B.; Huang, J.; Yu, G. Adsorption of perfluorinated compounds on aminated rice husk prepared by atom transfer radical polymerization. Chemosphere 2013, 91, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, L.; Li, Y.; Feng, Y.; Feng, W. Carbon-based functional nanomaterials: Preparation, properties and applications. Compos. Sci. Technol. 2019, 179, 10–40. [Google Scholar] [CrossRef]
- Zhang, C.; Tang, J.; Peng, C.; Jin, M. Degradation of perfluorinated compounds in wastewater treatment plant effluents by electrochemical oxidation with Nano-ZnO coated electrodes. J. Mol. Liq. 2016, 221, 1145–1150. [Google Scholar] [CrossRef]
- Schaefer, C.E.; Andaya, C.; Urtiaga, A.; McKenzie, E.R.; Higgins, C.P. Electrochemical treatment of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS) in groundwater impacted by aqueous film forming foams (AFFFs). J. Mol. Liq. 2015, 295, 170–175. [Google Scholar] [CrossRef]
- Liang, S.; Pierce Jr, R.D.; Lin, H.; Chiang, S.Y.; Huang, Q.J. Electrochemical oxidation of PFOA and PFOS in concentrated waste streams. Remediat. J. 2018, 28, 127–134. [Google Scholar] [CrossRef]
- Schaefer, C.E.; Andaya, C.; Burant, A.; Condee, C.W.; Urtiaga, A.; Strathmann, T.J.; Higgins, C.P. Electrochemical treatment of perfluorooctanoic acid and perfluorooctane sulfonate: Insights into mechanisms and application to groundwater treatment. Chem. Eng. J. 2017, 317, 424–432. [Google Scholar] [CrossRef]
- Qian, Y.; Guo, X.; Zhang, Y.; Peng, Y.; Sun, P.; Huang, C.H.; Niu, J.; Zhou, X.; Crittenden, J. Perfluorooctanoic acid degradation using uv-persulfate process: Modeling of the degradation and chlorate formation. Environ. Sci. Technol. 2016, 50, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Panchangam, S.C.; Chang, C.Y.; Andy Hong, P.K.; Hsueh, H.F. Removal of perfluorooctanoic acid and perfluorooctane sulfonate via ozonation under alkaline condition. J. Hazard. Mater. 2012, 243, 272–277. [Google Scholar] [CrossRef]
- Cheng, J.; Vecitis, C.D.; Park, H.; Mader, B.T.; Hoffmann, M.R. Sonochemical degradation of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in landfill groundwater: Environmental matrix effects. Environ. Sci. Technol. 2008, 42, 8057–8063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaplin, B.P. Critical review of electrochemical advanced oxidation processes for water treatment applications. Environ. Sci. Process. Impacts 2014, 16, 1182–1203. [Google Scholar] [CrossRef]
- Sirés, I.; Brillas, E.; Oturan, M.A.; Rodrigo, M.A.; Panizza, M. Electrochemical advanced oxidation processes: Today and tomorrow. A review. Environ. Sci. Pollut. Res. 2014, 21, 8336–8367. [Google Scholar] [CrossRef]
- Ma, Q.; Liu, L.; Cui, W.; Li, R.; Song, T.; Cui, Z. Electrochemical degradation of perfluorooctanoic acid (PFOA) by Yb-doped Ti/SnO2–Sb/PbO2 anodes and determination of the optimal conditions. RSC Adv. 2015, 5, 84856–84864. [Google Scholar] [CrossRef]
- Hou, J.; Li, G.; Liu, M.; Chen, L.; Yao, Y.; Fallgren, P.H.; Jin, S. Electrochemical destruction and mobilization of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) in saturated soil. Chemosphere 2022, 287, 132205. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.H.; Li Sip, Y.Y.; Kim, K.T.; Han, G.; Rodriguez, K.L.; Fox, D.W.; Afrin, S.; Burnstine-Townley, A.; Zhai, L.; Lee, W.H. Nanoparticle-embedded hydrogel synthesized electrodes for electrochemical oxidation of perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS). Chemosphere 2022, 296, 134001. [Google Scholar] [CrossRef]
- Trzcinski, A.P.; Harada, K. Adsorption of PFOS onto graphite intercalated compound and analysis of degradation by-products during electro-chemical oxidation. Chemosphere 2023, 323, 138268. [Google Scholar] [CrossRef] [PubMed]
- Pignatello, J.J.; Oliveros, E.; MacKay, A. Advanced oxidation processes for organic contaminant destruction based on the Fenton reaction and related chemistry. Crit. Rev. Environ. Sci. Technol. 2006, 36, 1–84. [Google Scholar] [CrossRef]
- Ertugay, N.; Kocakaplan, N.; Malkoç, E. Investigation of pH effect by Fenton-like oxidation with ZVI in treatment of the landfill leachate. Int. J. Min. Reclam. Environ. 2017, 31, 404–411. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J. Multivalent metal catalysts in Fenton/Fenton-like oxidation system: A critical review. Chem. Eng. J. 2023, 466, 143147. [Google Scholar] [CrossRef]
- Zhang, K.; Sumita; Li, C.; Sun, C.; Marmier, N. A review of the treatment process of perfluorooctane compounds in the waters: Adsorption, flocculation, and advanced oxidative process. Water 2022, 14, 2692. [Google Scholar] [CrossRef]
- Tang, H.; Xiang, Q.; Lei, M.; Yan, J.; Zhu, L.; Zou, J. Efficient degradation of perfluorooctanoic acid by UV–Fenton process. Chem. Eng. J. 2012, 184, 156–162. [Google Scholar] [CrossRef]
- Santos, A.; Rodriguez, S.; Pardo, F.; Romero, A. Use of Fenton reagent combined with humic acids for the removal of PFOA from contaminated water. Sci. Total Environ. 2016, 563, 657–663. [Google Scholar] [CrossRef]
- Jing, C.; Zhang, P.Y.; Jian, L. Photodegradation of perfluorooctanoic acid by 185 nm vacuum ultraviolet light. J. Environ. Sci. 2007, 19, 387–390. [Google Scholar]
- Matilainen, A.; Sillanpää, M. Removal of natural organic matter from drinking water by advanced oxidation processes. Chemosphere 2010, 80, 351–365. [Google Scholar] [CrossRef]
- Yuan, Y.; Feng, L.; He, X.; Wu, M.; Ai, Z.; Zhang, L.; Gong, J. Nitrate promoted defluorination of perfluorooctanoic acid in UV/sulfite system: Coupling hydrated electron/reactive nitrogen species-mediated reduction and oxidation. Environ. Pollut. 2022, 313, 120172. [Google Scholar] [CrossRef]
- Hu, Y.; Zhan, Y.; Wei, C.; Chen, F.; Cheng, J.; Shen, Y.; Zhou, Z.; Wang, L.; Liang, Y. Hydrochar coupled with iodide for efficient photodegradation of perfluorooctanoic acid and perfluorooctane sulfonic acid under ultraviolet light. Sci. Total Environ. 2023, 868, 161621. [Google Scholar] [CrossRef]
- Uriakhil, M.A.; Sidnell, T.; De Castro Fernandez, A.; Lee, J.; Ross, I.; Bussemaker, M. Per- and poly-fluoroalkyl substance remediation from soil and sorbents: A review of adsorption behaviour and ultrasonic treatment. Chemosphere 2021, 282, 131025. [Google Scholar] [CrossRef]
- Shende, T.; Andaluri, G.; Suri, R. Power density modulated ultrasonic degradation of perfluoroalkyl substances with and without sparging Argon. Ultrason. Sonochem. 2021, 76, 105639. [Google Scholar] [CrossRef] [PubMed]
- Shende, T.; Andaluri, G.; Suri, R. Frequency-dependent sonochemical degradation of perfluoroalkyl substances and numerical analysis of cavity dynamics. Sep. Purif. Technol. 2021, 261, 118250. [Google Scholar] [CrossRef]
- Xiong, X.; Shang, Y.; Bai, L.; Luo, S.; Seviour, T.W.; Guo, Z.; Ottosen, L.D.M.; Wei, Z. Complete defluorination of perfluorooctanoic acid (PFOA) by ultrasonic pyrolysis towards zero fluoro-pollution. Water Res. 2023, 235, 119829. [Google Scholar] [CrossRef]
- Li, Y.; He, L.; Lv, L.; Xue, J.; Wu, L.; Zhang, Z.; Yang, L. Review on plant uptake of PFOS and PFOA for environmental cleanup: Potential and implications. Environ. Sci. Pollut. Res. 2021, 28, 30459–30470. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Jaffe, P.R. Defluorination of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) by Acidimicrobium sp. strain A6. Environ. Sci. Technol. 2019, 53, 11410–11419. [Google Scholar] [CrossRef] [Green Version]
- Albert, K.; Hsieh, P.Y.; Chen, T.H.; Hou, C.H.; Hsu, H.Y. Diatom-assisted biomicroreactor targeting the complete removal of perfluorinated compounds. J. Hazard. Mater. 2020, 384, 121491. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Guo, C.S.; Zhang, Y. Removal efficiency of perfluorinated compounds with different microbial treatment techniques. Res. Environ. Sci. 2015, 28, 110–116. [Google Scholar]
- Olimattel, K.; Zhai, L.; Sadmani, A.H.M.A. Enhanced removal of perfluorooctane sulfonic acid and perfluorooctanoic acid via polyelectrolyte functionalized ultrafiltration membrane: Effects of membrane modification and water matrix. J. Hazard. Mater. Lett. 2021, 2, 100043. [Google Scholar] [CrossRef]
- Wang, Z.; Su, W.; Zhang, Y. Reverse osmosis membrane design for reclamation and removal of perfluorooctanoic acid. Desalin. Water Treat. 2021, 237, 32–36. [Google Scholar] [CrossRef]
- Kucharzyk, K.H.; Darlington, R.; Benotti, M.; Deeb, R.; Hawley, E. Novel treatment technologies for PFAS compounds: A critical review. J. Environ. Manag. 2017, 204, 757–764. [Google Scholar] [CrossRef]
- Eschauzier, C.; Beerendonk, E.; Scholte-Veenendaal, P.; De Voogt, P. Impact of treatment processes on the removal of perfluoroalkyl acids from the drinking water production chain. Environ. Sci. Technol. 2012, 46, 1708–1715. [Google Scholar] [CrossRef]
- Quiñones, O.; Snyder, S.A. Occurrence of perfluoroalkyl carboxylates and sulfonates in drinking water utilities and related waters from the United States. Environ. Sci. Technol. 2009, 43, 9089–9095. [Google Scholar] [CrossRef]
| Location | Concentration (ng/L) | Data Sources | |
|---|---|---|---|
| PFOS | PFOA | ||
| Pearl River | 0.90–99 | 0.85–13 | [25] |
| Yangtze River | 0.01–14 | 2.0–260 | [26] |
| Huaihe River | 4.7 | 18 | [27] |
| Yellow River | 82.3–261.8 | - | [28] |
| Haihe River | 2.0–7.6 | 4.4–42.0 | [29] |
| Liaohe River | 0.089–9.5 | 4.38–77.01 | [30] |
| Songhua River | 0.06–8.04 | 0.02–2.68 | [31] |
| Absorbent Materials | Types of Adsorbed Substances | Initial Concentration/(mg/L) | pH | Adsorption Capacity/(mg/g) | Data Sources |
|---|---|---|---|---|---|
| Powdered activated carbon | PFOA | 20–300 | 5–7 | 175–524 | [33] |
| Anion exchange resins | PFOS | 20–400 | 3–5 | 210–2575 | [34] |
| PFOA | 20–250 | 5 | 1206 | ||
| Non-ionic resins | PFOS | 0.01–5 | 6.4–6.9 | 37–41 | [35] |
| PFOA | 0.01–5 | 6.4–6.9 | 37–41 | ||
| BSSOs | PFOS | 100–400 | 7 | 23–907 | [36] |
| PFOA | 100–400 | 7 | 421–846 |
| Treatment Methods | Wastewater Sources | Wastewater Quality | Removal Efficiency (%) | Data Sources |
|---|---|---|---|---|
| EO (Nano ZnO as electrodes) | Wastewater treatment plant effluent. | Large range of PFCs, low levels of TOCs, etc. | Partial PFCs: 60. | [54] |
| EO (Ti/RuO2 as anodes) | Contains AFFFs Fire base groundwater. | High sulphate concentration, contains many PFCs. | PFOS:90, PFOA:90. | [55] |
| EO (Ti4O7 as anodes) | Ion exchange resin regeneration waste liquids. | High TOC/chlorine contents. | PFOS:100, PFOA:100. | [56] |
| EO (BDD as anodes) | Underground water. | Low TOC content, neutral. | PFOA:90. | [57] |
| Industrial wastewater treatment plants. | Wide range of PFCs, high content, neutral. | PFHpA:90, PFHxA:99, PFPeA:97, PFBA:75. | ||
| Waste leachate. | High COD/ammonia/chlorine content. | PFOS:75, PFOA:80. | ||
| UV/PS photocatalytic oxidation | Sewage treatment plants. | - | PFOS:50. | [58] |
| Ozone oxidation | Semiconductor industry wastewater. | - | PFOS:100, PFOA:92. | [59] |
| Ultrasonic oxidation | Groundwater at landfills. | Contains small amounts of organic compounds, neutral. | PFOS:90. | [60] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, J.; Huang, L.; Li, Y.; Zhang, Z.; Mu, R.; Liu, C.; Hu, S.; Xiao, Y.; Xu, M. A Review of Treatment Technologies for Perfluorooctane Sulfonate (PFOS) and Perfluorooctanoic Acid (PFOA) in Water. Processes 2023, 11, 2260. https://doi.org/10.3390/pr11082260
Cheng J, Huang L, Li Y, Zhang Z, Mu R, Liu C, Hu S, Xiao Y, Xu M. A Review of Treatment Technologies for Perfluorooctane Sulfonate (PFOS) and Perfluorooctanoic Acid (PFOA) in Water. Processes. 2023; 11(8):2260. https://doi.org/10.3390/pr11082260
Chicago/Turabian StyleCheng, Juntao, Liming Huang, Yunfeng Li, Zhen Zhang, Runzhi Mu, Changqing Liu, Shuncheng Hu, Yihua Xiao, and Mengchen Xu. 2023. "A Review of Treatment Technologies for Perfluorooctane Sulfonate (PFOS) and Perfluorooctanoic Acid (PFOA) in Water" Processes 11, no. 8: 2260. https://doi.org/10.3390/pr11082260
APA StyleCheng, J., Huang, L., Li, Y., Zhang, Z., Mu, R., Liu, C., Hu, S., Xiao, Y., & Xu, M. (2023). A Review of Treatment Technologies for Perfluorooctane Sulfonate (PFOS) and Perfluorooctanoic Acid (PFOA) in Water. Processes, 11(8), 2260. https://doi.org/10.3390/pr11082260







