Hybrid Materials for Tissue Repair and Replacement: Another Frontier in Biomaterial Exploitation Focusing on Cardiovascular and Urological Fields
Abstract
:1. Introduction: The Birth of Tissue Engineering and the Challenging Choice of Adequate Biomaterials
2. Moving toward a New Concept of Biocompatible Materials: Hybrid Materials
3. Urological Applications of Hybrid Materials: From Urological Conduits to the Regeneration of the Urinary Bladder
4. Hybrid Materials in the Cardiovascular Field: A Challenge for Material–Blood Interaction
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Skalak, R. Tissue Engineering: Proceedings of a Workshop Held at Granlibakken, Lake Tahoe, California, February 26–29, 1988; UCLA Symposia on Molecular and Cellular, Biology; Skalak, R., Fox, C.F., Eds.; Liss: New York, NY, USA, 1988; ISBN 978-0-8451-4706-1. [Google Scholar]
- Cohen, J. Biomaterials in Orthopedic Surgery. Am. J. Surg. 1967, 114, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Park, J.B. Biomaterials Science and Engineering; Springer: Boston, MA, USA, 1984; ISBN 978-1-4612-9710-9. [Google Scholar]
- Bergmann, C.P.; Stumpf, A. Dental Ceramics: Microstructure, Properties and Degradation; Topics in Mining, Metallurgy and Materials Engineering; Springer: Berlin/Heidelberg, Germany, 2013; ISBN 978-3-642-38223-9. [Google Scholar]
- Murphy, S.V.; Atala, A. Organ Engineering—Combining Stem Cells, Biomaterials, and Bioreactors to Produce Bioengineered Organs for Transplantation. BioEssays News Rev. Mol. Cell. Dev. Biol. 2013, 35, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Suh, H. Tissue Restoration, Tissue Engineering and Regenerative Medicine. Yonsei Med. J. 2000, 41, 681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Todros, S.; Todesco, M.; Bagno, A. Biomaterials and Their Biomedical Applications: From Replacement to Regeneration. Processes 2021, 9, 1949. [Google Scholar] [CrossRef]
- Place, E.S.; George, J.H.; Williams, C.K.; Stevens, M.M. Synthetic Polymer Scaffolds for Tissue Engineering. Chem. Soc. Rev. 2009, 38, 1139–1151. [Google Scholar] [CrossRef]
- Liu, S.; Qin, S.; He, M.; Zhou, D.; Qin, Q.; Wang, H. Current Applications of Poly(Lactic Acid) Composites in Tissue Engineering and Drug Delivery. Compos. Part B Eng. 2020, 199, 108238. [Google Scholar] [CrossRef]
- Reddy, M.S.B.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A Comparative Review of Natural and Synthetic Biopolymer Composite Scaffolds. Polymers 2021, 13, 1105. [Google Scholar] [CrossRef]
- O’Brien, F.J. Biomaterials & Scaffolds for Tissue Engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Nikolova, M.P.; Chavali, M.S. Recent Advances in Biomaterials for 3D Scaffolds: A Review. Bioact. Mater. 2019, 4, 271–292. [Google Scholar] [CrossRef]
- Gomez-Florit, M.; Pardo, A.; Domingues, R.M.A.; Graça, A.L.; Babo, P.S.; Reis, R.L.; Gomes, M.E. Natural-Based Hydrogels for Tissue Engineering Applications. Molecules 2020, 25, 5858. [Google Scholar] [CrossRef]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An Overview of Tissue and Whole Organ Decellularization Processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef] [Green Version]
- Keane, T.J.; Swinehart, I.T.; Badylak, S.F. Methods of Tissue Decellularization Used for Preparation of Biologic Scaffolds and in Vivo Relevance. Methods San Diego Calif. 2015, 84, 25–34. [Google Scholar] [CrossRef] [Green Version]
- Mendibil, U.; Ruiz-Hernandez, R.; Retegi-Carrion, S.; Garcia-Urquia, N.; Olalde-Graells, B.; Abarrategi, A. Tissue-Specific Decellularization Methods: Rationale and Strategies to Achieve Regenerative Compounds. Int. J. Mol. Sci. 2020, 21, 5447. [Google Scholar] [CrossRef]
- Rabbani, M.; Zakian, N.; Alimoradi, N. Contribution of Physical Methods in Decellularization of Animal Tissues. J. Med. Signals Sens. 2021, 11, 1–11. [Google Scholar] [CrossRef]
- Eberli, D.; Freitas Filho, L.; Atala, A.; Yoo, J.J. Composite Scaffolds for the Engineering of Hollow Organs and Tissues. Methods San Diego Calif. 2009, 47, 109–115. [Google Scholar] [CrossRef] [Green Version]
- Horst, M.; Madduri, S.; Milleret, V.; Sulser, T.; Gobet, R.; Eberli, D. A Bilayered Hybrid Microfibrous PLGA--Acellular Matrix Scaffold for Hollow Organ Tissue Engineering. Biomaterials 2013, 34, 1537–1545. [Google Scholar] [CrossRef] [Green Version]
- Adamowicz, J.; Pokrywczyńska, M.; Tworkiewicz, J.; Kowalczyk, T.; van Breda, S.V.; Tyloch, D.; Kloskowski, T.; Bodnar, M.; Skopinska-Wisniewska, J.; Marszałek, A.; et al. New Amniotic Membrane Based Biocomposite for Future Application in Reconstructive Urology. PLoS ONE 2016, 11, e0146012. [Google Scholar] [CrossRef] [Green Version]
- Adamowicz, J.; Pasternak, I.; Kloskowski, T.; Gniadek, M.; Van Breda, S.V.; Buhl, M.; Balcerczyk, D.; Gagat, M.; Grzanka, D.; Strupinski, W.; et al. Development of a Conductive Biocomposite Combining Graphene and Amniotic Membrane for Replacement of the Neuronal Network of Tissue-Engineered Urinary Bladder. Sci. Rep. 2020, 10, 5824. [Google Scholar] [CrossRef] [Green Version]
- Ananta, M.; Aulin, C.E.; Hilborn, J.; Aibibu, D.; Houis, S.; Brown, R.A.; Mudera, V. A Poly(Lactic Acid-Co-Caprolactone)–Collagen Hybrid for Tissue Engineering Applications. Tissue Eng. Part A 2009, 15, 1667–1675. [Google Scholar] [CrossRef]
- Franck, D.; Gil, E.S.; Adam, R.M.; Kaplan, D.L.; Chung, Y.G.; Estrada, C.R.; Mauney, J.R. Evaluation of Silk Biomaterials in Combination with Extracellular Matrix Coatings for Bladder Tissue Engineering with Primary and Pluripotent Cells. PLoS ONE 2013, 8, e56237. [Google Scholar] [CrossRef]
- Horst, M.; Milleret, V.; Nötzli, S.; Madduri, S.; Sulser, T.; Gobet, R.; Eberli, D. Increased Porosity of Electrospun Hybrid Scaffolds Improved Bladder Tissue Regeneration: The Role of Scaffold Porosity on Tissue Ingrowth. J. Biomed. Mater. Res. A 2014, 102, 2116–2124. [Google Scholar] [CrossRef] [PubMed]
- Ajalloueian, F.; Zeiai, S.; Fossum, M.; Hilborn, J.G. Constructs of Electrospun PLGA, Compressed Collagen and Minced Urothelium for Minimally Manipulated Autologous Bladder Tissue Expansion. Biomaterials 2014, 35, 5741–5748. [Google Scholar] [CrossRef] [PubMed]
- Todesco, M.; Zardin, C.; Iop, L.; Palmosi, T.; Capaldo, P.; Romanato, F.; Gerosa, G.; Bagno, A. Hybrid Membranes for the Production of Blood Contacting Surfaces: Physicochemical, Structural and Biomechanical Characterization. Biomater. Res. 2021, 25, 26. [Google Scholar] [CrossRef] [PubMed]
- Kickelbick, G. Hybrid Materials: Synthesis, Characterization, and Applications; Kickelbick, G., Ed.; Wiley-VCH: Weinheim, Germany, 2007; ISBN 978-3-527-31299-3. [Google Scholar]
- Kickelbick, G. Hybrid Materials—Past, Present and Future. Hybrid Mater. 2014, 1, 39–51. [Google Scholar] [CrossRef]
- Saleh, T.A. Hybrid Materials: Fundamentals and Classifications. In Polymer Hybrid Materials and Nanocomposites; Elsevier: Amsterdam, The Netherlands, 2021; pp. 147–176. ISBN 978-0-12-813294-4. [Google Scholar]
- Alemán, J.V.; Chadwick, A.V.; He, J.; Hess, M.; Horie, K.; Jones, R.G.; Kratochvíl, P.; Meisel, I.; Mita, I.; Moad, G.; et al. Definitions of Terms Relating to the Structure and Processing of Sols, Gels, Networks, and Inorganic-Organic Hybrid Materials (IUPAC Recommendations 2007). Pure Appl. Chem. 2007, 79, 1801–1829. [Google Scholar] [CrossRef]
- Chowdhury, S.; Pal, B.; Datta, P. Composite Biomaterials for Bone Grafting and Other Biomedical Applications. In Encyclopedia of Materials: Plastics and Polymers; Elsevier: Amsterdam, The Netherlands, 2022; pp. 697–716. ISBN 978-0-12-823291-0. [Google Scholar]
- Knight, M.; Curliss, D. Composite Materials. In Encyclopedia of Physical Science and Technology; Elsevier: Amsterdam, The Netherlands, 2003; pp. 455–468. ISBN 978-0-12-227410-7. [Google Scholar]
- Coenen, A.M.J.; Bernaerts, K.V.; Harings, J.A.W.; Jockenhoevel, S.; Ghazanfari, S. Elastic Materials for Tissue Engineering Applications: Natural, Synthetic, and Hybrid Polymers. Acta Biomater. 2018, 79, 60–82. [Google Scholar] [CrossRef]
- Zhang, Y.; Venugopal, J.R.; El-Turki, A.; Ramakrishna, S.; Su, B.; Lim, C.T. Electrospun Biomimetic Nanocomposite Nanofibers of Hydroxyapatite/Chitosan for Bone Tissue Engineering. Biomaterials 2008, 29, 4314–4322. [Google Scholar] [CrossRef]
- Lawrence, B.J.; Maase, E.L.; Lin, H.; Madihally, S.V. Multilayer Composite Scaffolds with Mechanical Properties Similar to Small Intestinal Submucosa. J. Biomed. Mater. Res. A 2009, 88A, 634–643. [Google Scholar] [CrossRef]
- Cardoso, G.B.; Machado-Silva, A.B.; Sabino, M.; Santos, A.R., Jr.; Zavaglia, C.A. Novel Hybrid Membrane of Chitosan/Poly (ε-Caprolactone) for Tissue Engineering. Biomatter 2014, 4, e29508. [Google Scholar] [CrossRef] [Green Version]
- Ghosal, K.; Thomas, S.; Kalarikkal, N.; Gnanamani, A. Collagen Coated Electrospun Polycaprolactone (PCL) with Titanium Dioxide (TiO2) from an Environmentally Benign Solvent: Preliminary Physico-Chemical Studies for Skin Substitute. J. Polym. Res. 2014, 21, 410. [Google Scholar] [CrossRef]
- Ajalloueian, F.; Zeiai, S.; Rojas, R.; Fossum, M.; Hilborn, J. One-Stage Tissue Engineering of Bladder Wall Patches for an Easy-to-Use Approach at the Surgical Table. Tissue Eng. Part C Methods 2013, 19, 688–696. [Google Scholar] [CrossRef]
- Heydarkhan-Hagvall, S.; Schenke-Layland, K.; Dhanasopon, A.P.; Rofail, F.; Smith, H.; Wu, B.M.; Shemin, R.; Beygui, R.E.; MacLellan, W.R. Three-Dimensional Electrospun ECM-Based Hybrid Scaffolds for Cardiovascular Tissue Engineering. Biomaterials 2008, 29, 2907–2914. [Google Scholar] [CrossRef] [Green Version]
- Kanatani, I.; Kanematsu, A.; Inatsugu, Y.; Imamura, M.; Negoro, H.; Ito, N.; Yamamoto, S.; Tabata, Y.; Ikada, Y.; Ogawa, O. Fabrication of an Optimal Urethral Graft Using Collagen-Sponge Tubes Reinforced with Copoly(L-Lactide/ε-Caprolactone) Fabric. Tissue Eng. 2007, 13, 2933–2940. [Google Scholar] [CrossRef]
- Engelhardt, E.-M.; Micol, L.A.; Houis, S.; Wurm, F.M.; Hilborn, J.; Hubbell, J.A.; Frey, P. A Collagen-Poly(Lactic Acid-Co-ɛ-Caprolactone) Hybrid Scaffold for Bladder Tissue Regeneration. Biomaterials 2011, 32, 3969–3976. [Google Scholar] [CrossRef]
- Geutjes, P.; Roelofs, L.; Hoogenkamp, H.; Walraven, M.; Kortmann, B.; de Gier, R.; Farag, F.; Tiemessen, D.; Sloff, M.; Oosterwijk, E.; et al. Tissue Engineered Tubular Construct for Urinary Diversion in a Preclinical Porcine Model. J. Urol. 2012, 188, 653–660. [Google Scholar] [CrossRef]
- Basu, J.; Jayo, M.J.; Ilagan, R.M.; Guthrie, K.I.; Sangha, N.; Genheimer, C.W.; Quinlan, S.F.; Payne, R.; Knight, T.; Rivera, E.; et al. Regeneration of Native-Like Neo-Urinary Tissue from Nonbladder Cell Sources. Tissue Eng. Part A 2012, 18, 1025–1034. [Google Scholar] [CrossRef]
- Horst, M.; Milleret, V.; Noetzli, S.; Gobet, R.; Sulser, T.; Eberli, D. Polyesterurethane and Acellular Matrix Based Hybrid Biomaterial for Bladder Engineering: Hybrid Biomaterial for Bladder Engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 658–667. [Google Scholar] [CrossRef]
- Azuraini, M.J.; Huong, K.-H.; Khalil, H.P.S.A.; Amirul, A.A. Fabrication and Characterization of P(3HB-Co-4HB)/Gelatine Biomimetic Nanofibrous Scaffold for Tissue Engineering Application. J. Polym. Res. 2019, 26, 257. [Google Scholar] [CrossRef]
- Casarin, M.; Todesco, M.; Sandrin, D.; Romanato, F.; Bagno, A.; Morlacco, A.; Dal Moro, F. A Novel Hybrid Membrane for Urinary Conduit Substitutes Based on Small Intestinal Submucosa Coupled with Two Synthetic Polymers. J. Funct. Biomater. 2022, 13, 222. [Google Scholar] [CrossRef]
- Alt, E.; Seliger, C. Antithrombotic Stent Coatings: Hirudin/Iloprost Combination. Semin. Interv. Cardiol. SIIC 1998, 3, 177–183. [Google Scholar]
- Herrmann, R.; Schmidmaier, G.; Märkl, B.; Resch, A.; Hähnel, I.; Stemberger, A.; Alt, E. Antithrombogenic Coating of Stents Using a Biodegradable Drug Delivery Technology. Thromb. Haemost. 1999, 82, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Heise, M.; Schmidmaier, G.; Husmann, I.; Heidenhain, C.; Schmidt, J.; Neuhaus, P.; Settmacher, U. PEG-Hirudin/Iloprost Coating of Small Diameter EPTFE Grafts Effectively Prevents Pseudointima and Intimal Hyperplasia Development. Eur. J. Vasc. Endovasc. Surg. 2006, 32, 418–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heidenhain, C.; Weichert, W.; Schmidmaier, G.; Wildemann, B.; Hein, M.; Neuhaus, P.; Heise, M. Polymer Coating of Porcine Decellularized and Cross-Linked Aortic Grafts. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 94B, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Reid, J.A.; Callanan, A. Hybrid Cardiovascular Sourced Extracellular Matrix Scaffolds as Possible Platforms for Vascular Tissue Engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 910–924. [Google Scholar] [CrossRef] [Green Version]
- Stamm, C.; Khosravi, A.; Grabow, N.; Schmohl, K.; Treckmann, N.; Drechsel, A.; Nan, M.; Schmitz, K.-P.; Haubold, A.; Steinhoff, G. Biomatrix/Polymer Composite Material for Heart Valve Tissue Engineering. Ann. Thorac. Surg. 2004, 78, 2084–2092, discussion 2092–2093. [Google Scholar] [CrossRef]
- Grabow, N.; Schmohl, K.; Khosravi, A.; Philipp, M.; Scharfschwerdt, M.; Graf, B.; Stamm, C.; Haubold, A.; Schmitz, K.-P.; Steinhoff, G. Mechanical and Structural Properties of a Novel Hybrid Heart Valve Scaffold for Tissue Engineering. Artif. Organs 2004, 28, 971–979. [Google Scholar] [CrossRef]
- Jahnavi, S.; Kumary, T.V.; Bhuvaneshwar, G.S.; Natarajan, T.S.; Verma, R.S. Engineering of a Polymer Layered Bio-Hybrid Heart Valve Scaffold. Mater. Sci. Eng. C 2015, 51, 263–273. [Google Scholar] [CrossRef]
- Jansen, P.; van Oeveren, W.; Capel, A.; Carpentier, A. In Vitro Haemocompatibility of a Novel Bioprosthetic Total Artificial Heart. Eur. J. Cardiothorac. Surg. 2012, 41, e166–e172. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Wei, K.-M.; Lin, F.; Kong, X.-D.; Yao, J.-M. Effect of Silicon on the Formation of Silk Fibroin/Calcium Phosphate Composite. J. Mater. Sci. Mater. Med. 2008, 19, 577–582. [Google Scholar] [CrossRef]
- Hong, Y.; Huber, A.; Takanari, K.; Amoroso, N.J.; Hashizume, R.; Badylak, S.F.; Wagner, W.R. Mechanical Properties and in Vivo Behavior of a Biodegradable Synthetic Polymer Microfiber–Extracellular Matrix Hydrogel Biohybrid Scaffold. Biomaterials 2011, 32, 3387–3394. [Google Scholar] [CrossRef] [Green Version]
- Casarin, M.; Morlacco, A.; Dal Moro, F. Bladder Substitution: The Role of Tissue Engineering and Biomaterials. Processes 2021, 9, 1643. [Google Scholar] [CrossRef]
- Casarin, M.; Morlacco, A.; Moro, F.D. Tissue Engineering and Regenerative Medicine in Pediatric Urology: Urethral and Urinary Bladder Reconstruction. Int. J. Mol. Sci. 2022, 26, 6360. [Google Scholar] [CrossRef]
- Kloskowski, T.; Kowalczyk, T.; Nowacki, M.; Drewa, T. Tissue Engineering and Ureter Regeneration: Is It Possible? Int. J. Artif. Organs 2013, 36, 392–405. [Google Scholar] [CrossRef]
- Serrano-Aroca, Á.; Vera-Donoso, C.D.; Moreno-Manzano, V. Bioengineering Approaches for Bladder Regeneration. Int. J. Mol. Sci. 2018, 19, 1796. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.-K.; Madihally, S.V.; Palmer, B.; Frimberger, D.; Fung, K.-M.; Kropp, B.P. Biomatrices for Bladder Reconstruction. Adv. Drug Deliv. Rev. 2015, 82–83, 47–63. [Google Scholar] [CrossRef]
- Sievert, K.D.; Tanagho, E.A. Organ-Specific Acellular Matrix for Reconstruction of the Urinary Tract. World J. Urol. 2000, 18, 19–25. [Google Scholar] [CrossRef]
- Lavelle, J.; Meyers, S.; Ramage, R.; Bastacky, S.; Doty, D.; Apodaca, G.; Zeidel, M.L. Bladder Permeability Barrier: Recovery from Selective Injury of Surface Epithelial Cells. Am. J. Physiol. Ren. Physiol. 2002, 283, F242–F253. [Google Scholar] [CrossRef] [Green Version]
- Baskin, L.S.; Hayward, S.W.; Young, P.; Cunha, G.R. Role of Mesenchymal-Epithelial Interactions in Normal Bladder Development. J. Urol. 1996, 156, 1820–1827. [Google Scholar] [CrossRef]
- Oberpenning, F.; Meng, J.; Yoo, J.J.; Atala, A. De Novo Reconstitution of a Functional Mammalian Urinary Bladder by Tissue Engineering. Nat. Biotechnol. 1999, 17, 149–155. [Google Scholar] [CrossRef]
- Lai, J.-Y.; Yoon, C.Y.; Yoo, J.J.; Wulf, T.; Atala, A. Phenotypic and Functional Characterization of in Vivo Tissue Engineered Smooth Muscle from Normal and Pathological Bladders. J. Urol. 2002, 168, 1853–1857, discussion 1858. [Google Scholar] [CrossRef]
- Nakanishi, Y.; Chen, G.; Komuro, H.; Ushida, T.; Kaneko, S.; Tateishi, T.; Kaneko, M. Tissue-Engineered Urinary Bladder Wall Using PLGA Mesh-Collagen Hybrid Scaffolds: A Comparison Study of Collagen Sponge and Gel as a Scaffold. J. Pediatr. Surg. 2003, 38, 1781–1784. [Google Scholar] [CrossRef] [PubMed]
- Atala, A.; Freeman, M.R.; Vacanti, J.P.; Shepard, J.; Retik, A.B. Implantation in Vivo and Retrieval of Artificial Structures Consisting of Rabbit and Human Urothelium and Human Bladder Muscle. J. Urol. 1993, 150, 608–612. [Google Scholar] [CrossRef] [PubMed]
- Atala, A.; Bauer, S.B.; Soker, S.; Yoo, J.J.; Retik, A.B. Tissue-Engineered Autologous Bladders for Patients Needing Cystoplasty. Lancet Lond. Engl. 2006, 367, 1241–1246. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Song, L.; Wang, J.; Fan, S.; Zhang, Y.; Xu, Y. Evaluation of Stretched Electrospun Silk Fibroin Matrices Seeded with Urothelial Cells for Urethra Reconstruction. J. Surg. Res. 2013, 184, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Algarrahi, K.; Franck, D.; Ghezzi, C.E.; Cristofaro, V.; Yang, X.; Sullivan, M.P.; Chung, Y.G.; Affas, S.; Jennings, R.; Kaplan, D.L.; et al. Acellular Bi-Layer Silk Fibroin Scaffolds Support Functional Tissue Regeneration in a Rat Model of Onlay Esophagoplasty. Biomaterials 2015, 53, 149–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, B.; Zhang, Y.; Zhou, L.; Sun, Z.; Zheng, J.; Chen, Y.; Dai, Y. Development of a Porcine Bladder Acellular Matrix with Well-Preserved Extracellular Bioactive Factors for Tissue Engineering. Tissue Eng. Part C Methods 2010, 16, 1201–1211. [Google Scholar] [CrossRef]
- Micol, L.A.; Arenas da Silva, L.F.; Geutjes, P.J.; Oosterwijk, E.; Hubbell, J.A.; Feitz, W.F.J.; Frey, P. In-Vivo Performance of High-Density Collagen Gel Tubes for Urethral Regeneration in a Rabbit Model. Biomaterials 2012, 33, 7447–7455. [Google Scholar] [CrossRef]
- Sayeg, K.; Freitas-Filho, L.G.; Waitzberg, Â.F.L.; Arias, V.E.A.; Laks, M.; Egydio, F.M.; Oliveira, A.S. Integration of Collagen Matrices into the Urethra When Implanted as Onlay Graft. Int. Braz. J. Urol. Off. J. Braz. Soc. Urol. 2013, 39, 414–423. [Google Scholar] [CrossRef] [Green Version]
- Pinnagoda, K.; Larsson, H.M.; Vythilingam, G.; Vardar, E.; Engelhardt, E.-M.; Thambidorai, R.C.; Hubbell, J.A.; Frey, P. Engineered Acellular Collagen Scaffold for Endogenous Cell Guidance, a Novel Approach in Urethral Regeneration. Acta Biomater. 2016, 43, 208–217. [Google Scholar] [CrossRef]
- Aufderklamm, S.; Vaegler, M.; Kelp, A.; Maurer, S.; Gustafsson, L.; Mundhenk, J.; Busch, S.; Daum, L.; Stenzl, A.; Amend, B.; et al. Collagen Cell Carriers Seeded with Human Urothelial Cells for Urethral Reconstructive Surgery: First Results in a Xenograft Minipig Model. World J. Urol. 2017, 35, 1125–1132. [Google Scholar] [CrossRef]
- Kropp, B.P.; Eppley, B.L.; Prevel, C.D.; Rippy, M.K.; Harruff, R.C.; Badylak, S.F.; Adams, M.C.; Rink, R.C.; Keating, M.A. Experimental Assessment of Small Intestinal Submucosa as a Bladder Wall Substitute. Urology 1995, 46, 396–400. [Google Scholar] [CrossRef]
- Kropp, B.P.; Rippy, M.K.; Badylak, S.F.; Adams, M.C.; Keating, M.A.; Rink, R.C.; Thor, K.B. Regenerative Urinary Bladder Augmentation Using Small Intestinal Submucosa: Urodynamic and Histopathologic Assessment in Long-Term Canine Bladder Augmentations. J. Urol. 1996, 155, 2098–2104. [Google Scholar] [CrossRef]
- Campodonico, F.; Benelli, R.; Michelazzi, A.; Ognio, E.; Toncini, C.; Maffezzini, M. Bladder Cell Culture on Small Intestinal Submucosa as Bioscaffold: Experimental Study on Engineered Urothelial Grafts. Eur. Urol. 2004, 46, 531–537. [Google Scholar] [CrossRef]
- Drewa, T. The Artificial Conduit for Urinary Diversion in Rats: A Preliminary Study. Transplant. Proc. 2007, 39, 1647–1651. [Google Scholar] [CrossRef]
- Ayyildiz, A.; Akgül, K.T.; Huri, E.; Nuhoğlu, B.; Kiliçoğlu, B.; Ustün, H.; Gürdal, M.; Germiyanoğlu, C. Use of Porcine Small Intestinal Submucosa in Bladder Augmentation in Rabbit: Long-Term Histological Outcome. ANZ J. Surg. 2008, 78, 82–86. [Google Scholar] [CrossRef]
- Wu, S.; Liu, Y.; Bharadwaj, S.; Atala, A.; Zhang, Y. Human Urine-Derived Stem Cells Seeded in a Modified 3D Porous Small Intestinal Submucosa Scaffold for Urethral Tissue Engineering. Biomaterials 2011, 32, 1317–1326. [Google Scholar] [CrossRef]
- Wang, Y.; Liao, L. Histologic and Functional Outcomes of Small Intestine Submucosa-Regenerated Bladder Tissue. BMC Urol. 2014, 14, 69. [Google Scholar] [CrossRef] [Green Version]
- Casarin, M.; Fortunato, T.M.; Imran, S.; Todesco, M.; Sandrin, D.; Borile, G.; Toniolo, I.; Marchesan, M.; Gerosa, G.; Bagno, A.; et al. Porcine Small Intestinal Submucosa (SIS) as a Suitable Scaffold for the Creation of a Tissue-Engineered Urinary Conduit: Decellularization, Biomechanical and Biocompatibility Characterization Using New Approaches. Int. J. Mol. Sci. 2022, 23, 2826. [Google Scholar] [CrossRef]
- Liu, Y.; Bharadwaj, S.; Lee, S.J.; Atala, A.; Zhang, Y. Optimization of a Natural Collagen Scaffold to Aid Cell-Matrix Penetration for Urologic Tissue Engineering. Biomaterials 2009, 30, 3865–3873. [Google Scholar] [CrossRef]
- Liao, W.-B.; Song, C.; Li, Y.-W.; Yang, S.-X.; Meng, L.-C.; Li, X.-H. Tissue-Engineered Conduit Using Bladder Acellular Matrix and Bladder Epithelial Cells for Urinary Diversion in Rabbits. Chin. Med. J. Engl. 2013, 126, 335–339. [Google Scholar]
- Chen, C.; Zheng, S.; Zhang, X.; Dai, P.; Gao, Y.; Nan, L.; Zhang, Y. Transplantation of Amniotic Scaffold-Seeded Mesenchymal Stem Cells and/or Endothelial Progenitor Cells From Bone Marrow to Efficiently Repair 3-Cm Circumferential Urethral Defect in Model Dogs. Tissue Eng. Part A 2018, 24, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, S.; Chapple, C.R.; Bullock, A.J.; Layton, C.; MacNeil, S. Tissue-Engineered Buccal Mucosa for Substitution Urethroplasty. BJU Int. 2004, 93, 807–811. [Google Scholar] [CrossRef] [PubMed]
- Kimuli, M.; Eardley, I.; Southgate, J. In Vitro Assessment of Decellularized Porcine Dermis as a Matrix for Urinary Tract Reconstruction. BJU Int. 2004, 94, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Ziats, N.P.; Miller, K.M.; Anderson, J.M. In Vitro and in Vivo Interactions of Cells with Biomaterials. Biomaterials 1988, 9, 5–13. [Google Scholar] [CrossRef]
- Chun, S.Y.; Lim, G.J.; Kwon, T.G.; Kwak, E.K.; Kim, B.W.; Atala, A.; Yoo, J.J. Identification and Characterization of Bioactive Factors in Bladder Submucosa Matrix. Biomaterials 2007, 28, 4251–4256. [Google Scholar] [CrossRef]
- Eberli, D.; Susaeta, R.; Yoo, J.J.; Atala, A. Tunica Repair with Acellular Bladder Matrix Maintains Corporal Tissue Function. Int. J. Impot. Res. 2007, 19, 602–609. [Google Scholar] [CrossRef]
- Atala, A. Tissue Engineering of Human Bladder. Br. Med. Bull. 2011, 97, 81–104. [Google Scholar] [CrossRef]
- Dong, Y.; Yong, T.; Liao, S.; Chan, C.K.; Stevens, M.M.; Ramakrishna, S. Distinctive Degradation Behaviors of Electrospun Polyglycolide, Poly(DL-Lactide-Co-Glycolide), and Poly(L-Lactide-Co-Epsilon-Caprolactone) Nanofibers Cultured with/without Porcine Smooth Muscle Cells. Tissue Eng. Part A 2010, 16, 283–298. [Google Scholar] [CrossRef]
- Dvir, T.; Timko, B.P.; Kohane, D.S.; Langer, R. Nanotechnological Strategies for Engineering Complex Tissues. Nat. Nanotechnol. 2011, 6, 13–22. [Google Scholar] [CrossRef]
- Coyan, G.N.; da Mota Silveira-Filho, L.; Matsumura, Y.; Luketich, S.K.; Katz, W.; Badhwar, V.; Wagner, W.R.; D’Amore, A. Acute In Vivo Functional Assessment of a Biodegradable Stentless Elastomeric Tricuspid Valve. J. Cardiovasc. Transl. Res. 2020, 13, 796–805. [Google Scholar] [CrossRef]
- Grunkemeier, G.L.; Furnary, A.P.; Wu, Y.; Wang, L.; Starr, A. Durability of Pericardial versus Porcine Bioprosthetic Heart Valves. J. Thorac. Cardiovasc. Surg. 2012, 144, 1381–1386. [Google Scholar] [CrossRef] [Green Version]
- Makkar, R.R.; Fontana, G.; Jilaihawi, H.; Chakravarty, T.; Kofoed, K.F.; De Backer, O.; Asch, F.M.; Ruiz, C.E.; Olsen, N.T.; Trento, A.; et al. Possible Subclinical Leaflet Thrombosis in Bioprosthetic Aortic Valves. N. Engl. J. Med. 2015, 373, 2015–2024. [Google Scholar] [CrossRef]
- Kheradvar, A.; Zareian, R.; The Regents of the University of California. Methods for Development of Hybrid Tissue Engineered Valve with Polyurethane Core. United States Patent US 2019/0151509 A1, 23 May 2019. [Google Scholar]
- Stephens, E.H.; de Jonge, N.; McNeill, M.P.; Durst, C.A.; Grande-Allen, K.J. Age-Related Changes in Material Behavior of Porcine Mitral and Aortic Valves and Correlation to Matrix Composition. Tissue Eng. Part A 2010, 16, 867–878. [Google Scholar] [CrossRef] [Green Version]
- Ong, S.H.; Mueller, R.; Iversen, S. Early Calcific Degeneration of a CoreValve Transcatheter Aortic Bioprosthesis. Eur. Heart J. 2012, 33, 586. [Google Scholar] [CrossRef]
- Conte, M.S. Critical Appraisal of Surgical Revascularization for Critical Limb Ischemia. J. Vasc. Surg. 2013, 57, 8S–13S. [Google Scholar] [CrossRef] [Green Version]
- Klinkert, P.; Post, P.N.; Breslau, P.J.; van Bockel, J.H. Saphenous Vein Versus PTFE for Above-Knee Femoropopliteal Bypass. A Review of the Literature. Eur. J. Vasc. Endovasc. Surg. 2004, 27, 357–362. [Google Scholar] [CrossRef] [Green Version]
- Pashneh-Tala, S.; MacNeil, S.; Claeyssens, F. The Tissue-Engineered Vascular Graft—Past, Present, and Future. Tissue Eng. Part B Rev. 2016, 22, 68–100. [Google Scholar] [CrossRef] [Green Version]
- Davies, M.G.; Hagen, P.-O. Pathophysiology of Vein Graft Failure: A Review. Eur. J. Vasc. Endovasc. Surg. 1995, 9, 7–18. [Google Scholar] [CrossRef] [Green Version]
- FitzGibbon, G.M.; Leach, A.J.; Kafka, H.P.; Keon, W.J. Coronary Bypass Graft Fate: Long-Term Angiographic Study. J. Am. Coll. Cardiol. 1991, 17, 1075–1080. [Google Scholar] [CrossRef] [Green Version]
- Vanderwal, A.; Becker, A.; Elbers, J.; Das, P. An Immunocytochemical Analysis of Rapidly Progressive Atherosclerosis in Human Vein Grafts. Eur. J. Cardiothorac. Surg. 1992, 6, 469–474. [Google Scholar] [CrossRef]
- Steinhoff, G.; Stock, U.; Karim, N.; Mertsching, H.; Timke, A.; Meliss, R.R.; Pethig, K.; Haverich, A.; Bader, A. Tissue Engineering of Pulmonary Heart Valves on Allogenic Acellular Matrix Conduits: In Vivo Restoration of Valve Tissue. Circulation 2000, 102, Iii-50–Iii-55. [Google Scholar] [CrossRef]
- Wilson, G.J.; Courtman, D.W.; Klement, P.; Michael Lee, J.; Yeger, H. Acellular Matrix: A Biomaterials Approach for Coronary Artery Bypass and Heart Valve Replacement. Ann. Thorac. Surg. 1995, 60, S353–S358. [Google Scholar] [CrossRef] [PubMed]
- Bader, A.; Schilling, T.; Teebken, O.E.; Brandes, G.; Herden, T.; Steinhoff, G.; Haverich, A. Tissue Engineering of Heart Valves—Human Endothelial Cell Seeding of Detergent Acellularized Porcine Valves1. Eur. J. Cardiothorac. Surg. 1998, 14, 279–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirsadraee, S.; Wilcox, H.E.; Korossis, S.A.; Kearney, J.N.; Watterson, K.G.; Fisher, J.; Ingham, E. Development and Characterization of an Acellular Human Pericardial Matrix for Tissue Engineering. Tissue Eng. 2006, 12, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Bechtel, J.F.M.; Müller-Steinhardt, M.; Schmidtke, C.; Brunswik, A.; Stierle, U.; Sievers, H.-H. Evaluation of the Decellularized Pulmonary Valve Homograft (SynerGraft). J. Heart Valve Dis. 2003, 12, 734–739, discussion 739–740. [Google Scholar] [PubMed]
- Hoerstrup, S.P.; Sodian, R.; Daebritz, S.; Wang, J.; Bacha, E.A.; Martin, D.P.; Moran, A.M.; Guleserian, K.J.; Sperling, J.S.; Kaushal, S.; et al. Functional Living Trileaflet Heart Valves Grown In Vitro. Circulation 2000, 102, III-44–III-49. [Google Scholar] [CrossRef]
- Shinoka, T.; Breuer, C.K.; Tanel, R.E.; Zund, G.; Miura, T.; Ma, P.X.; Langer, R.; Vacanti, J.P.; Mayer, J.E. Tissue Engineering Heart Valves: Valve Leaflet Replacement Study in a Lamb Model. Ann. Thorac. Surg. 1995, 60, S513–S516. [Google Scholar] [CrossRef]
- Stock, U.A.; Nagashima, M.; Khalil, P.N.; Nollert, G.D.; Herdena, T.; Sperling, J.S.; Moran, A.; Lien, J.; Martin, D.P.; Schoen, F.J.; et al. Tissue-Engineered Valved Conduits in the Pulmonary Circulation. J. Thorac. Cardiovasc. Surg. 2000, 119, 732–740. [Google Scholar] [CrossRef] [Green Version]
- Sodian, R.; Loebe, M.; Hein, A.; Martin, D.P.; Hoerstrup, S.P.; Potapov, E.V.; Hausmann, H.; Lueth, T.; Hetzer, R. Application of Stereolithography for Scaffold Fabrication for Tissue Engineered Heart Valves. ASAIO J. 2002, 48, 12–16. [Google Scholar] [CrossRef]
- Mudigonda, J.; Xu, D.; Amedi, A.; Lane, B.A.; Corporan, D.; Wang, V.; Padala, M. A Biohybrid Material With Extracellular Matrix Core and Polymeric Coating as a Cell Honing Cardiovascular Tissue Substitute. Front. Cardiovasc. Med. 2022, 9, 807255. [Google Scholar] [CrossRef]
- Zheng, Z.; Eglin, D.; Alini, M.; Richards, G.R.; Qin, L.; Lai, Y. Visible Light-Induced 3D Bioprinting Technologies and Corresponding Bioink Materials for Tissue Engineering: A Review. Engineering 2021, 7, 966–978. [Google Scholar] [CrossRef]
- Goldman, M.D.; Simpson, D.; Hawker, R.J.; Norcott, H.C.; Mccollum, C.N. Aspirin and Dipyridamole Reduce Platelet Deposition on Prosthetic Femoro-Popliteal Grafts in Man. Ann. Surg. 1983, 198, 713–716. [Google Scholar] [CrossRef]
- Pumphrey, C.W.; Chesebro, J.H.; Dewanjee, M.K.; Wahner, H.W.; Hollier, L.H.; Pairolero, P.C.; Fuster, V. In Vivo Quantitation of Platelet Deposition on Human Peripheral Arterial Bypass Grafts Using Indium-111-Labeled Platelets. Am. J. Cardiol. 1983, 51, 796–801. [Google Scholar] [CrossRef]
- Sayers, R.D.; Raptis, S.; Berce, M.; Miller, J.H. Long-Term Results of Femorotibial Bypass with Vein or Polytetrafluoroethylene. Br. J. Surg. 2003, 85, 934–938. [Google Scholar] [CrossRef]
- Salacinski, H.J.; Tiwari, A.; Hamilton, G.; Seifalian, A.M. Cellular Engineering of Vascular Bypass Grafts: Role of Chemical Coatings for Enhancing Endothelial Cell Attachment. Med. Biol. Eng. Comput. 2001, 39, 609–618. [Google Scholar] [CrossRef]
- Kolar, M.; Mozetič, M.; Stana-Kleinschek, K.; Fröhlich, M.; Turk, B.; Vesel, A. Covalent Binding of Heparin to Functionalized PET Materials for Improved Haemocompatibility. Materials 2015, 8, 1526–1544. [Google Scholar] [CrossRef] [Green Version]
- Calaitges, J.G.; Liem, T.K.; Spadone, D.; Nichols, W.K.; Silver, D. The Role of Heparin-Associated Antiplatelet Antibodies in the Outcome of Arterial Reconstruction. J. Vasc. Surg. 1999, 29, 779–785; discussion 785–786. [Google Scholar] [CrossRef] [Green Version]
- Curi, M.A.; Skelly, C.L.; Baldwin, Z.K.; Woo, D.H.; Baron, J.M.; Desai, T.R.; Katz, D.; McKinsey, J.F.; Bassiouny, H.S.; Gewertz, B.L.; et al. Long-Term Outcome of Infrainguinal Bypass Grafting in Patients with Serologically Proven Hypercoagulability. J. Vasc. Surg. 2003, 37, 301–306. [Google Scholar] [CrossRef] [Green Version]
- Liang, H.-C.; Chang, Y.; Hsu, C.-K.; Lee, M.-H.; Sung, H.-W. Effects of Crosslinking Degree of an Acellular Biological Tissue on Its Tissue Regeneration Pattern. Biomaterials 2004, 25, 3541–3552. [Google Scholar] [CrossRef]
- Schmidmaier, G.; Lucke, M.; Schwabe, P.; Raschke, M.; Haas, N.P.; Wildemann, B. Collective Review: Bioactive Implants Coated with Poly(D,L-Lactide) and Growth Factors IGF-I, TGF-Β1, or BMP-2 for Stimulation of Fracture Healing. J. Long. Term Eff. Med. Implants 2006, 16, 61–69. [Google Scholar] [CrossRef]
- Tamada, J.A.; Langer, R. Erosion Kinetics of Hydrolytically Degradable Polymers. Proc. Natl. Acad. Sci. USA 1993, 90, 552–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tapias, L.F.; Ott, H.C. Decellularized Scaffolds as a Platform for Bioengineered Organs. Curr. Opin. Organ Transplant. 2014, 19, 145–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, T.-Y.; Lin, B.; Kim, J.; Sullivan, M.; Tobita, K.; Salama, G.; Yang, L. Repopulation of Decellularized Mouse Heart with Human Induced Pluripotent Stem Cell-Derived Cardiovascular Progenitor Cells. Nat. Commun. 2013, 4, 2307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Meijden, P.E.J.; Munnix, I.C.A.; Auger, J.M.; Govers-Riemslag, J.W.P.; Cosemans, J.M.E.M.; Kuijpers, M.J.E.; Spronk, H.M.; Watson, S.P.; Renné, T.; Heemskerk, J.W.M. Dual Role of Collagen in Factor XII–Dependent Thrombus Formation. Blood 2009, 114, 881–890. [Google Scholar] [CrossRef] [Green Version]
- Nelson, T.; Kaufman, E.; Kline, J.; Sokoloff, L. The Extraneural Distribution of?-Hydroxybutyrate. J. Neurochem. 1981, 37, 1345–1348. [Google Scholar] [CrossRef]
- Wu, S.; Liu, Y.-L.; Cui, B.; Qu, X.-H.; Chen, G.-Q. Study on Decellularized Porcine Aortic Valve/Poly (3-Hydroxybutyrate-Co-3-Hydroxyhexanoate) Hybrid Heart Valve in Sheep Model. Artif. Organs 2007, 31, 689–697. [Google Scholar] [CrossRef]
- Todesco, M.; Pontara, E.; Cheng, C.; Gerosa, G.; Pengo, V.; Bagno, A. Preliminary Hemocompatibility Assessment of an Innovative Material for Blood Contacting Surfaces. J. Mater. Sci. Mater. Med. 2021, 32, 86. [Google Scholar] [CrossRef]
- Reed, A.M.; Potter, J.; Szycher, M. A Solution Grade Biostable Polyurethane Elastomer: ChronoFlex® AR. J. Biomater. Appl. 1994, 8, 210–236. [Google Scholar] [CrossRef]

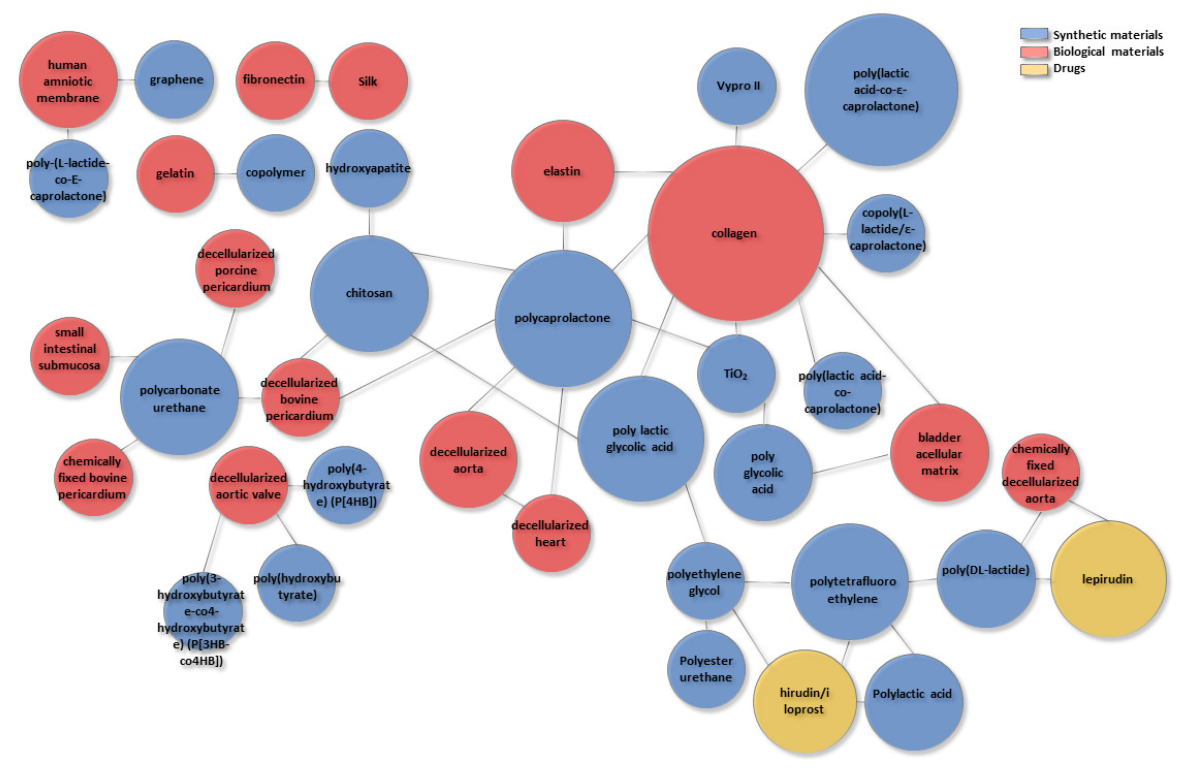
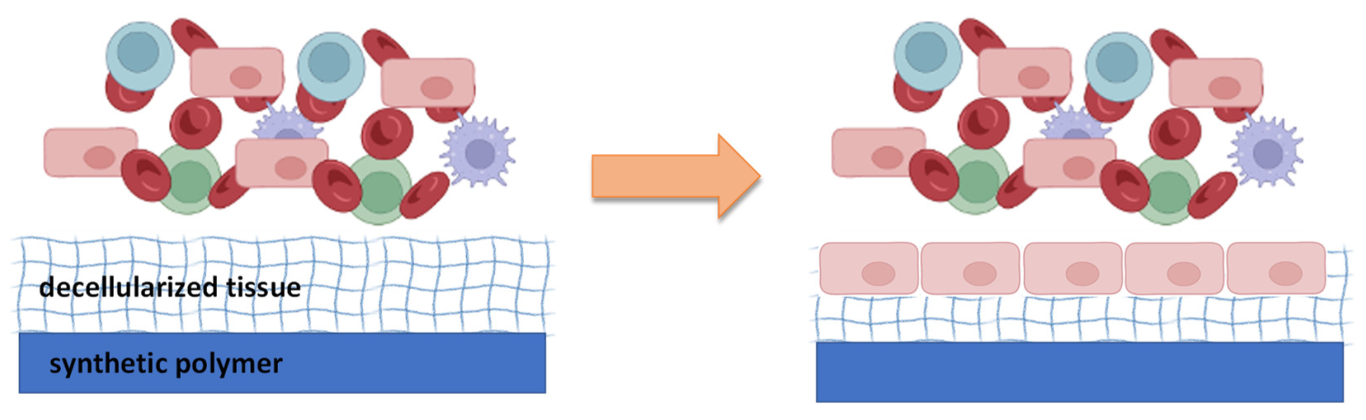
| Biological Materials | Synthetic Materials | Applications | Type of Study | References |
|---|---|---|---|---|
| Hydroxyapatite + Chitosan | - | Orthopedic | in vitro | [34] |
| Chitosan | Poly lactic glycolic acid | - | in vitro | [35] |
| Chitosan | Polycaprolactone | - | in vitro | [36] |
| Collagen | Polycaprolactone + TiO2 | Dermatological | in vitro | [37] |
| Collagen | Polycaprolactone | Urological | in vitro | [38] |
| Collagen and elastin | Polycaprolactone | Cardiovascular | in vitro | [39] |
| Collagen | Copoly(L-lactide/ε-caprolactone) | Urological | in vitro and in vivo | [40] |
| Collagen | Poly(lactic acid-co-caprolactone) | Dermatological | in vitro | [22] |
| Collagen | Poly(lactic acid-co-ε-caprolactone) | Urological | in vitro and in vivo | [41] |
| Collagen | Vypro II | Urological | in vitro and in vivo | [42] |
| Silk + collagen or fibronectin | - | Urological | in vitro | [23] |
| Collagen | Poly lactic glycolic acid | Urological | in vitro | [25] |
| - | poly glycolic acid + poly lactic glycolic acid | Urological | in vivo | [43] |
| Bladder acellular matrix + collagen | Poly glycolic acid | Urological | in vivo | [18] |
| Bladder acellular matrix | Poly lactic glycolic acid | Urological | in vitro and in vivo | [19,24] |
| - | Polyester urethane or poly lactic glycolic acid + Polyethylene glycol | Urological | in vivo | [44] |
| Human amniotic membrane | Poly-(L-lactide-co-E-caprolactone) | Urological | in vivo | [20] |
| Human amniotic membrane | Graphene | Urological | in vitro | [21] |
| Gelatin | Copolymer | Urological | in vitro | [45] |
| Small intestinal submucosa | Polycarbonate urethane | Urological | in vitro | [46] |
| - | Polytetrafluoroethylene + Polylactic acid | Cardiovascular | in vitro | [47] |
| - | Polytetrafluoroethylene + Poly(DL-lactide) | Cardiovascular | in vitro and in vivo | [48] |
| - | Polytetrafluoroethylene + polylactic acid with Polyethylene glycol-hirudin/iloprost | Cardiovascular | in vitro and in vivo | [49] |
| Chemically fixed decellularized porcine aorta | Poly(D,L-lactide) with lepirudin | Cardiovascular | in vitro and in vivo | [50] |
| Decellularized bovine heart and aorta | Polycaprolactone | Cardiovascular | in vitro | [51] |
| Decellularized porcine aortic valve | Poly(hydroxybutyrate) | Cardiovascular | in vitro and in vivo | [52] |
| Decellularized aortic valve | Poly(4-hydroxybutyrate) (P[4HB]) or poly(3-hydroxybutyrate-co4-hydroxybutyrate) (P[3HB-co4HB]) | Cardiovascular | in vitro | [53] |
| Decellularized bovine pericardium + Chitosan | Polycaprolactone | Cardiovascular | in vitro | [54] |
| Fixed bovine pericardium | Polycarbonate urethane | Cardiovascular | in vitro | [55] |
| Decellularized bovine or porcine pericardium | Polycarbonate urethane | Cardiovascular | in vitro | [26] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casarin, M.; Todesco, M.; Fontanella, C.G.; Morlacco, A.; Dal Moro, F.; Bagno, A. Hybrid Materials for Tissue Repair and Replacement: Another Frontier in Biomaterial Exploitation Focusing on Cardiovascular and Urological Fields. Processes 2023, 11, 2013. https://doi.org/10.3390/pr11072013
Casarin M, Todesco M, Fontanella CG, Morlacco A, Dal Moro F, Bagno A. Hybrid Materials for Tissue Repair and Replacement: Another Frontier in Biomaterial Exploitation Focusing on Cardiovascular and Urological Fields. Processes. 2023; 11(7):2013. https://doi.org/10.3390/pr11072013
Chicago/Turabian StyleCasarin, Martina, Martina Todesco, Chiara Giulia Fontanella, Alessandro Morlacco, Fabrizio Dal Moro, and Andrea Bagno. 2023. "Hybrid Materials for Tissue Repair and Replacement: Another Frontier in Biomaterial Exploitation Focusing on Cardiovascular and Urological Fields" Processes 11, no. 7: 2013. https://doi.org/10.3390/pr11072013







