Effects of Bio-Coal Briquette for Residential Combustion on Brown Carbon Emission Reduction
Abstract
:1. Introduction
1.1. Research Motivation
1.2. Research Advance
1.3. Research Contribution
1.4. Research Novelty
2. Materials and Methods
2.1. Materials
2.2. Experimental Methods
2.2.1. Experimental Scheme
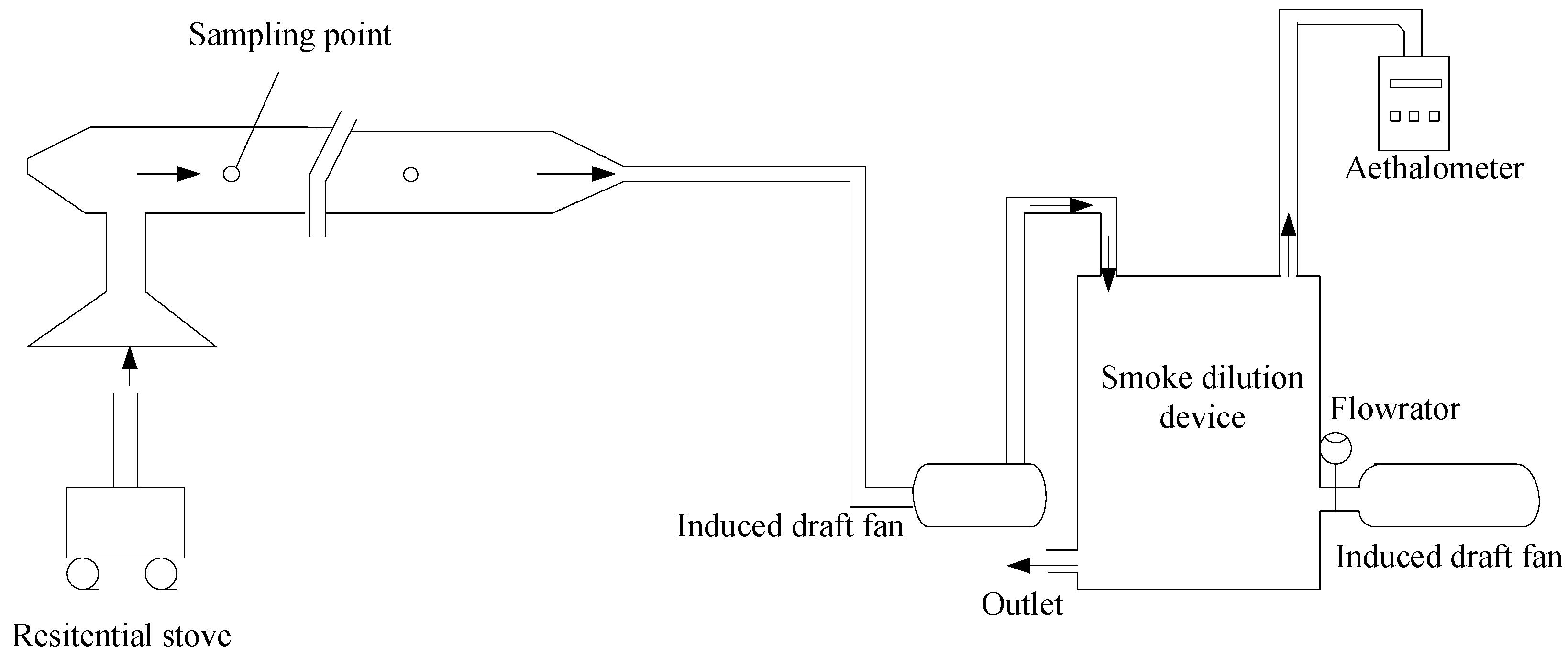
2.2.2. The Experimental System
2.2.3. Collected Sample Analysis
2.2.4. Calculation Method
- 1.
- EFs of BrC
- 2.
- Measurement of MAE and AAE
- 3.
- EFs and an interpolation value of 100% biomass and 100% coal
3. Results
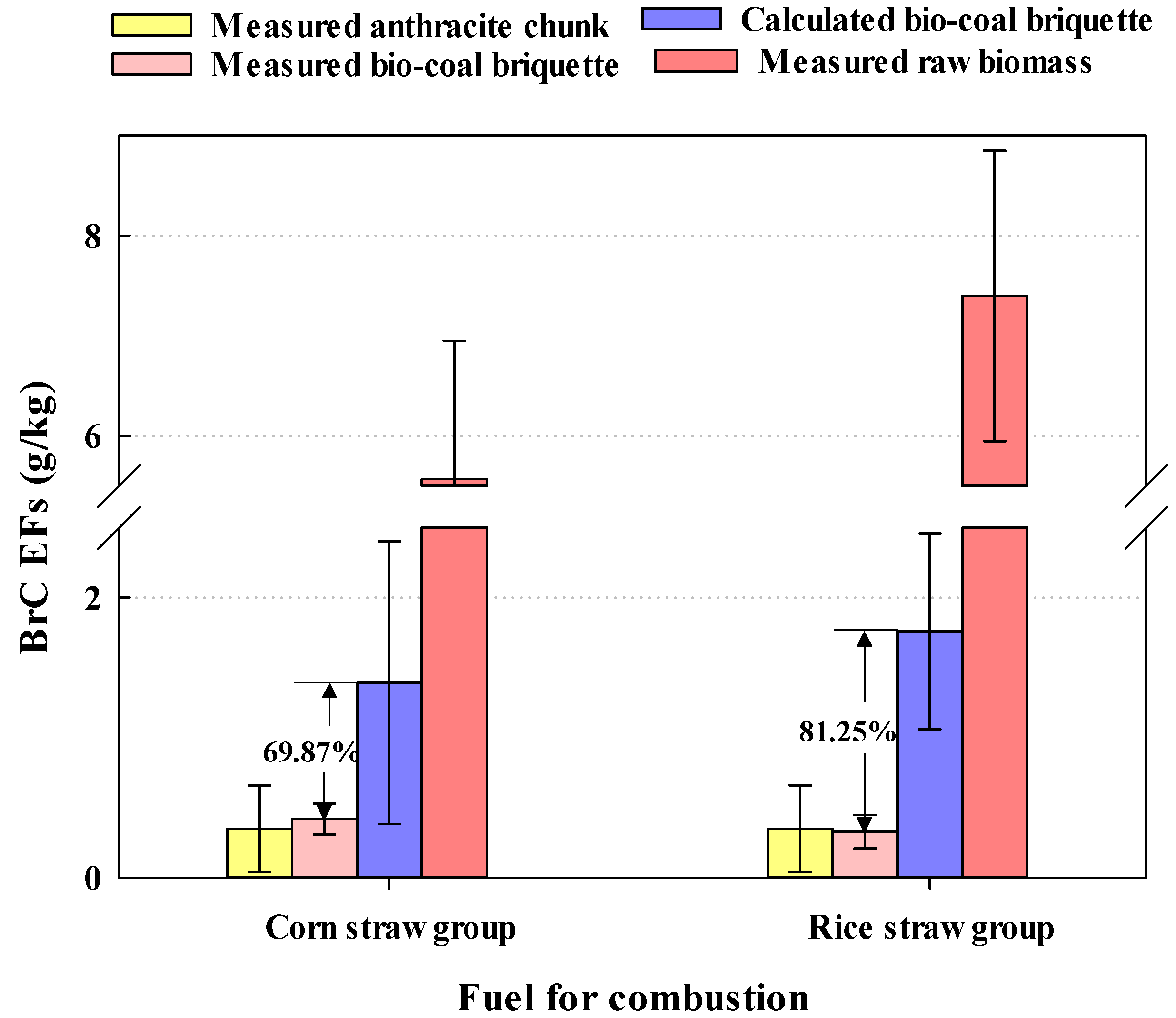
3.1. Analysis of the Results of Orthogonal Experiments
3.2. Optical Characteristics
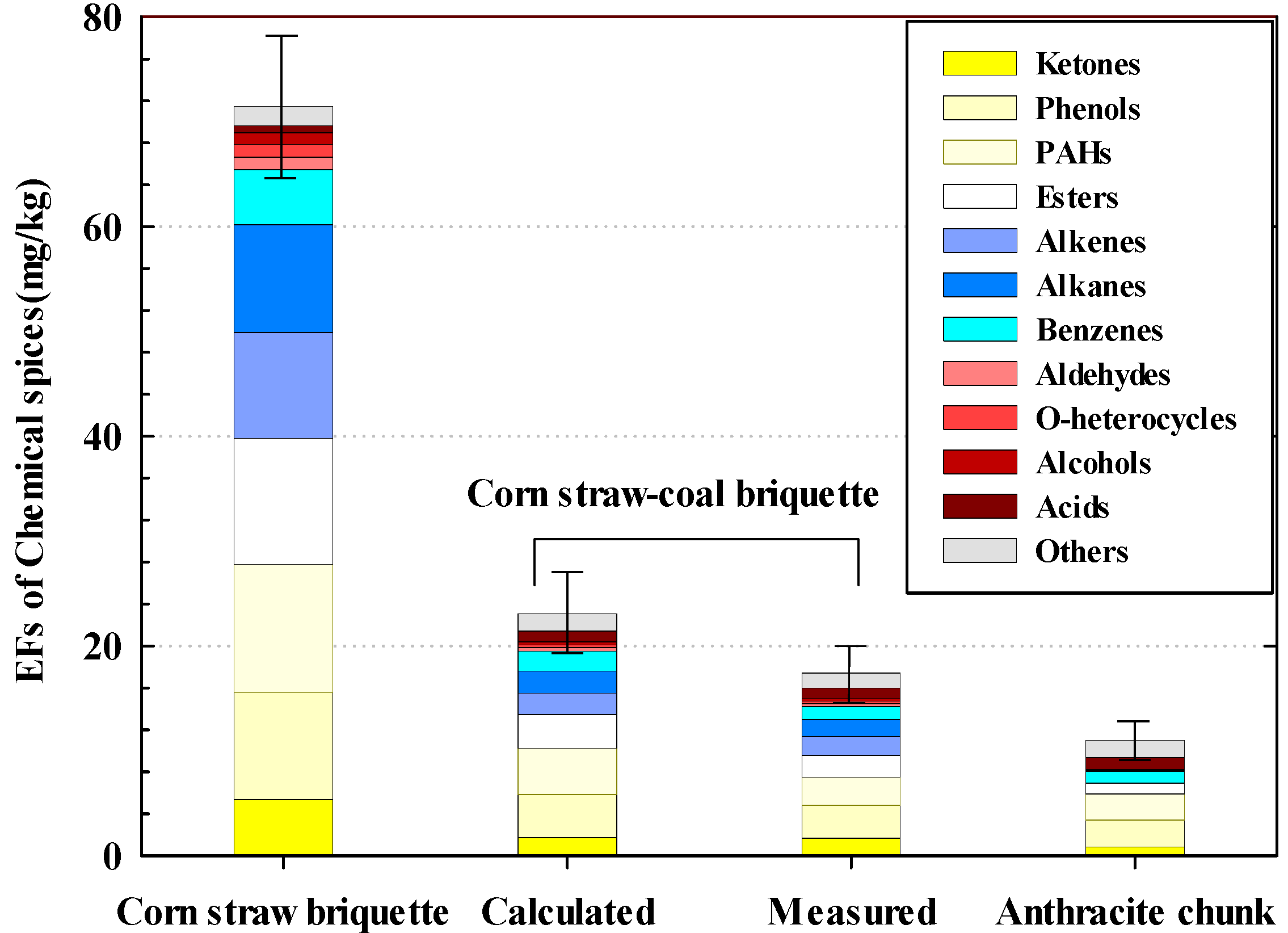
3.3. Analysis of the Results of GC–MS Testing
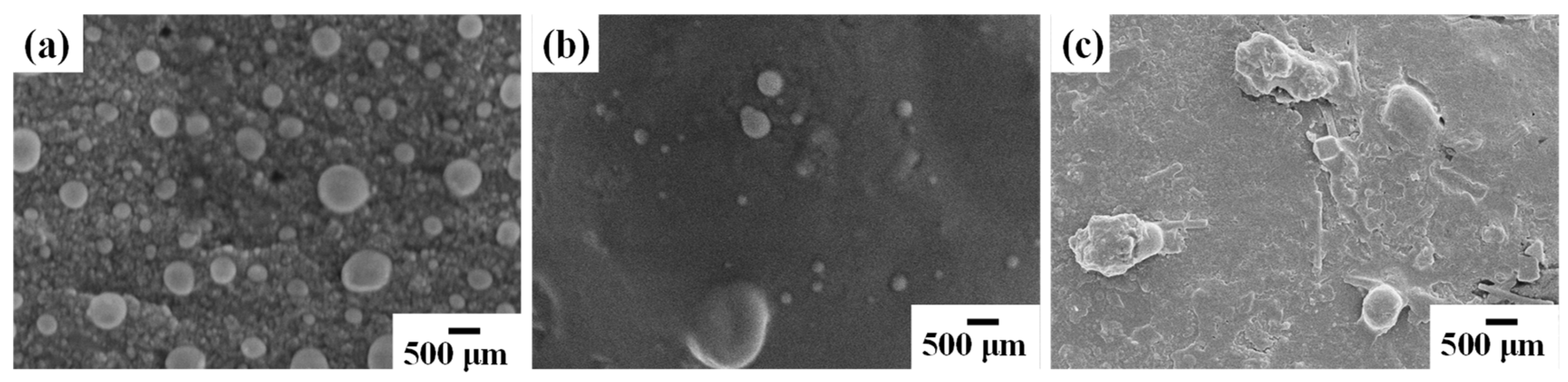
3.4. SEM Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoffer, A.; Gelencsér, A.; Guyon, P.; Kiss, G.; Schmid, O.; Frank, G.P.; Artaxo, P.; Andreae, M.O. Optical properties of humic-like substances (HULIS) in biomass-burning aerosols. Atmos. Chem. Phys. 2006, 6, 3563–3570. [Google Scholar] [CrossRef] [Green Version]
- Kirchstetter, T.W.; Novakov, T.; Hobbs, P.V. Evidence that the spectral dependence of light absorption by aerosols is affected by organic carbon. J. Geophys. Res. Atmos. 2004, 109, D21208. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Xie, C.; Xu, W.; Chen, C.; Sun, Y. Light absorption of black carbon and brown carbon in winter in North China Plain: Comparisons between urban and rural sites. Sci. Total. Environ. 2021, 770, 144821. [Google Scholar] [CrossRef] [PubMed]
- Jo, D.S.; Park, R.J.; Lee, S.; Kim, S.W.; Zhang, X. A global simulation of brown carbon: Implications for photochemistry and direct radiative effect. Atmos. Chem. Phys. 2016, 15, 27805–27852. [Google Scholar] [CrossRef] [Green Version]
- Kirchstetter, T.W.; Thatcher, T.L. Contribution of organic carbon to wood smoke particulate matter absorption of solar radiation. Atmos. Chem. Phys. 2012, 12, 6067–6072. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; Ramanathan, V.; Kotamarthi, V.R. Brown carbon: A significant atmospheric absorber of solar radiation? Atmos. Chem. Phys. 2013, 13, 8607–8621. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Wang, X.; Gu, R.; Hao, W.; Wang, W. Observations of fine particulate nitrated phenols in four sites in northern China: Concentrations, source apportionment, and secondary formation. Atmos. Chem. Phys. 2018, 18, 4349–4359. [Google Scholar] [CrossRef] [Green Version]
- Teich, M.; Pinxteren, D.V.; Wang, M.; Kecorius, S.; Wang, Z.; Müller, T.; Mocnik, G.; Herrmann, H. Contributions of nitrated aromatic compounds to the light absorption of water-soluble and particulate brown carbon in different atmospheric environments in Germany and China. Atmos. Chem. Phys. 2017, 17, 1653–1672. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Wang, X.; Lu, C.; Li, R.; Zhang, J.; Dong, S.; Yang, L.; Xue, L.; Chen, J.; Wang, W. Nitrated phenols and the phenolic precursors in the atmosphere in urban Jinan, China. Sci. Total Environ. 2020, 714, 136760. [Google Scholar] [CrossRef]
- Shrivastava, M.; Lou, S.; Zelenyuk, A.; Easter, R.C.; Corley, R.A.; Thrall, B.D.; Rasch, P.J.; Fast, J.D.; Massey Simonich, S.L.; Shen, H. Global long-range transport and lung cancer risk from polycyclic aromatic hydrocarbons shielded by coatings of organic aerosol. Proc. Natl. Acad. Sci. USA 2017, 114, 1246. [Google Scholar] [CrossRef] [Green Version]
- Harmon, A.; Hebert, V.; Cormier, S.; Subramanian, B.; Reed, J.; Backes, W.; Dugas, T. Particulate matter containing environmentally persistent free radicals induces AhR-dependent cytokine and reactive oxygen species production in human bronchial epithelial cells. PLoS ONE 2018, 13, e0205412. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.; Wang, X.; Dong, S.; Zhang, J.; Li, J.; Zhao, Y.; Liang, Y.; Xue, L.; Xie, H.; Zhang, Q.; et al. Emissions of fine particulate nitrated phenols from various on-road vehicles in China. Environ. Res. 2019, 179, 108709. [Google Scholar] [CrossRef]
- Yan, C.; Zheng, M.; Bosch, C.; Andersson, A.; Desyaterik, Y.; Sullivan, A.P.; Collett, J.L.; Zhao, B.; Wang, S.; He, K. Important fossil source contribution to brown carbon in Beijing during winter. Sci. Rep. 2017, 7, 43182. [Google Scholar] [CrossRef]
- Gelencsér, A.; Hoffer, A.; Kiss, G.; Tombácz, E.; Bencze, L. In-situ Formation of Light-Absorbing Organic Matter in Cloud Water. J. Atmos. Chem. 2003, 45, 25–33. [Google Scholar] [CrossRef]
- Phillips, S.M.; Smith, G.D. Light Absorption by Charge Transfer Complexes in Brown Carbon Aerosols. Environ. Sci. Technol. Let. 2014, 1, 382–386. [Google Scholar] [CrossRef] [Green Version]
- Lin, P.; Aiona, P.K.; Li, Y.; Shiraiwa, M.; Laskin, J.; Nizkorodov, S.A.; Laskin, A. Molecular Characterization of Brown Carbon in Biomass Burning Aerosol Particles. Environ. Sci. Technol. 2016, 50, 11815–11824. [Google Scholar] [CrossRef] [Green Version]
- Martinsson, J.; Eriksson, A.C.; Nielsen, I.E.; Malmborg, V.B.; Ahlberg, E.; Andersen, C.; Lindgren, R.; Nyström, R.; Nordin, E.Z.; Brune, W.H.; et al. Impacts of Combustion Conditions and Photochemical Processing on the Light Absorption of Biomass Combustion Aerosol. Environ. Sci. Technol. 2015, 49, 14663–14671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakrabarty, R.K.; Arnold, I.J.; Francisco, D.M.; Hatchett, B.; Hosseinpour, F.; Loria, M.; Pokharel, A.; Woody, B.M. Black and brown carbon fractal aggregates from combustion of two fuels widely used in Asian rituals. J. Quant. Spectros. Radiat. Transf. 2013, 122, 25–30. [Google Scholar] [CrossRef]
- Yan, C.; Zheng, M.; Sullivan, A.P.; Bosch, C.; Desyaterik, Y.; Andersson, A.; Li, X.; Guo, X.; Zhou, T.; Gustafsson, O. Chemical characteristics and light-absorbing property of water-soluble organic carbon in Beijing: Biomass burning contributions. Atmos. Environ. 2015, 121, 4–12. [Google Scholar] [CrossRef] [Green Version]
- Mcmeeking, G.R.; Fortner, E.; Onasch, T.B.; Taylor, J.W.; Flynn, M.; Coe, H.; Kreidenweis, S.M. Impacts of nonrefractory material on light absorption by aerosols emitted from biomass burning. J. Geophys. Res. Atmos. 2015, 119, 12, 272–12, 286. [Google Scholar] [CrossRef]
- Pokhrel, R.; Wagner, N.; Langridge, J.; Lack, D.; Jayarathne, T.; Stone, E.; Stockwell, C.; Yokelson, R.; Murphy, S. Parameterization of single-scattering albedo (SSA) and absorption Ångström exponent (AAE) with EC/OC for aerosol emissions from biomass burning. Atmos. Chem. Phys. 2016, 16, 9549–9561. [Google Scholar] [CrossRef] [Green Version]
- Tian, J.; Wang, Q.; Ni, H.; Wang, M.; Zhou, Y.; Han, Y.; Shen, Z.; Pongpiachan, S.; Zhang, N.; Zhao, Z.; et al. Emission Characteristics of Primary Brown Carbon Absorption from Biomass and Coal Burning: Development of an Optical Emission Inventory for China. J. Geophys. Res. Atmos. 2019, 124, 1879–1893. [Google Scholar] [CrossRef]
- Huang, R.-J.; Yang, L.; Shen, J.; Yuan, W.; Gong, Y.; Guo, J.; Cao, W.; Duan, J.; Ni, H.; Zhu, C.; et al. Water-Insoluble Organics Dominate Brown Carbon in Wintertime Urban Aerosol of China: Chemical Characteristics and Optical Properties. Environ. Sci. Technol. 2020, 54, 7836–7847. [Google Scholar] [CrossRef]
- Voliotis, A.; Prokes, R.; Lammel, G.; Samara, C. New insights on humic-like substances associated with wintertime urban aerosols from central and southern Europe: Size-resolved chemical characterization and optical properties. Atmos. Environ. 2017, 166, 286–299. [Google Scholar] [CrossRef]
- Krivácsy, Z.; Kiss, G.; Varga, B.; Galambos, I.; Sárvári, Z.; Gelencsér, A.; Molnár, á.; Fuzzi, S.; Facchini, M.C.; Zappoli, S. Study of humic-like substances in fog and interstitial aerosol by size-exclusion chromatography and capillary electrophoresis. Atmos. Environ. 2000, 34, 4273–4281. [Google Scholar] [CrossRef]
- Andreae, M.O.; Gelencsér, A. Black carbon or brown carbon? The nature of light-absorbing carbonaceous aerosols. Atmos. Chem. Phys. 2006, 6, 3131–3148. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Bond, T.C. Light absorption by organic carbon from wood combustion. Atmos. Chem. Phys. 2009, 10, 1773–1787. [Google Scholar] [CrossRef] [Green Version]
- Lin, P.; Fleming, L.T.; Nizkorodov, S.A.; Laskin, J.; Laskin, A. Comprehensive Molecular Characterization of Atmospheric Brown Carbon by High Resolution Mass Spectrometry with Electrospray and Atmospheric Pressure Photoionization. Anal. Chem. 2018, 90, 12493–12502. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, Y.H.; Surratt, J.D.; Weber, R.J. Sources, Composition and Absorption ngstrm Exponent of Light-absorbing Organic Components in Aerosol Extracts from the Los Angeles Basin. Environ. Sci. Technol. 2013, 47, 3685–3693. [Google Scholar] [CrossRef]
- Tang, J.; Li, J.; Su, T.; Han, Y.; Mo, Y.; Jiang, H.; Cui, M.; Jiang, B.; Chen, Y.; Tang, J.; et al. Molecular compositions and optical properties of dissolved brown carbon in biomass burning, coal combustion, and vehicle emission aerosols illuminated by excitation–emission matrix spectroscopy and Fourier transform ion cyclotron resonance mass spectrometry analysis. Atmos. Chem. Phys. 2020, 20, 2513–2532. [Google Scholar]
- Jiang, H.; Frie, A.L.; Lavi, A.; Chen, J.Y.; Zhang, H.; Bahreini, R.; Lin, Y.-H. Brown Carbon Formation from Nighttime Chemistry of Unsaturated Heterocyclic Volatile Organic Compounds. Environ. Sci. Technol. Let. 2019, 6, 184–190. [Google Scholar] [CrossRef]
- Liu, J.; Bergin, M.; Guo, H.; King, L.; Weber, R.J. Size-resolved measurements of brown carbon in water and methanol extracts and estimates of their contribution to ambient fine-particle light absorption. Atmos. Chem. Phys. 2013, 13, 18233–18276. [Google Scholar] [CrossRef] [Green Version]
- Chakrabarty, R.K.; Moosmüller, H.; Chen, L.; Lewis, K.; Arnott, W.P.; Mazzoleni, C.; Dubey, M.K.; Wold, C.E.; Hao, W.M.; Kreidenweis, S.M. Brown carbon in tar balls from smoldering biomass combustion. Atmos. Chem. Phys. 2010, 10, 6363–6370. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; He, Q.; Schade, J.; Passig, J.; Zimmermann, R.; Meidan, D.; Laskin, A.; Rudich, Y. Dynamic changes in optical and chemical properties of tar ball aerosols by atmospheric photochemical aging. Atmos. Chem. Phys. 2019, 19, 139–163. [Google Scholar] [CrossRef] [Green Version]
- Qi, J.; Li, Q.; Wu, J.; Jiang, J.; Miao, Z.; Li, D. Biocoal Briquettes Combusted in a Household Cooking Stove: Improved Thermal Efficiencies and Reduced Pollutant Emissions. Environ. Sci. Technol. 2017, 51, 1886–1892. [Google Scholar] [CrossRef]
- Qi, J.; Wu, J.; Zhang, L. Influence of Molding Technology on Thermal Efficiencies and Pollutant Emissions from Household Solid Fuel Combustion during Cooking Activities in Chinese Rural Areas. Symmetry 2021, 13, 2223. [Google Scholar] [CrossRef]
- Ikelle, I.; Eze, O. Study on the Combustion Properties of Bio-Coal Briquette Blends of Cassava Stalk. ChemSearch J. 2017, 8, 29–34. [Google Scholar]
- Balraj, A.; Krishnan, J.; Selvarajan, K.; Sukumar, K. Potential use of biomass and coal-fine waste for making briquette for sustainable energy and environment. Environ. Sci. Technol. 2021, 28, 63516–63522. [Google Scholar] [CrossRef]
- Sun, J.; Zhi, G.; Hitzenberger, R.; Chen, Y.; Tian, C.; Zhang, Y.; Feng, Y.; Cheng, M.; Zhang, Y.; Cai, J. Emission factors and light absorption properties of brown carbon from household coal combustion in China. Atmos. Chem. Phys. 2017, 17, 4769–4780. [Google Scholar] [CrossRef] [Green Version]
- Stockwell, C.E.; Jayarathne, T.; Cochrane, M.A.; Ryan, K.C.; Putra, E.I.; Saharjo, B.H.; Nurhayati, A.D.; Albar, I.; Blake, D.R.; Simpson, I.J. Field measurements of trace gases and aerosols emitted by peat fires inCentral Kalimantan, Indonesia, during the 2015 El Niño. Atmos. Chem. Phys. 2016, 16, 11711–11732. [Google Scholar] [CrossRef] [Green Version]
- Fan, X.J.; Cao, T.; Xu-Fang, Y.U.; Song, J.Z.; Wang, Y.; Xiao, X.; Xie, Y.; Fei-Yue, L.I. Emission characteristics and optical properties of extractable brown carbon from residential wood combustion. China Environ. Sci. 2019, 39, 3215–3224. [Google Scholar]
- Song, S.; Wang, Y.; Wang, Y.; Wang, T.; Tan, H. The characteristics of particulate matter and optical properties of Brown carbon in air lean condition related to residential coal combustion. Powder Technol. 2020, 379, 505–514. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, Y.; Zhi, G.; Hitzenberger, R.; Wu, C. Brown carbon’s emission factors and optical characteristics in household biomass burning: Developing a novel algorithm for estimating the contribution of brown carbon. Atmos. Chem. Phys. 2021, 21, 2329–2341. [Google Scholar] [CrossRef]
- Utry, N.; Ajtai, T.; Filep, Á.; Pintér, M.; Török, Z.; Bozóki, Z.; Szabo, G. Correlations between absorption Angström exponent (AAE) of wintertime ambient urban aerosol and its physical and chemical properties. Atmos. Environ. 2014, 91, 52–59. [Google Scholar] [CrossRef]
- Li, X.; Chen, Y.; Bond, T.C. Light absorption of organic aerosol from pyrolysis of corn stalk. Atmos. Environ. 2016, 144, 249–256. [Google Scholar] [CrossRef] [Green Version]
- Park, S.S.; Yu, J. Chemical and light absorption properties of humic-like substances from biomass burning emissions under controlled combustion experiments. Atmos. Environ. 2016, 136, 114–122. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, L.; Li, X.; Xin, J.; Liu, Z.; Sun, Y.; Liu, J.; Zhang, Y.; Du, W.; Jin, X.; et al. Emission characteristics of size distribution, chemical composition and light absorption of particles from field-scale crop residue burning in Northeast China. Sci. Total Environ. 2020, 710, 136304. [Google Scholar] [CrossRef]
- Li, M.; Fan, X.; Zhu, M.; Zou, C.; Song, J.; Wei, S.; Jia, W.; Peng, P.A. Abundance and Light Absorption Properties of Brown Carbon Emitted from Residential Coal Combustion in China. Environ. Sci. Technol. 2019, 53, 595–603. [Google Scholar] [CrossRef]
- Peng, L.; Laskin, J.; Nizkorodov, S.A.; Laskin, A. Revealing Brown Carbon Chromophores Produced in Reactions of Methylglyoxal with Ammonium Sulfate. Environ. Sci. Technol. 2015, 49, 14257. [Google Scholar]
- Bluvshtein, N.; Lin, P.; Flores, J.M.; Segev, L.; Mazar, Y.; Tas, E.; Snider, G.; Weagle, C.; Brown, S.S.; Laskin, A. Broadband optical properties of biomass burning aerosol and identification of brown carbon chromophores. J. Geophys. Res. 2017, 122, 5441–5456. [Google Scholar] [CrossRef]



| No. | Particle Size (mm) | Molding Pressure (MPa) | Biomass/Anthracite (m/m) |
|---|---|---|---|
| 1 | ≤1 | 15 | 1:9 |
| 2 | ≤2 | 25 | 1:4 |
| 3 | ≤3 | 35 | 3:7 |
| No. | Emission Source | EFs (g/kg) | Data Sources |
|---|---|---|---|
| 1 | Boio-coal briquette | 0.33–0.42 | This study |
| Anthracite | 0.35 ± 0.31 | ||
| Biomass briquette | 5.57–7.40 | ||
| 2 | Anthracite | 1.08 ± 0.80 | Sun et al. [39] |
| Coal briquette | 8.59 ± 2.70 | ||
| 3 | Peat | 0.85 | Stockwell et al. [40] |
| 4 | Firewood | 7.50–16.00 | Fan et al. [41] |
| 5 | Bituminous coal | 2.10–3.10 | Song et al. [42] |
| Lignite | 1.10–2.20 | ||
| 6 | Rice straw | 2.50 ± 3.06 | Sun et al. [43] |
| Corn straw | 0.45 ± 0.76 | ||
| Biomass pellet | 0.13 ± 0.06 |
| No. | Emission Source | Wavelength (nm) | MAE (m2/g) | AAE | Data Sources |
|---|---|---|---|---|---|
| 1 | Boio-coal briquette | 350–480 | 0.10–0.36 | 7.31–7.70 | This study |
| Anthracite | 350–480 | 1.33 ± 0.17 | 7.40 ± 0.23 | ||
| Biomass briquette | 350–480 | 1.17–1.28 | 5.30–6.23 | ||
| 2 | Corn straw | 400–550 | 7–7.7 | Li et al. [45] | |
| 3 | Rice straw | 300–400 | 1.37 ± 0.23 | 7.4–9.0 | Park et al. [46] |
| 4 | Coal | 365 | 1.5 ± 0.4 | 5.7 ± 0.2 | Huang et al. [23] |
| 5 | Corn straw | 375–625 | 2.10–3.10 | 1.5–4 | Wang et al. [47] |
| Rice straw | 375–625 | 1.10–2.20 | 5.0 ± 0.1 | ||
| 6 | Bituminous coal | 330–400 | 2.50 ± 3.06 | 7.7–11 | Li et al. [48] |
| Anthracite | 330–400 | 0.13 ± 0.06 | 8.2–12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, J.; Wu, J. Effects of Bio-Coal Briquette for Residential Combustion on Brown Carbon Emission Reduction. Processes 2023, 11, 1834. https://doi.org/10.3390/pr11061834
Qi J, Wu J. Effects of Bio-Coal Briquette for Residential Combustion on Brown Carbon Emission Reduction. Processes. 2023; 11(6):1834. https://doi.org/10.3390/pr11061834
Chicago/Turabian StyleQi, Juan, and Jianjun Wu. 2023. "Effects of Bio-Coal Briquette for Residential Combustion on Brown Carbon Emission Reduction" Processes 11, no. 6: 1834. https://doi.org/10.3390/pr11061834




