Effects of Lead Pollution on Photosynthetic Characteristics and Chlorophyll Fluorescence Parameters of Different Populations of Miscanthus floridulus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Materials
2.2. Experimental Design
2.3. Determination of Gas Exchange Parameters
2.4. Determination of Chlorophyll Fluorescence Parameters
2.5. Determination of Plant Morphology
2.6. Determination of Photosynthetic Pigment Content
2.7. Data Processing
3. Results
3.1. Growth Morphological Changes of M. floridulus under Pb Stress
3.2. The Changes of Photosynthesis Indexes
3.2.1. Effects of Pb on Net Photosynthetic Rate (Pn) and Stomatal Conductance (Gs) in Leaves of M. floridulus
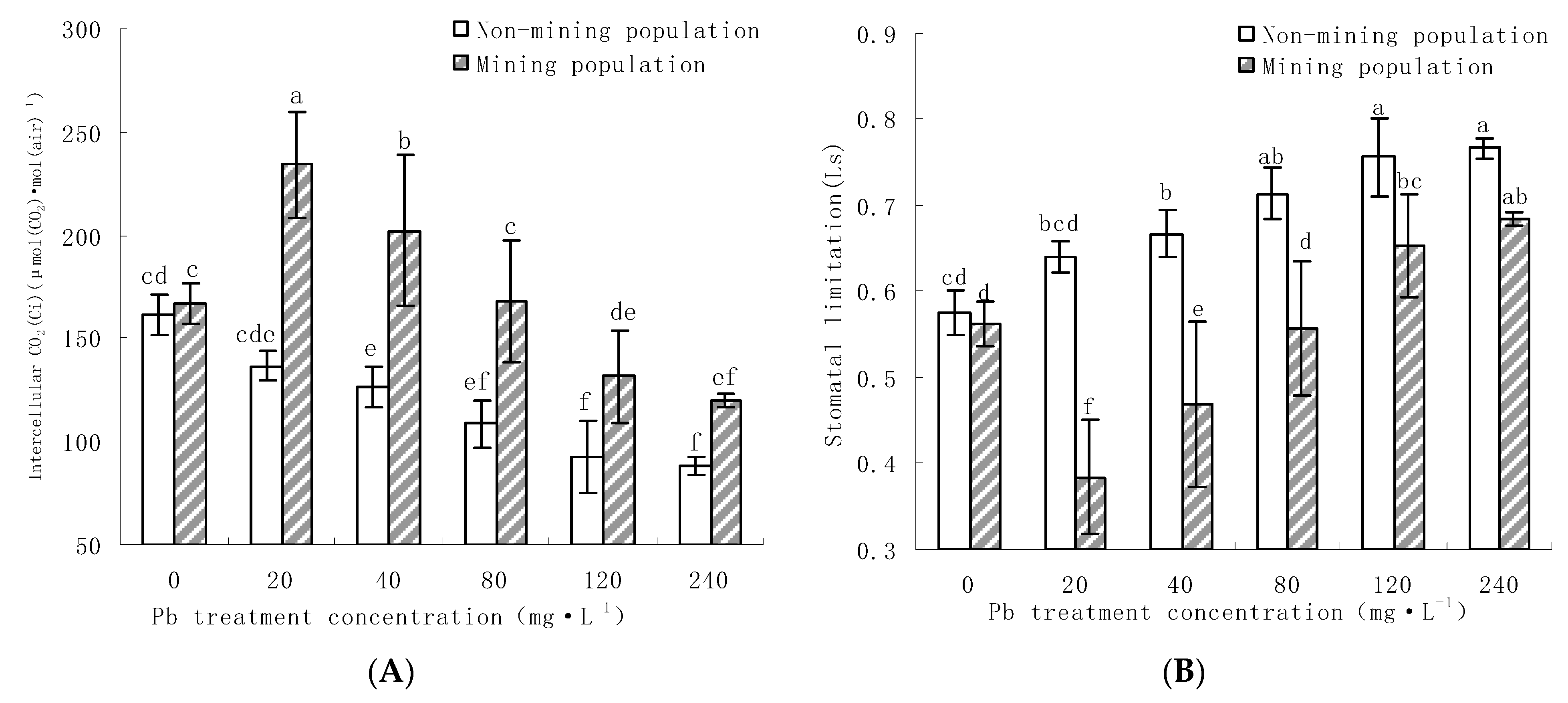
3.2.2. Effects of Pb on Intercellular Carbon Dioxide Concentration (Ci) and Stomatal Limit (Ls) in Leaves of M. floridulus
3.2.3. Effects of Pb on Transpiration Rate (Tr) and Water Use Efficiency (WUE) in Leaves of M. floridulus
3.3. Changes of Chlorophyll Fluorescence Parameters
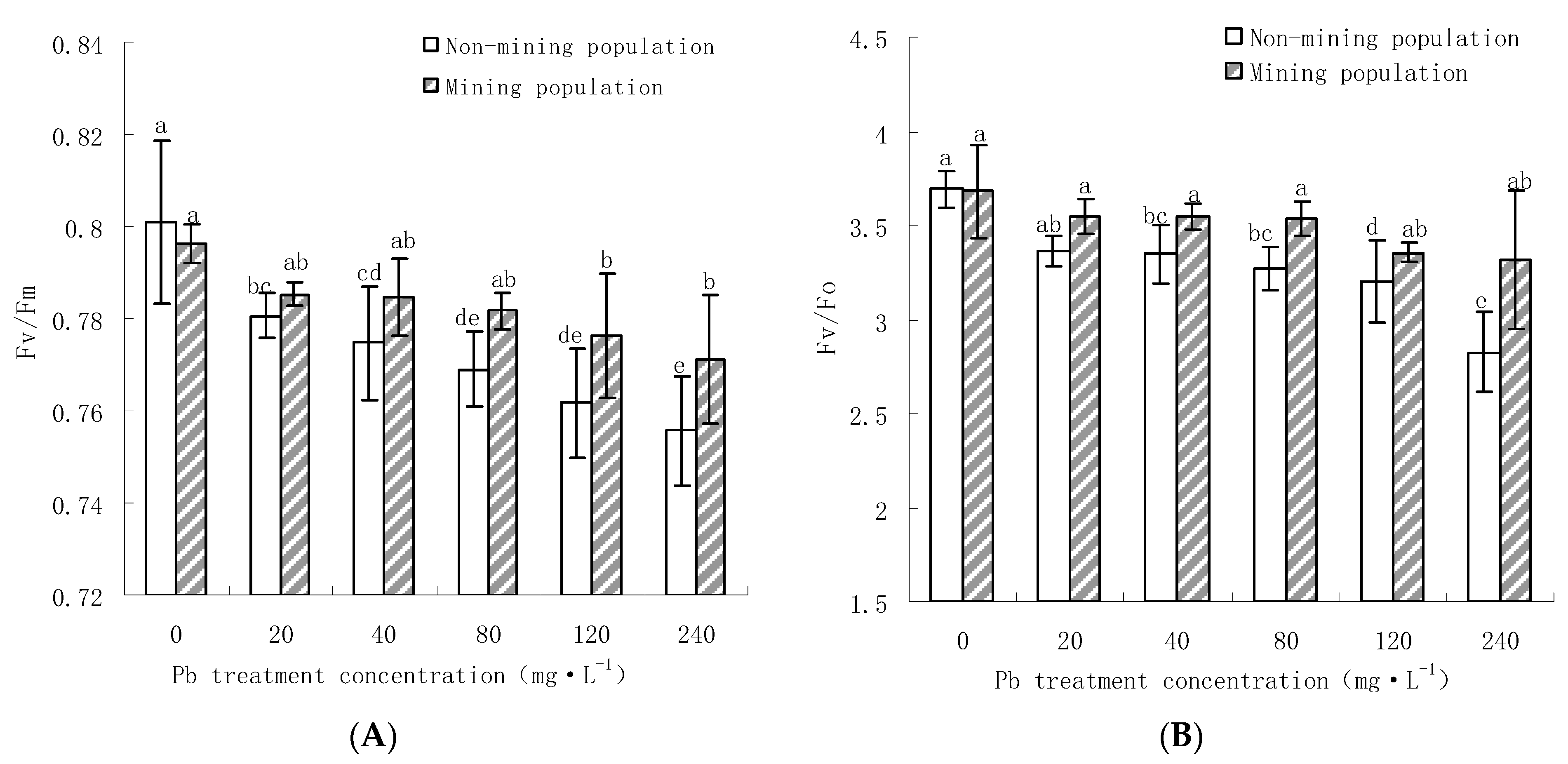
3.3.1. Effects of Pb on Fv/Fm and Fv/F0 of the Leaves of M. floridulus
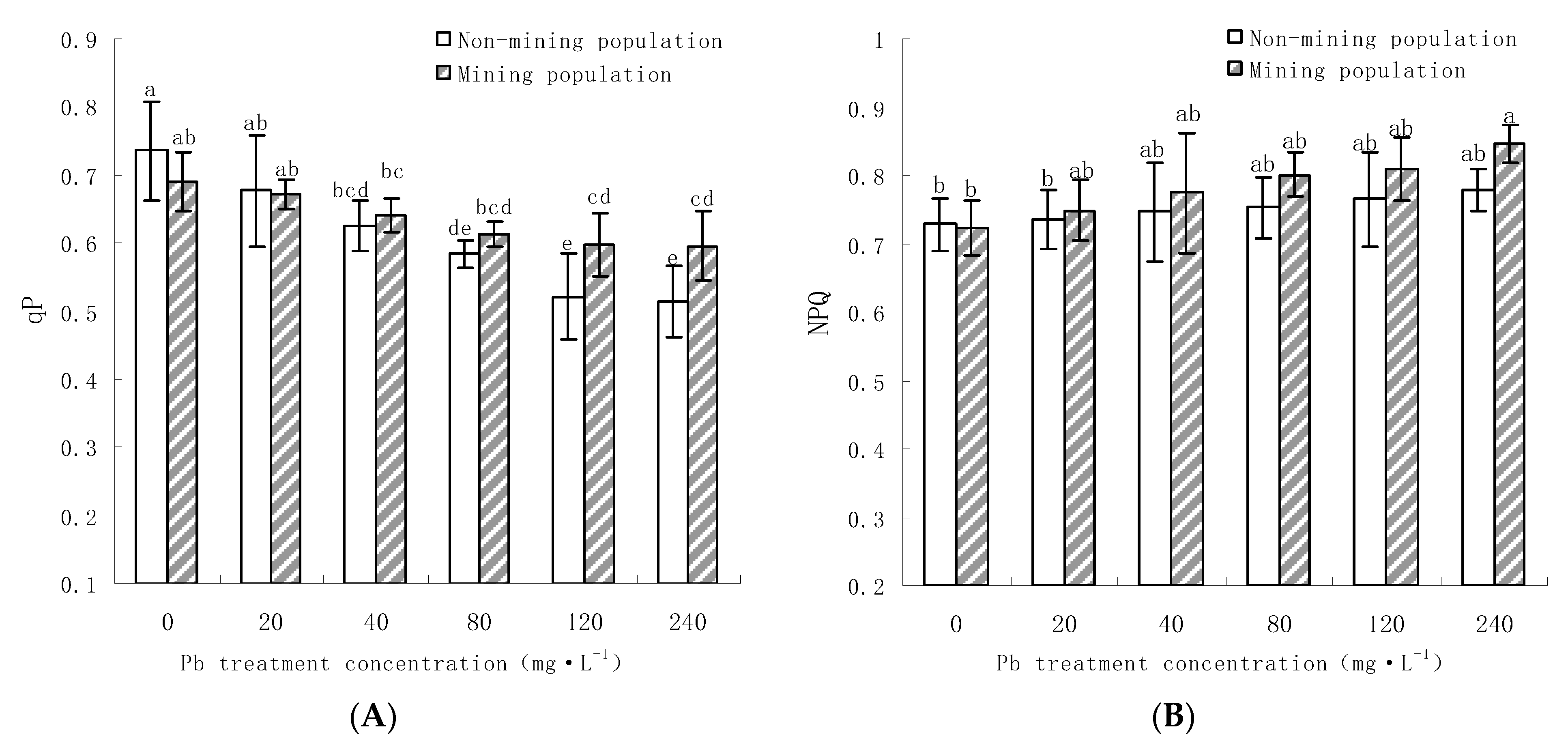
3.3.2. Effects of Pb on the qP and NPQ of the Leaves of M. floridulus
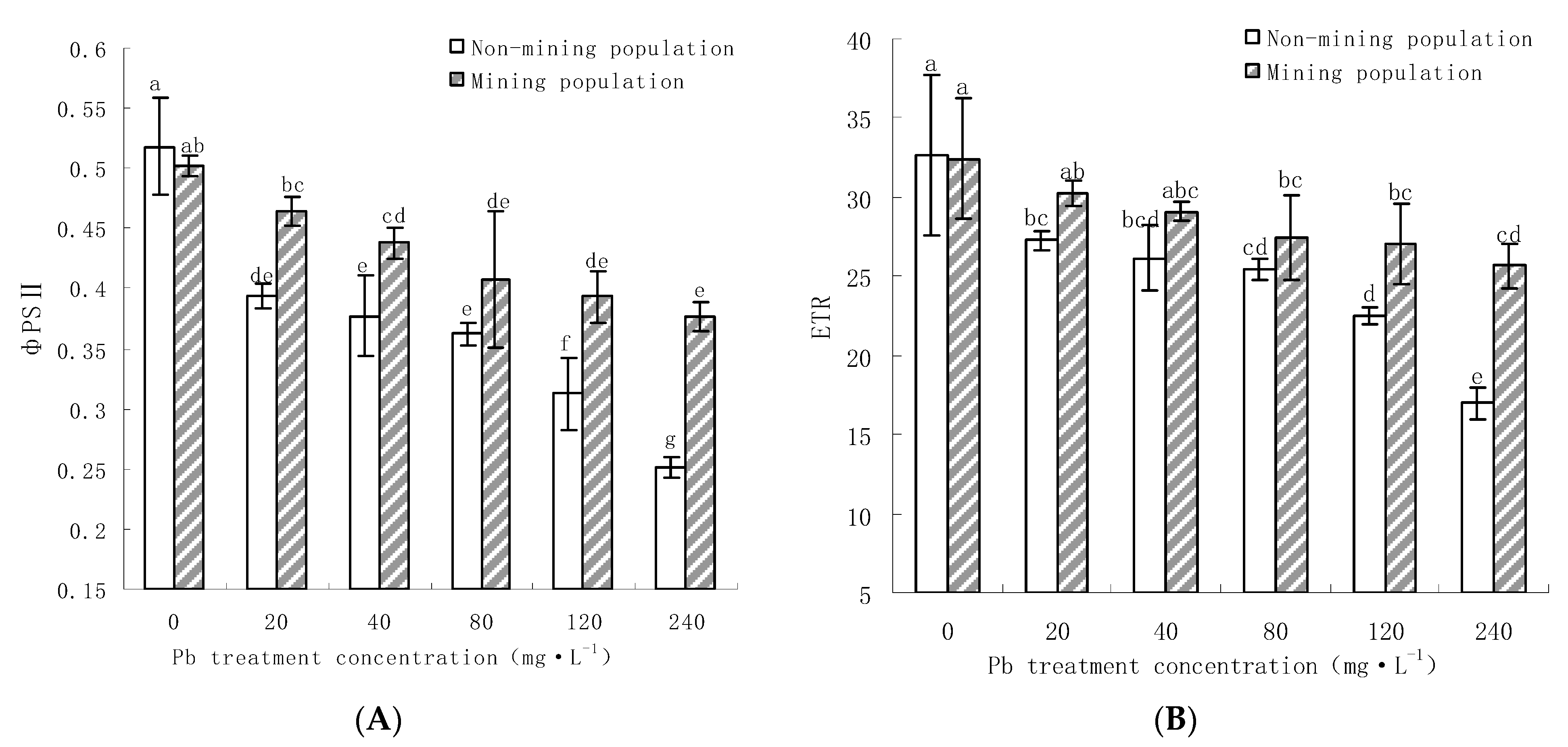
3.3.3. Effects of Pb on the Leaf of M. floridulus on фPSII and ETR
3.4. Effects of Pb on Photosynthetic Pigment Content in Leaves of M. floridulus
3.5. Correlation between Photosynthetic Physiological Indexes and Pb Concentration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, B.H.; Zhao, X.L.; Li, P.; Liang, J.J.; Fan, Q.H.; Ma, X.X.; Zheng, G.D.; Qiu, J. Lead isotopic fingerprinting as a tracer to identify the pollution sources of heavy metals in the southeastern zone of Baiyin, China. Sci. Total Environ. 2019, 660, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.Q.; Liu, X.; Ai, Y.W.; Chen, J.; Luo, X.M.; Chen, J.Y.; Shihong Zhong, S.H. Effects and mechanisms of revegetation modes on cadmium and lead pollution in artificial soil on railway rock-cut slopes. Sci. Total Environ. 2018, 644, 1602–1611. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.H.; Luo, S.H.; Luo, H.Y.; Huang, H.Y.; Xu, F.; Feng, S.; Xu, H. Improved phytoremediation of heavy metal contaminated soils by Miscanthus floridulus under a varied rhizosphere ecological characteristic. Sci. Total Environ. 2021, 808, 151995. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Li, S.; Chen, Z.; Wang, F.; Chen, J.; Wang, L.Q. A systemic ecological risk assessment based on spatial distribution and source apportionment in the abandoned lead acid battery plant zone, China. J. Hazard. Mater. 2018, 354, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.L.; Yuan, J.G.; Fang, W.; Yang, Z.Y. Differences of Pb accumulation among plant tissues of 25 Zay mays varieties. Acta Phytoeco. Sin. 2005, 29, 992–999. [Google Scholar]
- Song, J.; Shen, Q.; Wang, L.; Qiu, G.; Shi, J.; Xu, J.; Philip, C.B.; Liu, X. Effects of Cd, Cu, Zn and their combined action on microbial biomass and bacterial community structure. Environ. Pollut. 2018, 243, 510–518. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, H.; Li, J.; Liu, Y.; Shi, W.; Hu, H. The toxic factor of copper should be adjusted during the ecological risk assessment for soil bacterial community. Ecol. Indic. 2020, 111, 106072. [Google Scholar] [CrossRef]
- Zheng, B.J.; Xu, S.C.; Tu, Y.H.; Zou, F.; Liang, D.D.; Wang, H.; Mo, Y.W. Research progress on copper toxicity to crops. J. Shaoxing Univ. Arts Sci. 2022, 42, 58–64. [Google Scholar]
- Lin, M.Z.; Jin, M.F.; Chen, Y.; Zeng, Q.L. Photosynthetic characteristics and mechanism of wedelia chinensis under soil Cu pollution stress. J. Anhui Agric. Univ. 2018, 45, 657–663. [Google Scholar]
- Yang, K.; Huang, C.; Lu, S.; Peng, G.Y.; Huang, C.G. Selection of reference genes in real-time quantitative PCR of root tissue of Commelina purpurea under copper stress. Acta Phytophysiol. Sin. 2021, 57, 195–204. [Google Scholar]
- Qin, J.Q.; Zhao, H.R.; Dai, M.; Zhao, P.; Chen, X.; Liu, H.; Lu, B.Z. Speciation Distribution and Influencing Factors of Heavy Metals in Rhizosphere Soil of Miscanthus Floridulus in the Tailing Reservoir Area of Dabaoshan Iron Polymetallic Mine in Northern Guangdong. Processes 2022, 10, 1217. [Google Scholar] [CrossRef]
- Barbosa, B.; Boléo, S.; Sidella, S.; Costa, J.; Duarte, M.P.; Mendes, B.; Cosentino, S.L.; Fernando, A.L. Phytoremediation of heavy metal-contaminated soils using the perennial energy crops miscanthus spp. And Arundo donax L. Bioenerg. Res. 2015, 8, 1500–1511. [Google Scholar] [CrossRef]
- Zadel, U.; Nesme, J.; Michalke, B.; Vestergaard, G.; Plaza, G.A.; Schroder, P.; Radl, V.; Schloter, M. Changes induced by heavy metals in the plant-associated microbiome of miscanthus x giganteus. Sci. Total Environ. 2020, 711, 134433. [Google Scholar] [CrossRef]
- Ren, L.M.; Liu, P.; Zheng, Q.E. A survey of heavy metal content of plants growing on the soil polluted by manganese mine in Daxin County, Guangxi. Subtrop. Plant Sci. 2006, 35, 5–8. [Google Scholar]
- Sun, J.; Tie, B.Q.; Qin, P.F. Investigation of contaminated soil and plants by heavy metals in Pb-Zn mining area. J. Plant Resour. Environ. 2006, 15, 63–67. [Google Scholar]
- Li, Q.F.; Du, W.B.; Li, Z.A.; Wang, Z.F.; Peng, S.L. Heavy metals accumulation in mining area’s Miscanthus sinensis populations and its relationship with soil characters. Chin. J. Ecol. 2006, 25, 255–258. [Google Scholar]
- Chen, Z.J.; Tian, W.; Li, Y.J.; Sun, L.N.; Chen, Y.; Zhang, H.; Li, Y.Y.; Han, H. Responses of rhizosphere bacterial communities, their functions and their network interactions to Cd stress under phytostabilization by Miscanthus spp. Environ. Pollut. 2021, 287, 117663. [Google Scholar] [CrossRef]
- Ye, Z.H.; Baker, A.J.M.; Wong, M.H.; Willis, A.J. Copper tolerance, uptake and accumulation by Phragmites australis. Chemosphere 2003, 50, 795–800. [Google Scholar] [CrossRef]
- Rascio, N.; Navari-Izzo, F. Heavy metal hyperaccumulating plants: How and why do they do it? And what makes them so interesting. Plant Sci. 2011, 189, 169–181. [Google Scholar] [CrossRef]
- Fu, S.; Zhou, Y.; Zhao, Y.; Gao, Q.Z.; Peng, X.Z.; Dang, Z.; Zhang, C.B.; Yang, X.Q.; Yang, Z.J.; Dou, L.; et al. Study on heavy metals in soils contaminated by acid mine drainage from Dabaoshan Mine, Guangdong. Environ. Sci. 2007, 28, 805–812. [Google Scholar]
- Zhao, H.R.; Wang, D.; Zhu, B.T.; Jin, X. Evaluation of heavy metal pollution of soil in the Dabaoshan mining area based on the information diffusion theory. J. Agro-Environ.Sci. 2019, 38, 79–86. [Google Scholar]
- Chen, J.Y.; Liu, G.B.; Cui, J.L.; Xiao, T.F. Migration process and risk assessment of heavy metals in soil plant system of Dabaoshan mining area in Guangdong Province. Environ. Sci. 2019, 40, 5629–5639. [Google Scholar]
- Qin, J.Q.; Xia, B.C.; Hu, M.; Zhao, P.; Zhao, H.R.; Lin, X.F. Analysis of the vegetation succession of tailing wasteland of Dabaoshan Mine, Guangdong Province. J. Agro-Environ. Sci. 2009, 28, 2085–2091. [Google Scholar]
- Qin, J.Q.; Zhao, H.R.; Liu, H.; Dai, M.; Zhao, P.; Chen, X.; Wu, X.G. The Difference of Lead Accumulation and Transport in Different Ecotypes of Miscanthus floridulus. Processes 2022, 10, 2219. [Google Scholar] [CrossRef]
- Zhao, H.R.; Xia, B.C.; Qin, J.Q.; Zhang, J. Hydrogeochemical and mineralogical characteristics related to heavy metal attenuation in a stream polluted by acid mine drainage: A case study in Dabaoshan mine, China. J. Environ. Sci. 2012, 24, 979–989. [Google Scholar] [CrossRef]
- Zhao, H.R.; Xia, B.C.; Fan, C.; Zhao, P.; Shen, S. Human health risk from soil heavy metal contamination under different land uses near Dabaoshan mine, southern China. Sci. Total Environ. 2012, 417, 45–54. [Google Scholar] [CrossRef]
- Qin, J.; Zhao, H.; Liu, H.; Dai, M.; Zhao, P.; Chen, X.; Wu, X. Analysis on characteristics of phytogeochemistry of Elsholtzia haichowensisin Daye Tonglushan copper mine. Acta Ecol. Sin. 2001, 21, 907–912. [Google Scholar]
- Wen, C.H.; Deng, C.Q.; Chang, X.X.; Wang, H.B.; Wang, H.X. Differentiation in Datura stramonium L.populations exposed to heavy-metal pollution at different durations: RAPD analysis. Acta Ecol. Sin. 2001, 21, 1239–1245. [Google Scholar]
- Tian, S.K.; Lu, L.L.; Yang, X.E. The impact of EDTA on lead distribution and speciation in the accumulator Sedum alfredii by synchrotron X-ray investigation. Environ. Pollut. 2011, 159, 782–789. [Google Scholar] [CrossRef]
- Farooqui, A.; Kulshreshtha, K.; Srivastavaa, K.; Singh, S.N.; Farooqui, S.A.; Pandey, V.; Ahmad, P.J. Photosynthesis, stomata1 response and metal accumulation in Cineraria maritima L. and Centauria moschata L. grown in metal-rich soil. Sci. Total Environ. 1995, 164, 203–207. [Google Scholar] [CrossRef]
- He, B.; Yang, X.E. Seudm alrfedii: A New Lead Accumulatnig Ecotype. Acta Bot. Sin. 2002, 44, 1356–1370. [Google Scholar]
- Berry, J.A.; Downton, W.J.S. Environmental regulation of photosynthesis. In Photosynthesis: Development, Carbon Metabolism and Plant Productivity, Vol II; Academic Press: NewYork, NY, USA, 1982; pp. 263–343. [Google Scholar]
- Fischer, R.A.; Turner, N.C. Plant productivity in the arid and semiarid zones. Annual Rev. Plant Physi. 1978, 29, 227–317. [Google Scholar] [CrossRef]
- Ralph, P.J.; Schreiber, U.; Gademann, R. Coral photobiology studied with a new imaging pulse amplitude modulated fluorometer. Phycol. Res. 2005, 41, 335–342. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams, W.W., III. Xanthophyll cycle and light stress in nature: Uniform response to excess direct sunlight among higher plant species. Planta 1996, 198, 460–470. [Google Scholar] [CrossRef]
- Li, H.S.; Sui, Q.; Zhao, S.J.; Zhang, W.H. Experimental Principle and Technology of Plant Physiology and Biochemistry; Higher Education Press: Beijing, China, 2000; pp. 134–138. [Google Scholar]
- Ma, J.; Sun, M.; Qiu, L.; Xie, Y.; Ma, Y.; Liang, W. The 5-Aminolevulinic Acid (5-ALA) Supplement Enhances PSII Photochemical Activity and Antioxidant Activity in the Late Growth Promotion of Pseudostellaria heterophylla. Plants 2022, 11, 3035. [Google Scholar] [CrossRef]
- Zhou, Y.M.; Han, S.J.; Hu, Y.L.; Zhang, H.-S.; Zheng, J. Effects of elevated CO2 concentration on photosynthetic parameters of Pinus koraiensis needles. Acta Ecol. Sin. 2008, 28, 423–429. [Google Scholar]
- Huang, H.; Li, S.; Guo, J.L. Effects of cadmium stress on antioxidant system and photosynthesis of maize seedlings. J. Agro-Environ. Sci. 2010, 29, 211–215. [Google Scholar]
- Li, X.W.; Chen, S.L. Effect of shading on photosynthetic characteristics and chlorophyll fluorescence parameters in leaves of Fritillaria cirrhosa. Acta Ecol. Sin. 2008, 28, 3438–3445. [Google Scholar]
- Fen, D.L.; Liu, Y. Photosynthesis and chlorophyⅡfluorescence parameters of the reed (Phragmites communis)grown in the hydro-fluctuation belt of Three Gorges Reservoir Area. Acta Ecol. Sin. 2008, 28, 2013–2021. [Google Scholar]
- Ayyaz, A.; Amir, M.; Umer, S.; Iqbal, M.; Bano, H.; Gul, H.S.; Farooq, M.A. Melatonin induced changes in photosynthetic efficiency as probed by OJIP associated with improved chromium stress tolerance in canola (Brassica napus L.). Heliyon 2020, 6, e04364. [Google Scholar] [CrossRef]
- Hoque, M.N.; Tahjib-Ul-Arif, M.; Hannan, A.; Sultana, N.; Akhter, S.; Hasanuzzaman, M.; Akter, F.; Hossain, M.S.; Sayed, M.A.; Hasan, M.T.; et al. Melatonin Modulates Plant Tolerance to Heavy Metal Stress: Morphological Responses to Molecular Mechanisms. Int. J. Mol. Sci. 2021, 22, 11445. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Li, Y.M.; Li, J.W.; Teng, R.M.; Chen, Y.; Wang, Y.H.; Zhuang, J. Effects of Exogenous 5-Aminolevulinic acid on the Chlorophyll Synthesis and Fluorescence Characteristics and Gene Expression of Key Enzymes in Tea Plants under Drought Stress. Tea Sci. 2022, 42, 187–199. (In Chinese) [Google Scholar]
- Bussotti, F.; Gerosa, G.; Digrado, A.; Pollastrini, M. Selection of chlorophyll fluorescence parameters as indicators of photosynthetic efficiency in large scale plant ecological studies. Ecol. Indic. 2020, 108, 105686. [Google Scholar] [CrossRef]
- Li, Y.T.; Xu, W.W.; Ren, B.Z.; Zhao, B.; Wang, J.W.; Liu, P.; Zhang, Z.S. High temperature reduces photosynthesis in maize leaves by damaging chloroplast ultrastructure and photosystem II. J. Agron. Crop Sci. 2020, 206, 548–564. [Google Scholar] [CrossRef]
- Yin, C.Y.; Berninger, F.; Li, C.Y. Photosynthetic responses of Populus przewalski subjected to drought stress. Photosynthetica 2006, 44, 62–68. [Google Scholar] [CrossRef]
- Garnier, E.; Shipley, B.; Roumet, C.; Laurent, G. Standardized protocol for the determination of specific leaf area and leaf dry matter content. Funct. Ecol. 2001, 15, 688–695. [Google Scholar] [CrossRef]
- Cornelissen, J.H.C.; Diez, P.C.; Hunt, R. Seedling growth, allocation and leaf attributes in a wide range of woody plant species and types. J. Ecol. 1996, 84, 755–765. [Google Scholar] [CrossRef]
- Wright, I.J.; Wearoby, M. Cross-species relationship between seedling relative growth rate, nitrogen productivity and root vs. leaf function in 28 Australian woody species. Funct. Ecol. 2000, 14, 97–107. [Google Scholar] [CrossRef]
- Penning, F.W.T.; Zhu, D.F.; Cheng, S.H. Simulation of Ecophysiological Processes of Growth in Several Annual Crops; Chinese Agricultural Science and Technology Press: Beijing, China, 1991; pp. 34–36. [Google Scholar]
- Zhao, C.G. The relationship between root/shoot ratio and quality in tobacco. Sci. Tech. Infor. 2006, 18, 133. [Google Scholar]
- Maxwell, K.; Johnson, G.N. Chlorohpyll fluorescence: A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Appenroth, K.J.; Stöckel, J.; Srivastava, A.; Strasser, R.J. Multiple effects of chromate on the photosynthetic apparatus of Spirodela polyrhiza as probed by OJIP chlorophyII a fluorescence measurement. Environ. Pollut. 2001, 115, 49–64. [Google Scholar] [CrossRef]
- Yuan, Z.L.; Li, C.M.; Xiong, S.P.; Li, H.; Zhang, G.L.; Ma, X.M. Effect of Cd and Pb pollution on chlorophyll content, activity of protectiase and cell membrance lipid peroxidation change in tobacco leaves. J. Henan Agric.Univ. 2005, 39, 15–19. [Google Scholar]
- Hura, T.; Hura, K.; Ostrowska, A.; Dziurka, K. Rapid plant rehydration initiates permanent and adverse changes in the photosynthetic apparatus of triticale. Plant Soil 2015, 397, 127–145. [Google Scholar] [CrossRef]
- Hu, W.H.; Yan, X.H.; Li, X.H.; Cao, Z.G. Effect of 24-Epibrassionolide on the ChlorophyII Fluorescence Triansient in leaves of Pepper under drought stress. Bull. Bot. Res. 2021, 41, 53–59. [Google Scholar]
- Fghire, R.; Anaya, F.; Ali, O.I.; Benlhabib, O.; Ragab, R.; Wahbi, S. Physiological and photosynthetic response of quinoa to drought stress. Chil. J. Agric. Res. 2015, 75, 174–183. [Google Scholar] [CrossRef]
- Zhang, J.; Li, T.L. Effects of daytime sub-high temperature on photosynthesis and chloroplast ultrastructure of tomato leaves in green house. Acta Hortic. Sin. 2005, 32, 614–619. [Google Scholar]
- Ralph, P.J.; Gademann, R. Rapid light curves: A powerful tool to assess photosynthetic activity. Aquat. Bot. 2005, 82, 222–237. [Google Scholar] [CrossRef]
- Da-Matta, F.M.; Maestri, M.; Mosquim, P.R.; Barros, R.S. Photosynthesis in coffee (Coffea arabica and C. canephora) as affected by winter and summer conditions. Plant Sci. 1997, 128, 43–50. [Google Scholar] [CrossRef]
- Zhu, H.; Teng, Y.; Wang, X.; Zhao, L.; Ren, W.; Luo, Y.; Christie, P. Changes in clover rhizosphere microbial community and diazotrophs in mercury-contaminated soils. Sci. Total Environ. 2021, 767, 145473. [Google Scholar] [CrossRef]
- Karavatas, S.; Manetas, Y. Seasonal patterns of photosystemⅡphotochemical efficiency in evergreen sclerophylls and drought semi-deciduous shrubs under Mediterranean field conditions. Photosynthetica 1999, 36, 41–49. [Google Scholar] [CrossRef]
- Lima, J.D.; Mosquim, P.R.; Damattta, F.M. Leaf gas exchange and chlorophyll fluorescence parameters in Phaseolus vulgaris as affected by nitrogen and phosphorus deficiency. Photosynthetica 1999, 37, 113–121. [Google Scholar] [CrossRef]
- Du, Y.L.; Zhao, Q.; Chen, L.W.; Yao, X.D.; Zhang, W.; Zhang, B.; Xie, F.T. Effect of drought stress on sugar metabolism in leaves and roots of soybean seedlings. Plant Physiol. Biochem. 2020, 146, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.Q.; Huang, Z.R.; Huang, W.L.; Huang, W.T.; Chen, H.H.; Yang, L.T.; Ye, X.; Chen, L.S. Physiological and molecular adaptations of Citrus grandis roots to long-term copper excess revealed by physiology, metabolome and transcriptome. Environ. Exp. Bot. 2022, 203, 105049. [Google Scholar] [CrossRef]


| Soil Sample Point | pH | Organic C (g·kg−1) | Available P (mg·kg−1) | Available N (mg·kg−1) | Heavy Metal Contents (mg·kg−1) | |||
|---|---|---|---|---|---|---|---|---|
| Zn | Pb | Cu | Cd | |||||
| Dabaoshan Mining Area | 6.2 | 14.7 ± 0.9 b | 32.2 ± 2.0 b | 30.2 ± 1.9 b | 1768.7 ± 91.1 a | 1253.3 ± 71.3 a | 1701.3 ± 77.5 a | 9.1 ± 0.9 a |
| Boluo County | 6.6 | 13.8 ± 0.9 b | 26.6 ± 1.8 b | 28.4 ± 2.7 b | 135.2 ± 13.1 b | 242.6 ± 44.1 b | 48.4 ± 9.5 b | 1.1 ± 0.2 b |
| Population | Pb Treatment (mg·L−1) | Plant Height (cm) | Plant Biomass (g) | Specific Leaf Area (cm2·mg−1) | Root-Shoot Ratio |
|---|---|---|---|---|---|
| Non-mining population | 0 | 41.1 ± 3.15 ab | 4.25 ± 0.20 a | 193.3 ± 3.1 a | 0.40 ± 0.07 ef |
| 20 | 32.1 ± 1.00 c | 3.28 ± 0.02 bc | 181.8 ± 8.1 ab | 0.41 ± 0.04 def | |
| 40 | 26.0 ± 1.45 de | 2.62 ± 0.34 de | 172.3 ± 9.1 bc | 0.45 ± 0.03 cde | |
| 80 | 20.1 ± 2.15 f | 2.32 ± 0.04 e | 152.5 ± 9.5 d | 0.49 ± 0.08 bgd | |
| 120 | 16.2 ± 1.76 g | 1.69 ± 0.42 fg | 143.4 ± 20.2 d | 0.55 ± 0.03 ab | |
| 240 | 13.0 ± 1.95 g | 1.25 ± 0.19 g | 113.4 ± 10.6 e | 0.61 ± 0.03 a | |
| Mining population | 0 | 40.8 ± 1.85 ab | 4.22 ± 0.31 a | 191.8 ± 8.2 a | 0.39 ± 0.04 ef |
| 20 | 42.0 ± 1.95 a | 4.32 ± 0.22 a | 196.7 ± 5.0 a | 0.37 ± 0.02 f | |
| 40 | 38.1 ± 1.45 b | 3.65 ± 0.59 b | 183.7 ± 9.1 ab | 0.42 ± 0.01 def | |
| 80 | 29.0 ± 1.95 cd | 2.97 ± 0.27 cd | 172.1 ± 9.9 bc | 0.45 ± 0.04 def | |
| 120 | 23.1 ± 1.00 ef | 2.31 ± 0.20 e | 162.3 ± 9.6 cd | 0.48 ± 0.08 bcd | |
| 240 | 21.0 ± 2.55 f | 2.15 ± 0.07 ef | 145.5 ± 14.2 d | 0.51 ± 0.02 bc |
| Population | Pb Treatment (mg·L−1) | Chl-a Content (mg·g−1 FW) | Chl-b Content (mg·g−1 FW) | Chl a/b Value (mg·g−1) | Car Content (mg·g−1 FW) |
|---|---|---|---|---|---|
| Non-mining population | 0 | 1.31 ± 0.010 a | 0.75 ± 0.048 a | 1.74 ± 0.155 d | 0.43 ± 0.005 a |
| 20 | 1.25 ± 0.013 ab | 0.68 ± 0.055 a | 1.84 ± 0.091 cd | 0.41 ± 0.006 a | |
| 40 | 1.23 ± 0.032 b | 0.52 ± 0.102 b | 2.36 ± 0.472 bcd | 0.39 ± 0.029 b | |
| 80 | 1.11 ± 0.015 c | 0.43 ± 0.041 b | 2.58 ± 0.185 bc | 0.39 ± 0.025 b | |
| 120 | 1.01 ± 0.071 d | 0.29 ± 0.081 c | 3.48 ± 0.523 a | 0.34 ± 0.026 c | |
| 240 | 0.76 ± 0.017 e | 0.25 ± 0.006 c | 3.04 ± 0.663 ab | 0.31 ± 0.033 c | |
| Mining population | 0 | 1.26 ± 0.026 a | 0.69 ± 0.009 a | 1.82 ± 0.319 c | 0.39 ± 0.015 ab |
| 20 | 1.29 ± 0.011 a | 0.72 ± 0.003 a | 1.78 ± 0.061 c | 0.41 ± 0.006 a | |
| 40 | 1.28 ± 0.010 a | 0.75 ± 0.018 a | 1.71 ± 0.063 c | 0.40 ± 0.006 ab | |
| 80 | 1.20 ± 0.020 b | 0.57 ± 0.044 b | 2.11 ± 0.179 bc | 0.38 ± 0.011 ab | |
| 120 | 1.18 ± 0.011 b | 0.51 ± 0.081 bc | 2.31 ± 0.603 ab | 0.36 ± 0.044 ab | |
| 240 | 1.15 ± 0.008 c | 0.45 ± 0.005 c | 2.56 ± 0.075 a | 0.36 ± 0.006 b |
| Type | Item | Pb | Biomass | Pn | Gs | Ci | Tr | Fv/Fm | Fv/F0 | qP | NPQ | ETR | Chla |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-mining population | Pb | 1 | |||||||||||
| Biomass | −0.868 ** | 1 | |||||||||||
| Pn | −0.818 ** | 0.936 ** | 1 | ||||||||||
| Gs | −0.777 ** | 0.916 ** | 0.897 ** | 1 | |||||||||
| Ci | −0.820 ** | 0.921 ** | 0.900 ** | 0.839 ** | 1 | ||||||||
| Tr | −0.779 ** | 0.917 ** | 0.980 ** | 0.877 ** | 0.865 ** | 1 | |||||||
| Fv/Fm | −0.711 ** | 0.890 ** | 0.778 ** | 0.812 ** | 0.750 ** | 0.774 ** | 1 | ||||||
| Fv/F0 | −0.846 ** | 0.841 ** | 0.873 ** | 0.788 ** | 0.802 ** | 0.811 ** | 0.701 ** | 1 | |||||
| qP | −0.750 ** | 0.862 ** | 0.773 ** | 0.786 ** | 0.863 ** | 0.702 ** | 0.728 ** | 0.736 ** | 1 | ||||
| NPQ | 0.352 | −0.397 | −0.439 | −0.333 | −0.439 | −0.356 | −0.264 | −0.449 | −0.450 | 1 | |||
| ETR | −0.885 ** | 0.893 ** | 0.813 ** | 0.839 ** | 0.801 ** | 0.770 ** | 0.816 ** | 0.819 ** | 0.857 ** | −0.290 | 1 | ||
| Chla | −0.986 ** | 0.877 ** | 0.819 ** | 0.773 ** | 0.832 ** | 0.775 ** | 0.750 ** | 0.847 ** | 0.759 ** | −0.375 | 0.878 ** | 1 | |
| Mining population | Pb | 1 | |||||||||||
| Biomass | −0.860 ** | 1 | |||||||||||
| Pn | −0.866 ** | 0.856 ** | 1 | ||||||||||
| Gs | −0.881 ** | 0.891 ** | 0.861 ** | 1 | |||||||||
| Ci | −0.686 ** | 0.745 ** | 0.699 ** | 0.685 ** | 1 | ||||||||
| Tr | −0.902 ** | 0.944 ** | 0.873 ** | 0.935 ** | 0.716 ** | 1 | |||||||
| Fv/Fm | −0.456 | 0.665 ** | 0.471 * | 0.716 ** | 0.278 | 0.699 ** | 1 | ||||||
| Fv/F0 | −0.565 * | 0.545 * | 0.405 | 0.519 * | 0.288 | 0.548 * | 0.680 ** | 1 | |||||
| qP | −0.651 ** | 0.705 ** | 0.771 ** | 0.744 ** | 0.491 * | 0.733 ** | 0.320 | 0.103 | 1 | ||||
| NPQ | 0.653 ** | −0.664 ** | −0.460 | −0.679 ** | −0.261 | −0.582 * | −0.796 ** | −0.604 ** | −0.292 | 1 | |||
| ETR | −0.684 ** | 0.716 ** | 0.695 ** | 0.616 ** | 0.432 | 0.710 ** | 0.615 ** | 0.617 ** | 0.376 | −0.538 * | 1 | ||
| Chla | −0.861 ** | 0.904 ** | 0.833 ** | 0.795 ** | 0.771 ** | 0.829 ** | 0.524 * | 0.412 | 0.669 ** | −0.588 * | 0.637 ** | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, J.; Jiang, X.; Qin, J.; Zhao, H.; Dai, M.; Liu, H.; Chen, X. Effects of Lead Pollution on Photosynthetic Characteristics and Chlorophyll Fluorescence Parameters of Different Populations of Miscanthus floridulus. Processes 2023, 11, 1562. https://doi.org/10.3390/pr11051562
Qin J, Jiang X, Qin J, Zhao H, Dai M, Liu H, Chen X. Effects of Lead Pollution on Photosynthetic Characteristics and Chlorophyll Fluorescence Parameters of Different Populations of Miscanthus floridulus. Processes. 2023; 11(5):1562. https://doi.org/10.3390/pr11051562
Chicago/Turabian StyleQin, Jianqiao, Xueding Jiang, Jianhua Qin, Huarong Zhao, Min Dai, Hao Liu, and Xi Chen. 2023. "Effects of Lead Pollution on Photosynthetic Characteristics and Chlorophyll Fluorescence Parameters of Different Populations of Miscanthus floridulus" Processes 11, no. 5: 1562. https://doi.org/10.3390/pr11051562






