Experimental Study on Migration and Intrusion Characteristics of Pulverized Coal in Propped Fractures
Abstract
:1. Introduction
2. Experimental Design
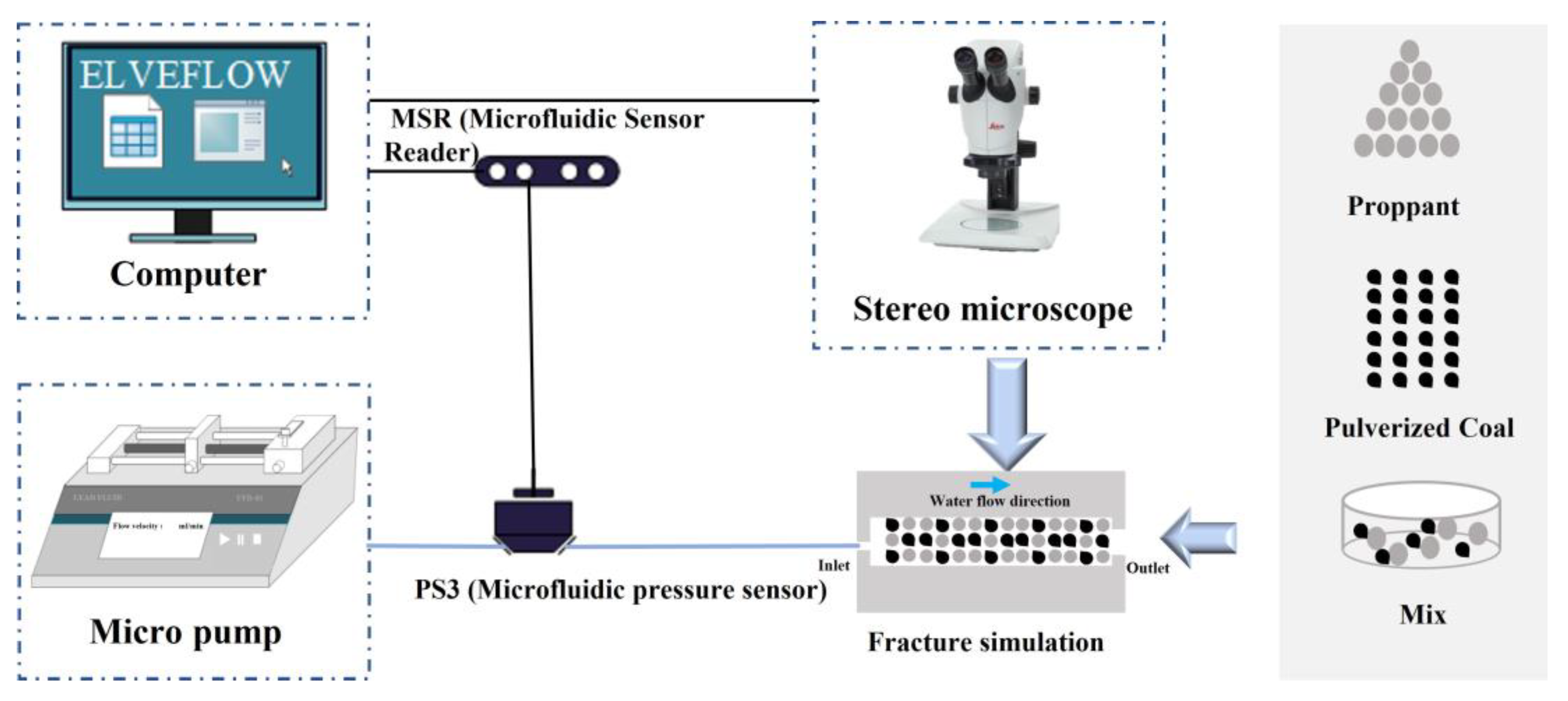
2.1. Experimental Setup
2.2. Coal and Solution Samples
2.3. Solution Surface Tension Test
2.4. Methods
3. Results and Analysis
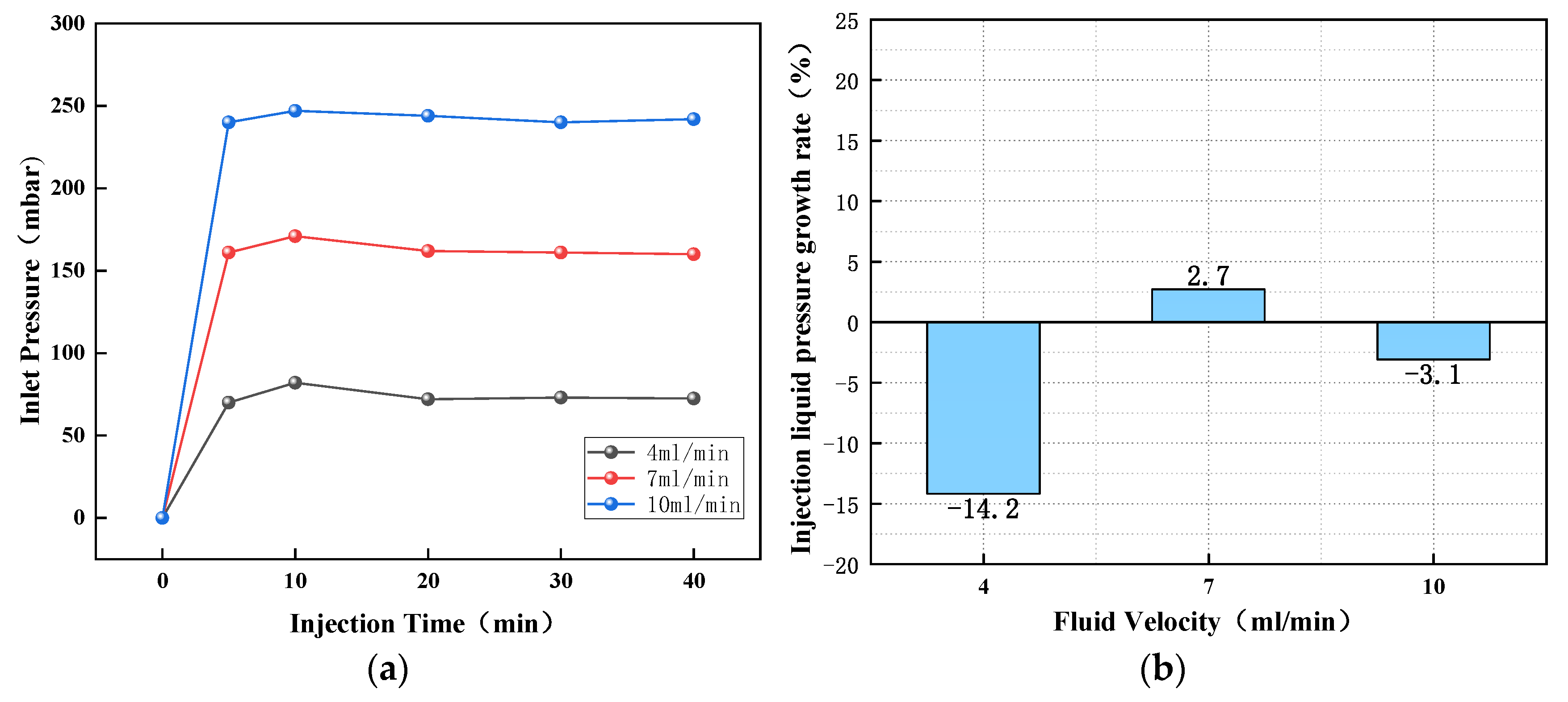
3.1. Injection Pressure Changes of Different Surfactant Solutions at Different Flow Rates
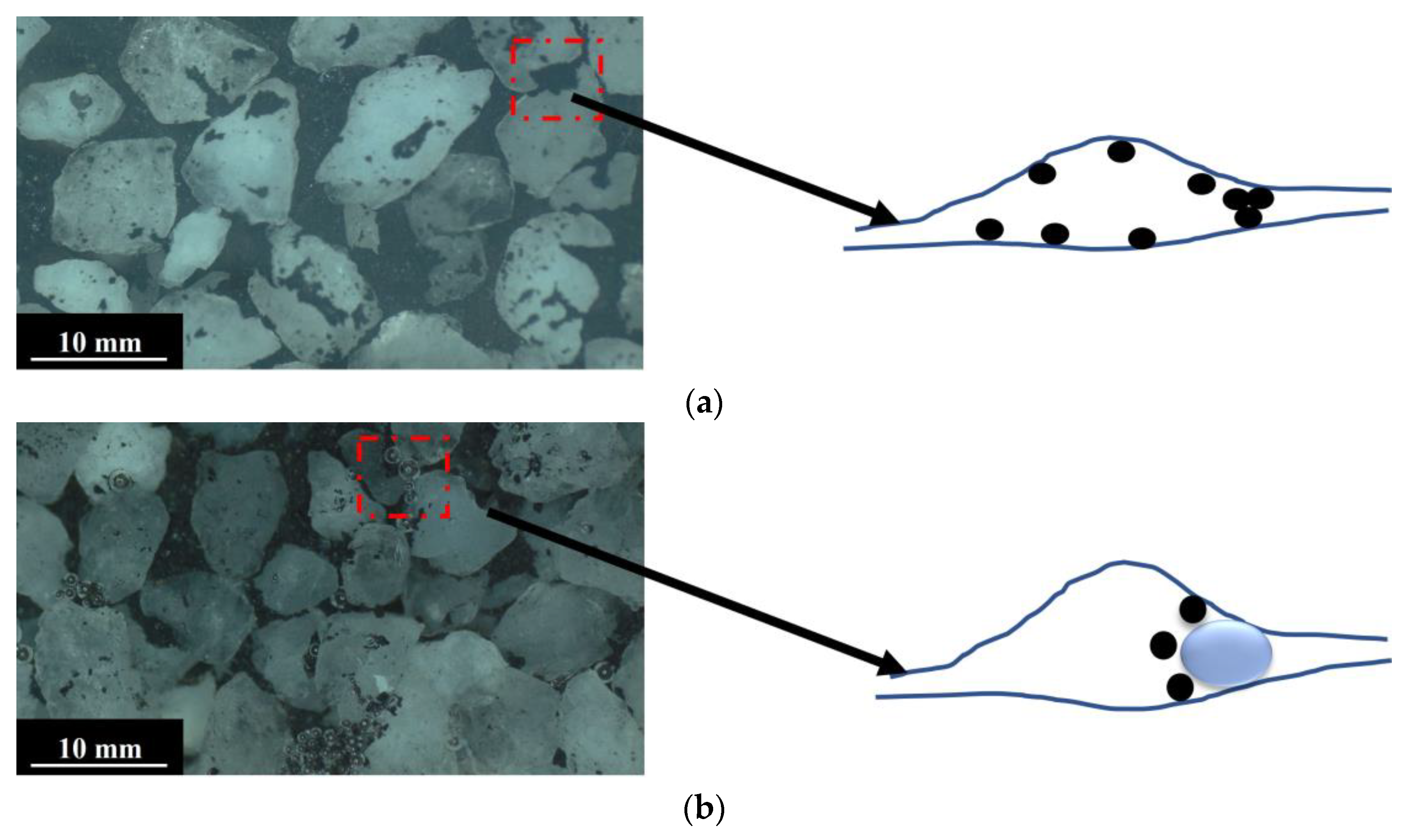
3.2. Images Obtained with Solutions of Different Surfactants at Different Flow Rates
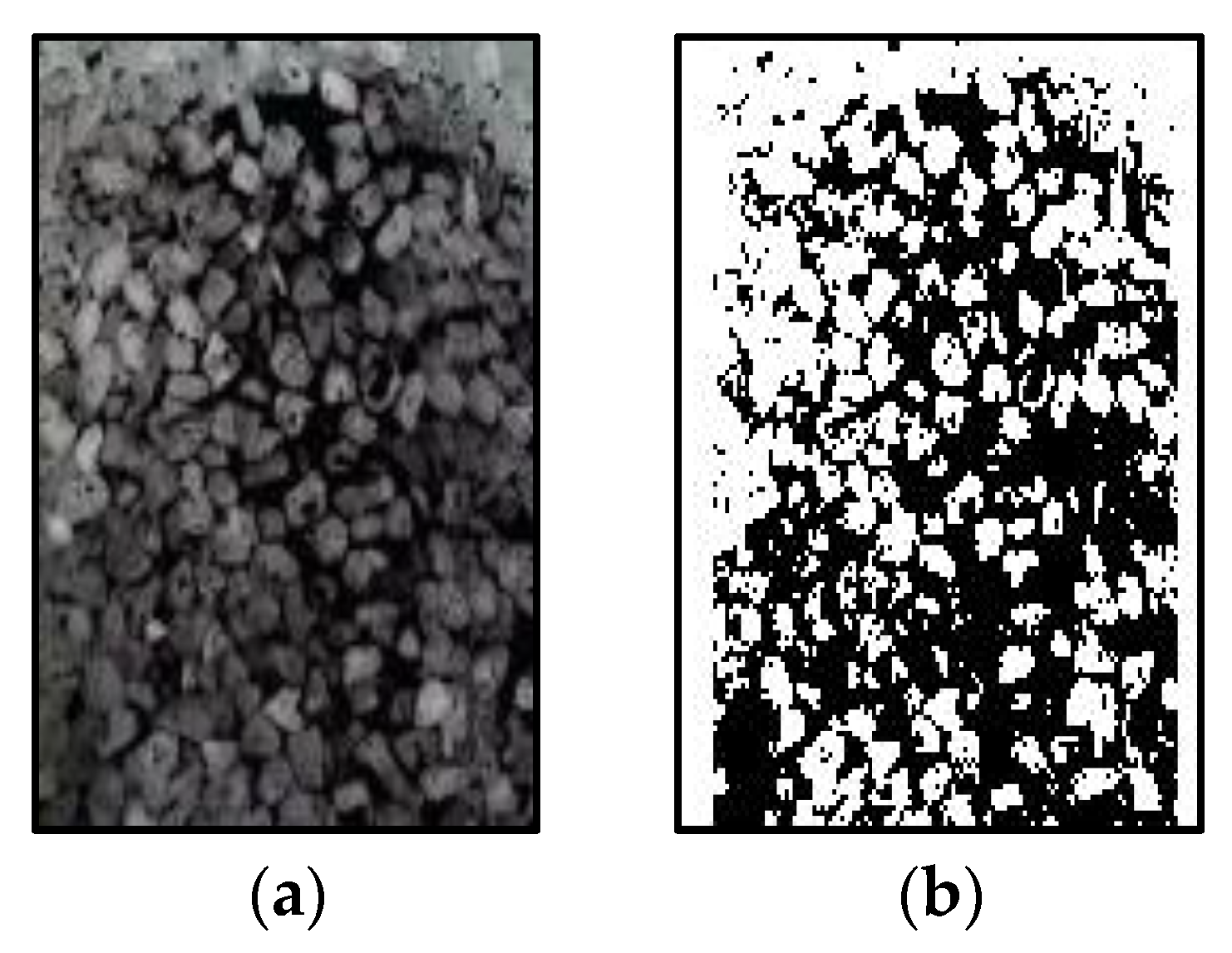
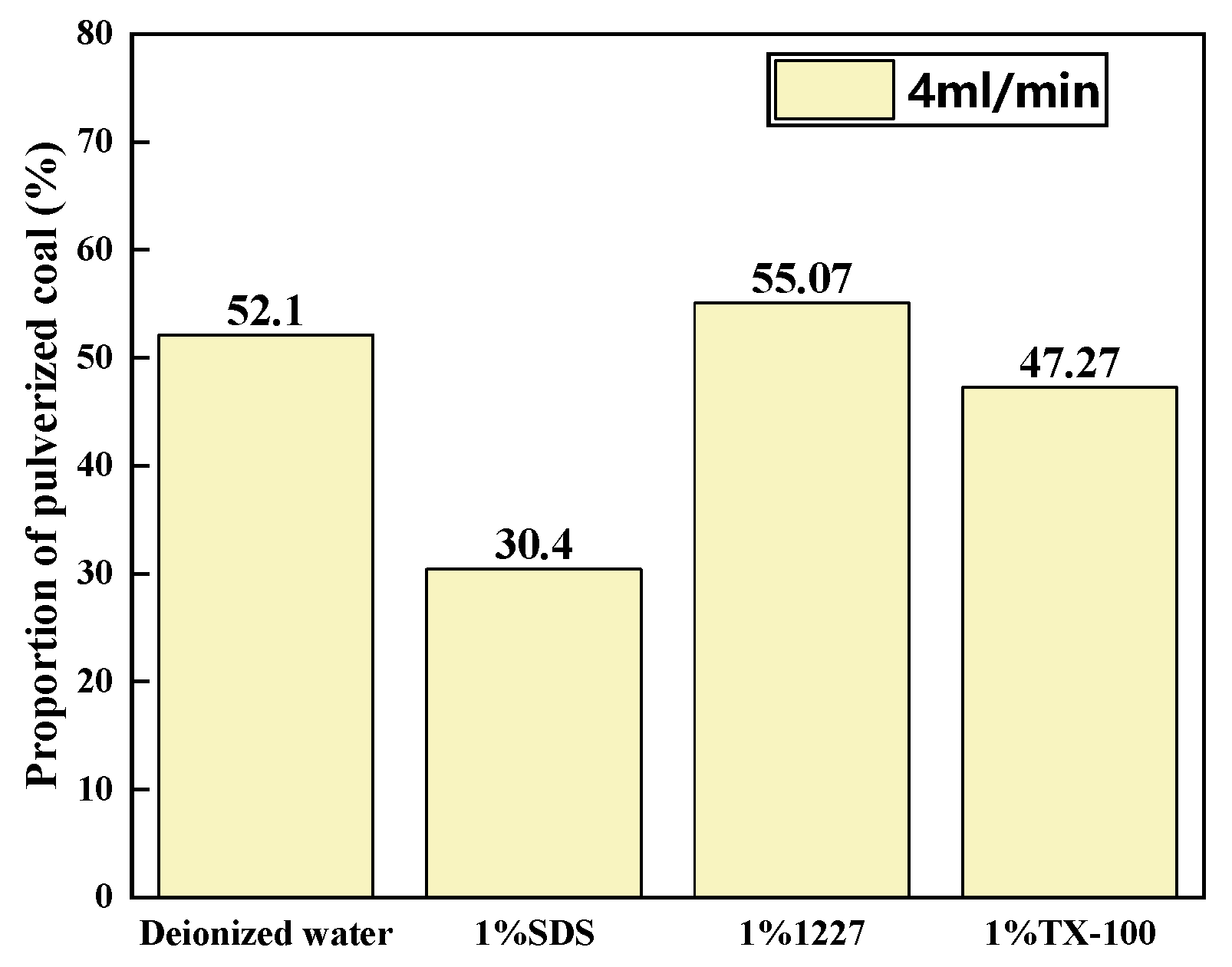
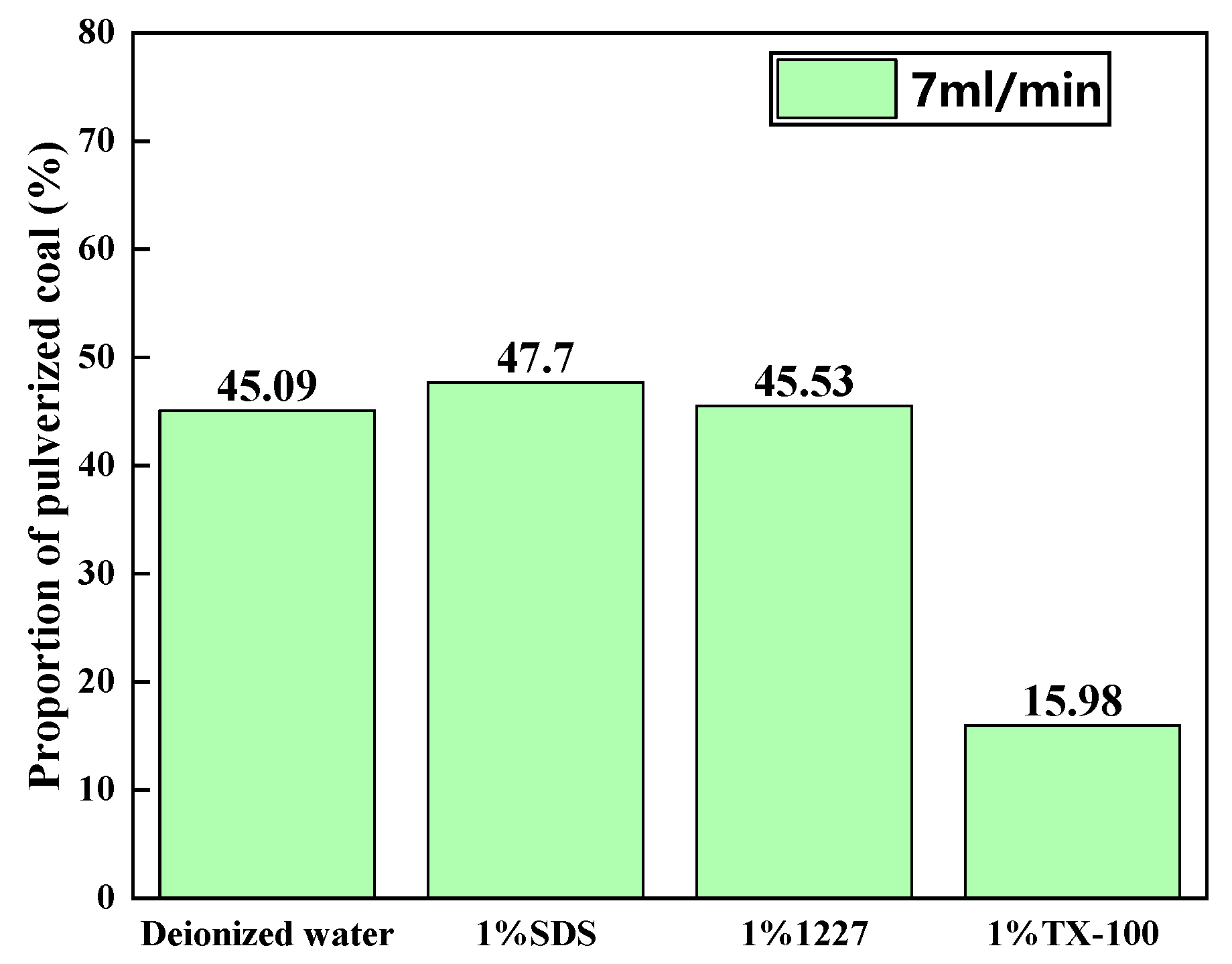
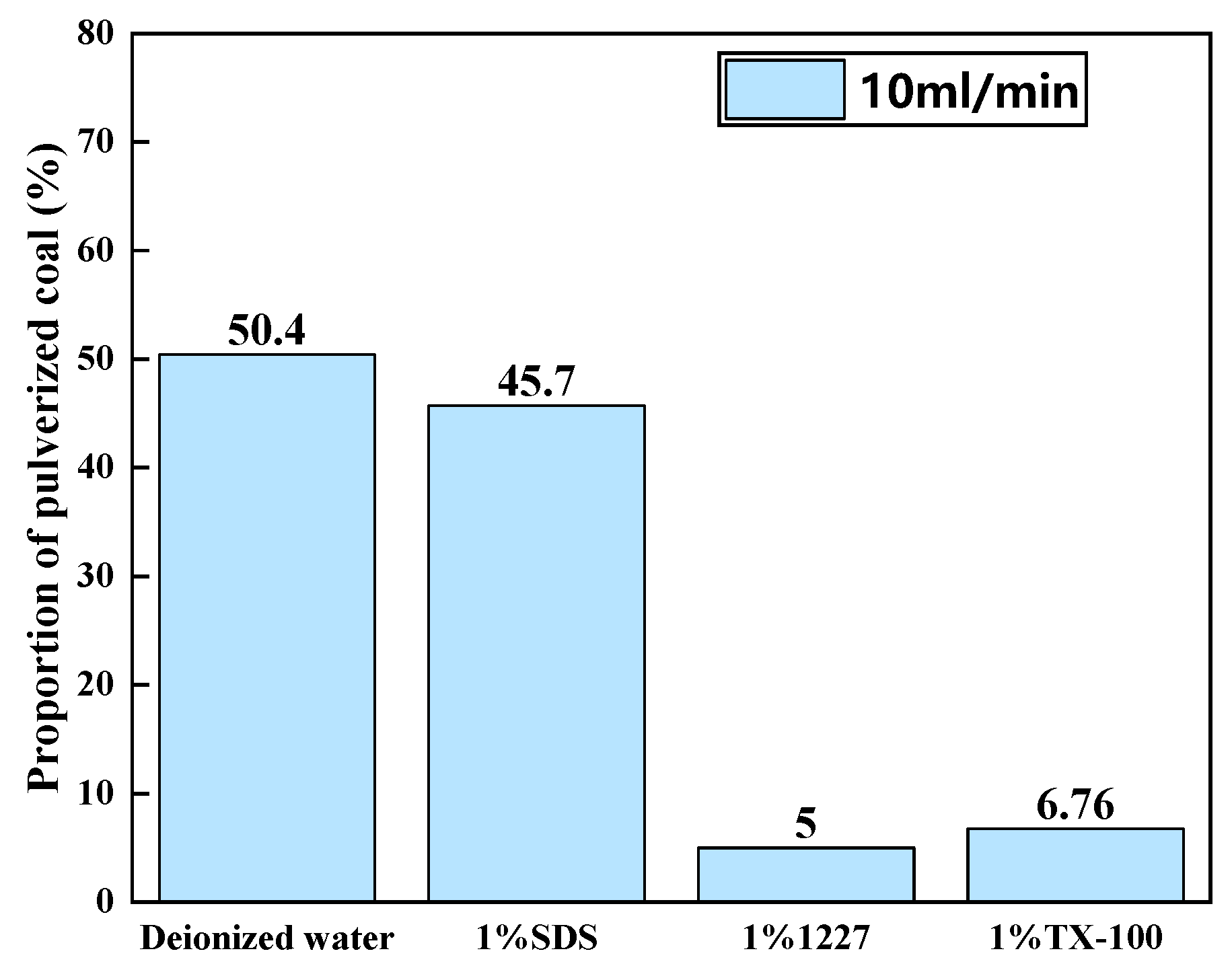
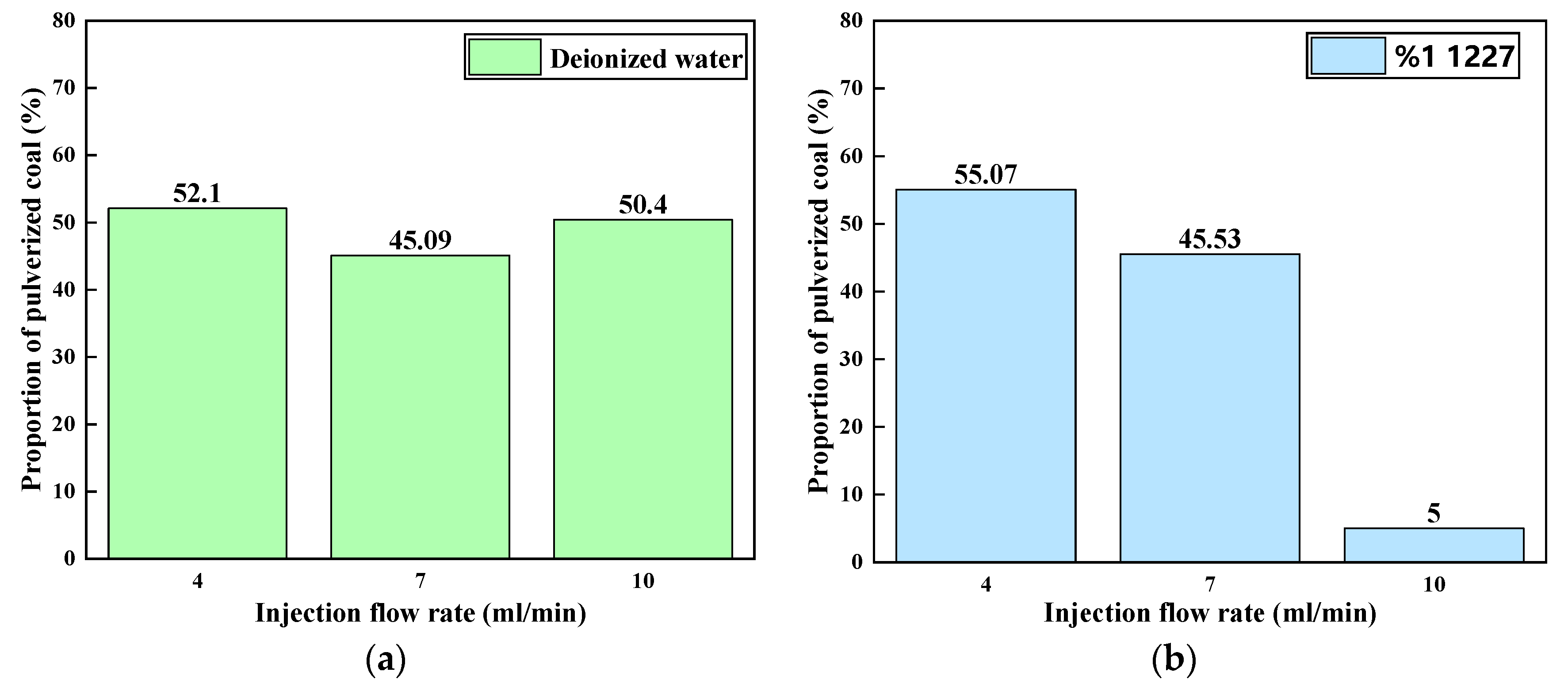
3.2.1. Ratios of Pulverized Coal at Different Flow Rates
3.2.2. Ratios of Pulverized Coal in Solutions of Different Surfactants
3.3. Formula Derivation and Experimental Verification of Inlet Pressure
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Q | The volume of liquid injected | 4 mL/min = 0.067 cm3/s 7 mL/min = 0.117 cm3/s 10 mL/min = 0.167 cm3/s |
| A | Fracture cross-sectional area | 0.7 cm2 |
| L | Crack length | 3 cm |
| The viscosity of the liquid | SDS (0.00098 pa·s) 1227 (0.00102 pa·s) TX-100 (0.00106 pa·s) | |
| r | Effective flow radius | 0.002634 R4 = r4 |
| R | The average radius of proppant particle size | 1.105 mm |
| τ | The tortuosity of effective flow pores | 5.08 |
| The ratio of circumference to diameter | 3.14 | |
| The pressure difference between the inlet and the outlet |
| NO. | Fluid | Error |
|---|---|---|
| 1 | water | 9.5 |
| 2 | water | 6.8 |
| 3 | water | 17.6 |
| 4 | 1% SDS | 8.1 |
| 5 | 1% SDS | 10 |
| 6 | 1% SDS | 12 |
| 7 | 1% 1227 | 8 |
| 8 | 1% 1227 | 7.6 |
| 9 | 1% 1227 | 10.8 |
| 10 | 1% TX-100 | 8.9 |
| 11 | 1% TX-100 | 9 |
| 12 | 1% TX-100 | 10 |
References
- Qin, Y.-J.; Su, W.-W.; Tian, F.-X.; Chen, Y.-P. Research status and development direction of microcosmic effect under coal seam water injection. J. China Univ. Min. Technol. 2020, 49, 428–444. [Google Scholar] [CrossRef]
- Cheng, W.-M.; Zhou, G.; Chen, L.-J.; Wang, G.; Nie, W.; Zheng, Q.-T. Research progress and prospect of dust control theory and technology in China’s coal mines in the past 20 years. Coal Sci. Technol. 2020, 48, 1–20. [Google Scholar] [CrossRef]
- Cheng, W.-M.; Nie, W.; Zhou, G.; Yu, Y.-B.; Ma, Y.-Y.; Xue, J. Research and practice on fluctuation water injection technology at low permeability coal seam. Saf. Sci. 2012, 50, 851–856. [Google Scholar] [CrossRef]
- Awan, F.U.R.; Arif, M.; Iglauer, S.; Keshavarz, A. Coal fines migration: A holistic review of influencing factors. Adv. Colloid Interface Sci. 2021, 301, 102595. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, H. Study on the Mechanism of Fiber Fracturing Fluid Controlling Pulverized Coal Transportation. Energies 2022, 15, 2165. [Google Scholar] [CrossRef]
- Dontsov, E.V.; Peirce, A.P. Proppant transport in hydraulic fracturing: Crack tip screen-out in KGD and P3D models. Int. J. Solids Struct. 2015, 63, 206–218. [Google Scholar] [CrossRef]
- Shi, J.; Feng, Z.; Zhou, D.; Meng, Q.; Hu, L.; Li, X. Experimental study on coal blockage removal based on pulverized coal blockage. J. Pet. Sci. Eng. 2022, 217, 110885. [Google Scholar] [CrossRef]
- Weng, A.-Q.; Yuan, S.-J.; Wang, X.-N.; Ma, R.-F. Study on application of surfactant compound in coal seam water injection for dust reduction. China Saf. Sci. J. 2020, 30, 90–95. [Google Scholar] [CrossRef]
- Xu, L.M.; Li, Y.J.; Du, L.L.; Yang, F.S.; Zhang, R.J.; Wei, H.; Wang, G.; Hao, Z. Study on the effect of SDBS and SDS on deep coal seam water injection. Sci. Total Environ. 2023, 856, 158930. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.P.; Jiang, W.; Si, L.L.; Xu, X.Y.; Wen, Z.W. Experimental research of the surfactant effect on seepage law in coal seam water injection. J. Nat. Gas Sci. Eng. 2022, 103, 104612. [Google Scholar] [CrossRef]
- Wang, G.; Wang, E.M.; Huang, Q.M.; Li, S.P. Effects of cationic and anionic surfactants on long flame coal seam water injection. Fuel 2022, 309, 122233. [Google Scholar] [CrossRef]
- Xu, L.M.; Lu, K.X.; Li, Y.J.; Li, Y.W.; Pan, Y.S.; Wei, H.; Li, N.; Yang, F.S.; Lv, X.F. Study on the infiltration mechanism of injecting chelating agent into deep low permeable coal seam. Fuel 2022, 310, 122164. [Google Scholar] [CrossRef]
- Jiang, H.B.; Xiao, Y.L.; Zhao, W. Study on the wetting property of a novel surfactant solution on coal dust. J. Saf. Sci. Technol. 2013, 9, 11–15. [Google Scholar] [CrossRef]
- Xu, L.M.; Pan, Y.S.; Lv, X.F. Experimental research on increasing effect of water injection in coal seam with chelating agent. Energy Sources Part A Recovery Util. Environ. Eff. 2021, 1–11. [Google Scholar] [CrossRef]
- Zhang, Q.T.; Xing, X.Y.; Zhou, G.; Hu, Y.Y.; Shang, S.C.; Fu, M.M.; Ma, H.; Li, H.F.; Men, Y.Q. Preparation and micro-wetting mechanism analysis of highly permeable-moistening additive for coal seam water injection based on plant extraction technology. Fuel 2022, 322, 124125. [Google Scholar] [CrossRef]
- Qi, Y.G.; Zang, F.N.; Liu, B.; Meng, S.-Z.; Zhu, H.-Y.; Du, J.-Y.; Mo, R.-H. Calculation on discharge flow of pulverized coal in gas production channel for coalbed methane well. J. China Coal Soc. 2013, 38, 1627–1633. [Google Scholar] [CrossRef]
- Wang, G.; Wang, Q.N.; Li, H.X.; Yan, S.; Huang, T.Y.; Liu, X.L. Experimental study on the optimized anion fracturing fluid for improving coal samples permeability. Energy Sources Part A Recovery Util. Environ. Eff. 2021, 1–15. [Google Scholar] [CrossRef]
- Wang, G.; Li, Y.Q.; Wang, E.M.; Huang, Q.M.; Wang, S.B.; Li, H.X. Experimental study on preparation of nanoparticle-surfactant nanofluids and their effects on coal surface wettability. Int. J. Min. Sci. Technol. 2022, 32, 387–397. [Google Scholar] [CrossRef]
- Wei, Y.C.; Cui, M.L.; Zang, J.; Meng, T.; Yao, Z. Aggregation and sedimentation experiments of coal fines with different particle sizes during CBM development. Coal Geol. Explor. 2020, 48, 40–47. [Google Scholar] [CrossRef]
- Huang, Q.M.; Liu, S.M.; Cheng, W.M.; Wang, G. Fracture permeability damage and recovery behaviors with fracturing fluid treatment of coal: An experimental study. Fuel 2020, 282, 118809. [Google Scholar] [CrossRef]
- Wang, Z.W.; Liu, S.M.; Qin, Y. Coal wettability in coalbed methane production: A critical review. Fuel 2021, 303, 121277. [Google Scholar] [CrossRef]
- Zou, Y.S.; Zhang, S.C.; Zhang, J.; Wang, X.; Lu, H.B. Damage mechanism of coal powder on fracture conductivity. J. China Coal Soc. 2012, 37, 1890–1894. [Google Scholar] [CrossRef]
- Liu, Y.; Zang, S.A.; CAO, L.H.; Meng, S.Z.; Zhang, T.L. Rules of coal powder migration and deposition in the proppant fracture. J. China Coal Soc. 2014, 39, 1333–1337. [Google Scholar] [CrossRef]
- Zou, Y.S.; Zhang, S.C.; Zhang, J. Experimental Method to Simulate Coal Fines Migration and Coal Fines Aggregation Prevention in the Hydraulic Fracture. Transp. Porous Media 2014, 101, 17–34. [Google Scholar] [CrossRef]
- Hu, S.Y.; Hao, Y.X.; Chen, Y.B.; Feng, G.R.; Li, G.F.; Zhang, X.T. Dynamic influence law of coal powder migration and deposition on propped fracture permeability. Coal Sci. Technol. 2021, 46, 1288–1296. [Google Scholar] [CrossRef]
- Zhao, Z.; Ni, X.M.; Liu, Z.D.; Wu, X. Experimental study on the attenuation characteristics of coal fissure induced by gas-water two-phase drive. Coal Sci. Technol. 2020, 45, 3853–3863. [Google Scholar] [CrossRef]
- Shi, J.T.; Wu, J.Y.; Fang, Y.X.; Lu, J.G.; Hou, C.H.; Li, X.F.; Zhang, S.A.; Xiong, X.Y. A new coal reservoir permeability model considering the influence of pulverized coal blockage and its application. Nat. Gas Ind. B 2021, 8, 67–78. [Google Scholar] [CrossRef]
- Zhao, T.T.; Xu, H.; Tang, D.Z.; Zong, P. The theoretical basis of model building for coal reservoir permeability: A review and improvement. J. Nat. Gas Sci. Eng. 2022, 106, 104744. [Google Scholar] [CrossRef]
- Li, H.; Huang, B.X. A new permeability model of fracture containing proppants. J. Nat. Gas Sci. Eng. 2022, 104, 104661. [Google Scholar] [CrossRef]
- Dahi-Taleghani, A.; Olson, J.E. Numerical modeling of multistranded-hydraulic-fracture propagation: Accounting for the interaction between induced and natural fractures. SPE J. 2011, 16, 575–581. [Google Scholar] [CrossRef]
- Nguyen, P.D.; Dusterhoft, R.G.; Clarkson, B. Control of formation fines to provide long-term conductivity in weak, unconsolidated reser voirs. In Proceedings of the Offshore Technology Conference, Houston, TX, USA, 2–5 May 2005. [Google Scholar]
- Lee, K.S.; Ivanova, N.; Starov, V.M.; Hilal, N.; Dutschk, V. Kinetics of wetting and spreading by aqueous surfactant solutions. Adv. Colloid Interface Sci. 2008, 144, 54–65. [Google Scholar] [CrossRef]
- Kovalchuk, N.M.; Simmons, M.J.H. Surfactant-mediated wetting and spreading: Recent advances and applications. Curr. Opin. Colloid Interface Sci. 2021, 51, 101375. [Google Scholar] [CrossRef]
- Li, S.; Zhao, B.; Lin, H.; Shuang, H.; Kong, X.; Yang, E. Review and prospects of surfactant-enhanced spray dust suppression: Mechanisms and effectiveness. Process Saf. Environ. Prot. 2021, 154, 410–424. [Google Scholar] [CrossRef]
- Xu, C.; Wang, D.; Wang, H.; Ma, L.; Zhu, X.; Zhu, Y.; Zhang, Y.; Liu, F. Experimental investigation of coal dust wetting ability of anionic surfactants with different structures. Process Saf. Environ. Prot. 2019, 121, 69–76. [Google Scholar] [CrossRef]
- Kilau, H.W. The Influence of Sulfate Ion on the Coal-Wetting Performance of Anionic Surfactants; US Department of the Interior, Bureau of Mines: Washington, DC, USA, 1990.
- Li, J.; Zhou, F.; Liu, H. The selection and application of a compound wetting agent to the coal seam water infusion for dust control. Int. J. Coal Prep. Util. 2016, 36, 192–206. [Google Scholar] [CrossRef]
- Torkzaban, S.; Bradford, S.A.; Vanderzalm, J.L.; Patterson, B.M.; Harris, B.; Prommer, H. Colloid release and clogging in porous media: Effects of solution ionic strength and flow velocity. J. Contam. Hydrol. 2015, 181, 161–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doroshchuk, V.A.; Kulichenko, S.A. Preconcentration of Cadmium with OP-10Nonionic Surfactant Phases at the Cloud Point. J. Anal. Chem. 2005, 60, 400–403. [Google Scholar] [CrossRef]
- Liu, Y.; Su, X.; Zhang, S. Influencing characteristics and control of coal powder to proppant fracture conductivity. J. China Coal Soc. 2017, 42, 687–693. [Google Scholar] [CrossRef]
- Kozeny, J. Ueber kapillare Leitung des Wassers im Boden. Sitzungsberichte Der Akad. Der Wiss. Wien 1927, 136, 271–306. [Google Scholar]
- Carman, P.C. Permeability of saturated sands, soils and clays. J. Agric. Sci. 1939, 29, 263–273. [Google Scholar] [CrossRef]






| English Synonym | Formula | CAS No. | Manufacturer | Purity | Molecular Weight |
|---|---|---|---|---|---|
| SDS | C12H25SO4Na | 151-21-3 | Shanghai Macklin Biochemical Co., Ltd., Shanghai, China | AR, 92.5–100.5% | 288.38 |
| 1227 | C21H38ClN | 139-07-1 | Shanghai Macklin Biochemical Co., Ltd., Shanghai, China | AR, 99% | 339.99 |
| TX-100 | C34H62O11 | 9002-93-1 | Beijing Solarbio Science & Technology Co., Ltd., Beijing, China | >98% | 647 |
| wt% | 0.1 | 0.25 | 0.5 | 0.75 | 1 |
|---|---|---|---|---|---|
| Surface tension of 1% SDS solution/mN·m−1 | 40.98 | 34.12 | 33.66 | 32.58 | 32.49 |
| Surface tension of 1% 1227 solution/mN·m−1 | 32.01 | 31.64 | 31.96 | 31.96 | 31.78 |
| Surface tension of 1% TX-100 solution/mN·m−1 | 39.35 | 36.85 | 36.6 | 36.65 | 36.6 |
| No. | Fluid | Coal Powder (g) | Particle Size of Proppant (Mesh) | Particle Size of Coal Powder (Mesh) | Flow Rate (mL/min) | Time (min) |
|---|---|---|---|---|---|---|
| 1 | water | 0.2 | 16–20 | >120 | 4 | 40 |
| 2 | water | 0.2 | 16–20 | >120 | 7 | 40 |
| 3 | water | 0.2 | 16–20 | >120 | 10 | 40 |
| 4 | 1% SDS | 0.2 | 16–20 | >120 | 4 | 40 |
| 5 | 1% SDS | 0.2 | 16–20 | >120 | 7 | 40 |
| 6 | 1% SDS | 0.2 | 16–20 | >120 | 10 | 40 |
| 7 | 1% 1227 | 0.2 | 16–20 | >120 | 4 | 40 |
| 8 | 1% 1227 | 0.2 | 16–20 | >120 | 7 | 40 |
| 9 | 1% 1227 | 0.2 | 16–20 | >120 | 10 | 40 |
| 10 | 1% TX-100 | 0.2 | 16–20 | >120 | 4 | 40 |
| 11 | 1% TX-100 | 0.2 | 16–20 | >120 | 7 | 40 |
| 12 | 1% TX-100 | 0.2 | 16–20 | >120 | 10 | 40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Q.; Yin, L.; Huang, Q.; Wang, E.; Hou, Z. Experimental Study on Migration and Intrusion Characteristics of Pulverized Coal in Propped Fractures. Processes 2023, 11, 2074. https://doi.org/10.3390/pr11072074
Zhu Q, Yin L, Huang Q, Wang E, Hou Z. Experimental Study on Migration and Intrusion Characteristics of Pulverized Coal in Propped Fractures. Processes. 2023; 11(7):2074. https://doi.org/10.3390/pr11072074
Chicago/Turabian StyleZhu, Qingao, Liming Yin, Qiming Huang, Enmao Wang, and Zhiguo Hou. 2023. "Experimental Study on Migration and Intrusion Characteristics of Pulverized Coal in Propped Fractures" Processes 11, no. 7: 2074. https://doi.org/10.3390/pr11072074




