Advancing Process Intensification with High-Frequency Ultrasound: A Mini-Review of Applications in Biofuel Production and Beyond
Abstract
:1. Introduction
| Low-Frequency Ultrasound | Characteristics | High-Frequency Ultrasound |
|---|---|---|
| 20–100 kHz [2] | Frequency Range | 1000–10,000 kHz [2] |
| Production of larger bubbles [20] | Cavitation Bubbles Size | Production of smaller bubbles [21] |
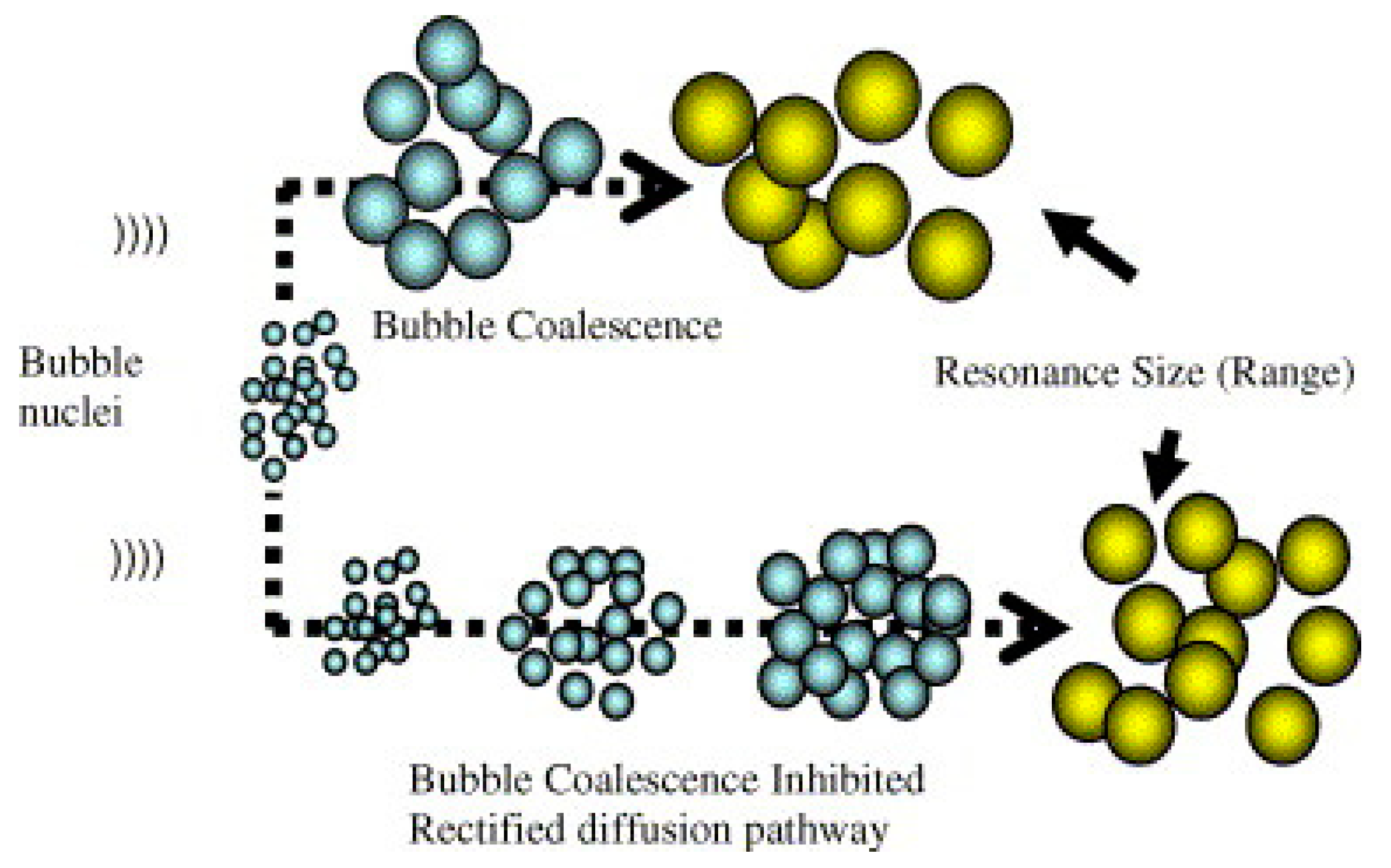
| Lesser [22] | Quantity of Bubbles | Higher [22] |
| Higher intensity [20] | Bubble Collapse Intensity | Lower intensity [20] |
| Lesser free radical formed [26] | Amount of Free Radical Formation | Higher free radical formed [26] |
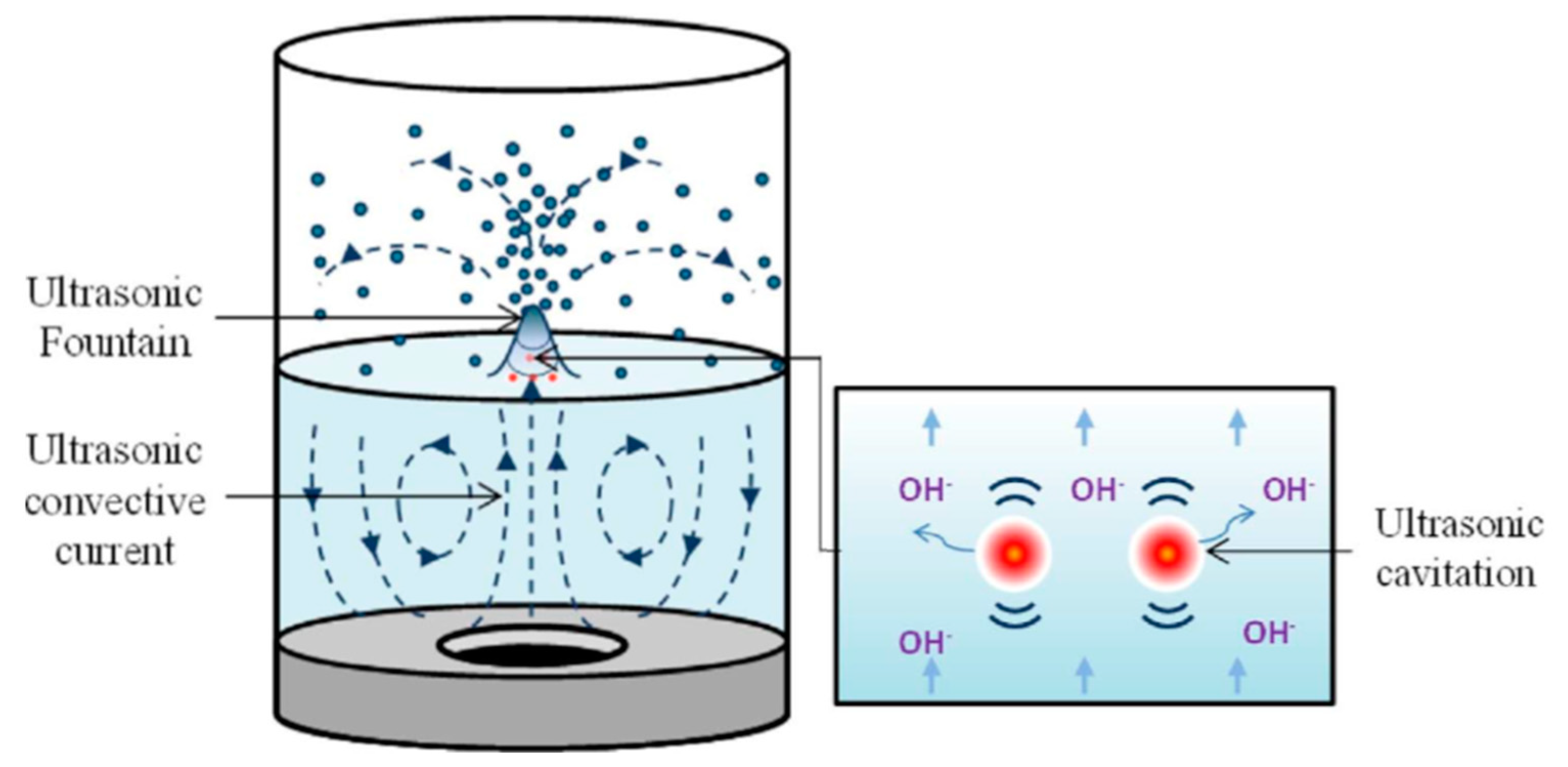
| None or weak fountain formation [27] | Formation of Fountain (Figure 3) | Yes [28] |
2. High-Frequency Ultrasound Technology for Biofuel Production
2.1. High-Frequency Ultrasound-Assisted Transesterification Process for Biodiesel Production
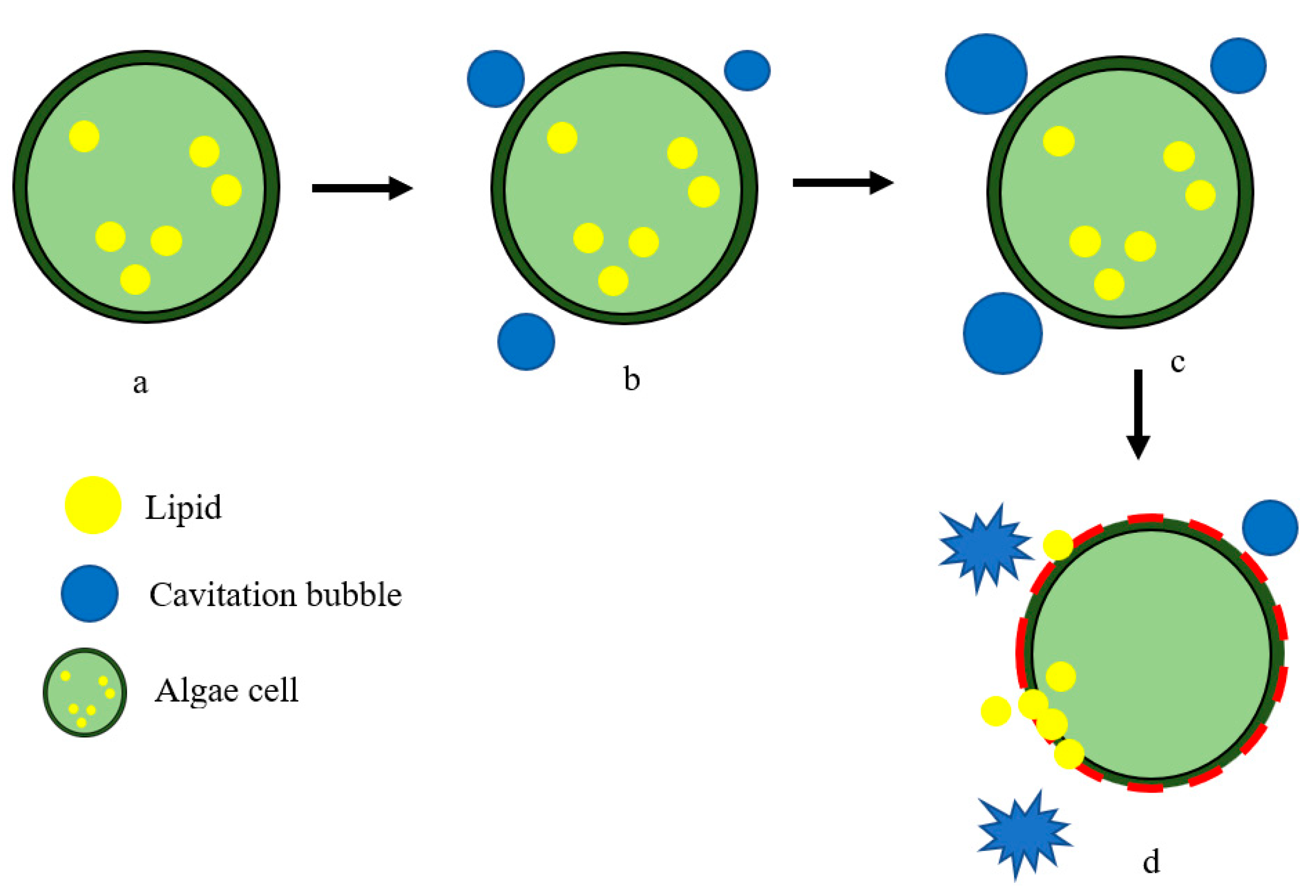
2.2. High-Frequency Ultrasound on Microalgal Cell Disruption for Biofuel Production
3. Other Applications of High-Frequency Ultrasound
3.1. Chemical Absorption of CO2
3.2. Inactivation of Harmful Microorganisms in Water
4. Challenges to Be Addressed in Scaling up HFU for Biofuel Production
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vernès, L.; Vian, M.; Chemat, F. Chapter 12—Ultrasound and Microwave as Green Tools for Solid-Liquid Extraction. In Liquid-Phase Extraction; Poole, C.F., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 355–374. [Google Scholar]
- Chuah, L.F.; Klemeš, J.J.; Yusup, S.; Bokhari, A.; Akbar, M.M. A review of cleaner intensification technologies in biodiesel production. J. Clean. Prod. 2017, 146, 181–193. [Google Scholar] [CrossRef]
- Tay, W.H.; Lau, K.K.; Shariff, A.M. High performance promoter-free CO2 absorption using potassium carbonate solution in an ultrasonic irradiation system. J. CO2 Util. 2017, 21, 383–394. [Google Scholar] [CrossRef]
- Yusof, S.M.M.; Shariff, A.M.; Tay, W.H.; Lau, K.K.; Mustafa, N.F.A. Mass transfer intensification of CO2 absorption in monoethanolamine using high frequency ultrasonic technology in continuous system. Int. J. Greenh. Gas Control 2020, 102, 103157. [Google Scholar] [CrossRef]
- Shokrollahi, F.; Lau, K.K.; Tay, W.H. Performance comparison of ultrasonic-assisted and magnetic stirred absorption methods for CO2 separation. SN Appl. Sci. 2020, 2, 1217. [Google Scholar] [CrossRef]
- Tan, S.X.; Lim, S.; Ong, H.C.; Pang, Y.L. State of the art review on development of ultrasound-assisted catalytic transesterification process for biodiesel production. Fuel 2019, 235, 886–907. [Google Scholar] [CrossRef]
- Mohammadi, P.; Karami, N.; Zinatizadeh, A.A.; Falahi, F.; Aghamohammadi, N.; Almasi, A. Using high frequency and low-intensity ultrasound to enhance activated sludge characteristics. Ultrason. Sonochem. 2019, 54, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Mason, T.J. Sonochemistry and sonoprocessing: The link, the trends and (probably) the future. Ultrason. Sonochem. 2003, 10, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yuan, W.; Jiang, X.; Jing, Y.; Wang, Z. Disruption of microalgal cells using high-frequency focused ultrasound. Bioresour. Technol. 2014, 153, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Somnuk, K.; Phanyusoh, D.; Thawornprasert, J.; Oo, Y.M.; Prateepchaikul, G. Continuous Ultrasound-Assisted Esterification and Transesterification of Palm Fatty Acid Distillate for Ethyl Ester Production. Processes 2021, 9, 449. [Google Scholar] [CrossRef]
- Yang, F.; Shi, C.; Yan, L.; Xu, Y.; Dai, Y.; Bi, S.; Liu, Y. Low-frequency ultrasonic treatment: A potential strategy to improve the flavor of fresh watermelon juice. Ultrason. Sonochem. 2022, 91, 106238. [Google Scholar] [CrossRef]
- Miladi, M.; Martins, A.A.; Mata, T.M.; Vegara, M.; Pérez-Infantes, M.; Remmani, R.; Ruiz-Canales, A.; Núñez-Gómez, D. Optimization of Ultrasound-Assisted Extraction of Spent Coffee Grounds Oil Using Response Surface Methodology. Processes 2021, 9, 2085. [Google Scholar] [CrossRef]
- Kasaai, M.R. Input power-mechanism relationship for ultrasonic irradiation: Food and polymer applications. Nat. Sci. 2013, 5, 14–22. [Google Scholar] [CrossRef]
- Thanh Nguyen, T.; Asakura, Y.; Koda, S.; Yasuda, K. Dependence of cavitation, chemical effect, and mechanical effect thresholds on ultrasonic frequency. Ultrason. Sonochem. 2017, 39, 301–306. [Google Scholar] [CrossRef]
- Martinez-Guerra, E.; Gude, V.G. Continuous and pulse sonication effects on transesterification of used vegetable oil. Energy Convers. Manag. 2015, 96, 268–276. [Google Scholar] [CrossRef]
- Asakura, Y.; Yasuda, K. Frequency and power dependence of the sonochemical reaction. Ultrason. Sonochem. 2021, 81, 105858. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.C.; Sapozhnikov, O.A.; Khokhlova, V.A.; Crum, L.A.; Bailey, M.R. Ultrasonic atomization of liquids in drop-chain acoustic fountains. J. Fluid Mech. 2015, 766, 129–146. [Google Scholar] [CrossRef]
- Mahamuni, N.N.; Adewuyi, Y.G. Optimization of the Synthesis of Biodiesel via Ultrasound-Enhanced Base-Catalyzed Transesterification of Soybean Oil Using a Multifrequency Ultrasonic Reactor. Energy Fuels 2009, 23, 2757–2766. [Google Scholar] [CrossRef]
- Reuter, F.; Lesnik, S.; Ayaz-Bustami, K.; Brenner, G.; Mettin, R. Bubble size measurements in different acoustic cavitation structures: Filaments, clusters, and the acoustically cavitated jet. Ultrason. Sonochem. 2019, 55, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Tiehm, A.; Nickel, K.; Zellhorn, M.; Neis, U. Ultrasonic waste activated sludge disintegration for improving anaerobic stabilization. Water Res. 2001, 35, 2003–2009. [Google Scholar] [CrossRef]
- Wayment, D.G.; Casadonte, D.J. Frequency effect on the sonochemical remediation of alachlor. Ultrason. Sonochem. 2002, 9, 251–257. [Google Scholar] [CrossRef]
- Bhangu, S.K.; Gupta, S.; Ashokkumar, M. Ultrasonic enhancement of lipase-catalysed transesterification for biodiesel synthesis. Ultrason. Sonochem. 2017, 34, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Ashokkumar, M.; Lee, J.; Kentish, S.; Grieser, F. Bubbles in an acoustic field: An overview. Ultrason. Sonochem. 2007, 14, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Loh, B.G.; Hyun, S.; Ro, P.I.; Kleinstreuer, C. Acoustic streaming induced by ultrasonic flexural vibrations and associated enhancement of convective heat transfer. J. Acoust. Soc. Am. 2002, 111, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Naji, O.; Al-juboori, R.A.; Khan, A.; Yadav, S.; Altaee, A.; Alpatova, A.; Soukane, S.; Ghaffour, N. Ultrasound-assisted membrane technologies for fouling control and performance improvement: A review. J. Water Process Eng. 2021, 43, 102268. [Google Scholar] [CrossRef]
- Crum, L.A. Comments on the evolving field of sonochemistry by a cavitation physicist. Ultrason. Sonochem. 1995, 2, S147–S152. [Google Scholar] [CrossRef]
- Kim, G.; Cheng, S.; Hong, L.; Kim, J.T.; Li, K.C.; Chamorro, L.P. On the acoustic fountain types and flow induced with focused ultrasound. J. Fluid Mech. 2021, 909, R2. [Google Scholar] [CrossRef]
- Gondrexon, N.; Renaudin, V.; Petrier, C.; Clement, M.; Boldo, P.; Gonthier, Y.; Bernis, A. Experimental study of the hydrodynamic behaviour of a high frequency ultrasonic reactor. Ultrason. Sonochem. 1998, 5, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Schueller, B.S.; Yang, R.T. Ultrasound enhanced adsorption and desorption of phenol on activated carbon and polymeric resin. Ind. Eng. Chem. Res. 2001, 40, 4912–4918. [Google Scholar] [CrossRef]
- Degfie, T.A.; Mamo, T.T.; Mekonnen, Y.S. Optimized Biodiesel Production from Waste Cooking Oil (WCO) using Calcium Oxide (CaO) Nano-catalyst. Sci. Rep. 2019, 9, 18982. [Google Scholar] [CrossRef]
- Oliveira, P.A.; Baesso, R.M.; Morais, G.C.; Alvarenga, A.V.; Costa-Félix, R.P.B. Ultrasound-assisted transesterification of soybean oil using low power and high frequency and no external heating source. Ultrason. Sonochem. 2021, 78, 105709. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.; Abedin, M.Z.; Amin, M.B.; Nekmahmud, M.; Oláh, J. Sustainable biofuel economy: A mapping through bibliometric research. J. Environ. Manag. 2023, 336, 117644. [Google Scholar] [CrossRef] [PubMed]
- Callegari, A.; Bolognesi, S.; Cecconet, D.; Capodaglio, A.G. Production technologies, current role, and future prospects of biofuels feedstocks: A state-of-the-art review. Crit. Rev. Environ. Sci. Technol. 2020, 50, 384–436. [Google Scholar] [CrossRef]
- Atabani, A.E.; Silitonga, A.S.; Badruddin, I.A.; Mahlia, T.M.I.; Masjuki, H.H.; Mekhilef, S. A comprehensive review on biodiesel as an alternative energy resource and its characteristics. Renew. Sustain. Energy Rev. 2012, 16, 2070–2093. [Google Scholar] [CrossRef]
- Aghbashlo, M.; Tabatabaei, M.; Hosseinpour, S.; Hosseini, S.S.; Ghaffari, A.; Khounani, Z.; Mohammadi, P. Development and evaluation of a novel low power, high frequency piezoelectric-based ultrasonic reactor for intensifying the transesterification reaction. Biofuel Res. J. 2016, 3, 528–535. [Google Scholar] [CrossRef]
- Demirbas, A. Biofuels sources, biofuel policy, biofuel economy and global biofuel projections. Energy Convers. Manag. 2008, 49, 2106–2116. [Google Scholar] [CrossRef]
- Raboni, M.; Viotti, P.; Capodaglio, A.G. A comprehensive analysis of the current and future role of biofuels for transport in the European union (EU). Rev. Ambiente Agua 2015, 10, 9–21. [Google Scholar] [CrossRef]
- Saengsawang, B.; Bhuyar, P.; Manmai, N.; Ponnusamy, V.K.; Ramaraj, R.; Unpaprom, Y. The optimization of oil extraction from macroalgae, Rhizoclonium sp. by chemical methods for efficient conversion into biodiesel. Fuel 2020, 274, 117841. [Google Scholar] [CrossRef]
- Velasquez-Orta, S.B.; Lee, J.G.M.; Harvey, A. Alkaline in situ transesterification of Chlorella vulgaris. Fuel 2012, 94, 544–550. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, S.; Wang, Y.; Adhikari, S.; Dempster, T.A.; Wang, Y. Direct biodiesel production from wet microalgae assisted by radio frequency heating. Fuel 2019, 256, 115994. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Yoo, C.; Jun, S.-Y.; Ahn, C.-Y.; Oh, H.-M. Comparison of several methods for effective lipid extraction from microalgae. Bioresour. Technol. 2010, 101, S75–S77. [Google Scholar] [CrossRef] [PubMed]
- Bargole, S.S.; Singh, P.K.; George, S.; Saharan, V.K. Valorisation of low fatty acid content waste cooking oil into biodiesel through transesterification using a basic heterogeneous calcium-based catalyst. Biomass Bioenergy 2021, 146, 105984. [Google Scholar] [CrossRef]
- Gupta, A.R.; Yadav, S.V.; Rathod, V.K. Enhancement in biodiesel production using waste cooking oil and calcium diglyceroxide as a heterogeneous catalyst in presence of ultrasound. Fuel 2015, 158, 800–806. [Google Scholar] [CrossRef]
- Shobhana, G.; Asikin-Mijan, N.; AbdulKareem-Alsultan, G.; Sivasangar, S.; Izham, S.M.; Taufiq-Yap, Y.H. Biodiesel production via simultaneous esterification and transesterification of chicken fat oil by mesoporous sulfated Ce supported activated carbon. Biomass Bioenergy 2020, 141, 105714. [Google Scholar] [CrossRef]
- El-Shafay, A.S.; Ağbulut, Ü.; Attia, E.-A.; Touileb, K.L.; Gad, M.S. Waste to energy: Production of poultry-based fat biodiesel and experimental assessment of its usability on engine behaviors. Energy 2023, 262, 125457. [Google Scholar] [CrossRef]
- Costa, E.; Almeida, M.F.; Alvim-Ferraz, M.C.; Dias, J.M. Exploiting the Complementary Potential of Rice Bran Oil as a Low-Cost Raw Material for Bioenergy Production. Processes 2022, 10, 2460. [Google Scholar] [CrossRef]
- Rao, T.; Rao, G.; Reddy, K. Experimental Investigation of Pongamia, Jatropha and Neem Methyl Esters as Biodiesel on C.I. Engine. Jordan J. Mech. Ind. Eng. 2008, 2, 117–122. [Google Scholar]
- Li, J.; Fu, Y.-J.; Qu, X.-J.; Wang, W.; Luo, M.; Zhao, C.-J.; Zu, Y.-G. Biodiesel production from yellow horn (Xanthoceras sorbifolia Bunge.) seed oil using ion exchange resin as heterogeneous catalyst. Bioresour. Technol. 2012, 108, 112–118. [Google Scholar] [CrossRef]
- Georgogianni, K.G.; Kontominas, M.G.; Pomonis, P.J.; Avlonitis, D.; Gergis, V. Conventional and in situ transesterification of sunflower seed oil for the production of biodiesel. Fuel Process. Technol. 2008, 89, 503–509. [Google Scholar] [CrossRef]
- Vital-López, L.; Mercader-Trejo, F.; Rodríguez-Reséndiz, J.; Zamora-Antuñano, M.A.; Rodríguez-López, A.; Esquerre-Verastegui, J.E.; Farrera Vázquez, N.; García-García, R. Electrochemical Characterization of Biodiesel from Sunflower Oil Produced by Homogeneous Catalysis and Ultrasound. Processes 2023, 11, 94. [Google Scholar] [CrossRef]
- Mahbub, N.; Gemechu, E.; Zhang, H.; Kumar, A. The life cycle greenhouse gas emission benefits from alternative uses of biofuel coproducts. Sustain. Energy Technol. Assess. 2019, 34, 173–186. [Google Scholar] [CrossRef]
- Xue, J.; Grift, T.E.; Hansen, A.C. Effect of biodiesel on engine performances and emissions. Renew. Sustain. Energy Rev. 2011, 15, 1098–1116. [Google Scholar] [CrossRef]
- Musthafa, M.M.; Kumar, T.A.; Mohanraj, T.; Chandramouli, R. A comparative study on performance, combustion and emission characteristics of diesel engine fuelled by biodiesel blends with and without an additive. Fuel 2018, 225, 343–348. [Google Scholar] [CrossRef]
- Silitonga, A.S.; Shamsuddin, A.H.; Mahlia, T.M.I.; Milano, J.; Kusumo, F.; Siswantoro, J.; Dharma, S.; Sebayang, A.H.; Masjuki, H.H.; Ong, H.C. Biodiesel synthesis from Ceiba pentandra oil by microwave irradiation-assisted transesterification: ELM modeling and optimization. Renew. Energy 2020, 146, 1278–1291. [Google Scholar] [CrossRef]
- Demirbaş, A. Biodiesel fuels from vegetable oils via catalytic and non-catalytic supercritical alcohol transesterifications and other methods: A survey. Energy Convers. Manag. 2003, 44, 2093–2109. [Google Scholar] [CrossRef]
- Babadi, A.A.; Rahmati, S.; Fakhlaei, R.; Barati, B.; Wang, S.; Doherty, W.; Ostrikov, K. Emerging technologies for biodiesel production: Processes, challenges, and opportunities. Biomass Bioenergy 2022, 163, 106521. [Google Scholar] [CrossRef]
- Mishra, V.K.; Goswami, R. A review of production, properties and advantages of biodiesel. Biofuels 2018, 9, 273–289. [Google Scholar] [CrossRef]
- Karmakar, B.; Halder, G. Progress and future of biodiesel synthesis: Advancements in oil extraction and conversion technologies. Energy Convers. Manag. 2019, 182, 307–339. [Google Scholar] [CrossRef]
- Mahmudul, H.M.; Hagos, F.Y.; Mamat, R.; Adam, A.A.; Ishak, W.F.W.; Alenezi, R. Production, characterization and performance of biodiesel as an alternative fuel in diesel engines—A review. Renew. Sustain. Energy Rev. 2017, 72, 497–509. [Google Scholar] [CrossRef]
- Badday, A.S.; Abdullah, A.Z.; Lee, K.T.; Khayoon, M.S. Intensification of biodiesel production via ultrasonic-assisted process: A critical review on fundamentals and recent development. Renew. Sustain. Energy Rev. 2012, 16, 4574–4587. [Google Scholar] [CrossRef]
- Qiu, Z.; Zhao, L.; Weatherley, L. Process intensification technologies in continuous biodiesel production. Chem. Eng. Process. Process Intensif. 2010, 49, 323–330. [Google Scholar] [CrossRef]
- Hingu, S.M.; Gogate, P.R.; Rathod, V.K. Synthesis of biodiesel from waste cooking oil using sonochemical reactors. Ultrason. Sonochem. 2010, 17, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Badday, A.S.; Abdullah, A.Z.; Lee, K.-T. Ultrasound-assisted transesterification of crude Jatropha oil using cesium doped heteropolyacid catalyst: Interactions between process variables. Energy 2013, 60, 283–291. [Google Scholar] [CrossRef]
- Kumar, D.; Kumar, G.; Poonam; Singh, C.P. Ultrasonic-assisted transesterification of Jatropha curcus oil using solid catalyst, Na/SiO2. Ultrason. Sonochem. 2010, 17, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Mootabadi, H.; Salamatinia, B.; Bhatia, S.; Abdullah, A.Z. Ultrasonic-assisted biodiesel production process from palm oil using alkaline earth metal oxides as the heterogeneous catalysts. Fuel 2010, 89, 1818–1825. [Google Scholar] [CrossRef]
- Gallego-Juarez, J.; Graff, K.F. Power Ultrasonics: Applications of High-Intensity Ultrasound; Elsevier: Amsterdam, The Netherlands, 2014; pp. 1–1142. [Google Scholar]
- Ashokkumar, M.; Hodnett, M.; Zeqiri, B.; Grieser, F.; Price, G.J. Acoustic Emission Spectra from 515 kHz Cavitation in Aqueous Solutions Containing Surface-Active Solutes. J. Am. Chem. Soc. 2007, 129, 2250–2258. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.W.S.; Ng, H.K.; Gan, S. Advances in ultrasound-assisted transesterification for biodiesel production. Appl. Therm. Eng. 2016, 100, 553–563. [Google Scholar] [CrossRef]
- Veljković, V.B.; Avramović, J.M.; Stamenković, O.S. Biodiesel production by ultrasound-assisted transesterification: State of the art and the perspectives. Renew. Sustain. Energy Rev. 2012, 16, 1193–1209. [Google Scholar] [CrossRef]
- Bendicho, C.; Lavilla, I. EXTRACTION | Ultrasound Extractions. In Encyclopedia of Separation Science; Wilson, I.D., Ed.; Academic Press: Oxford, UK, 2000; pp. 1448–1454. [Google Scholar]
- Yin, Z.; Zhu, L.; Li, S.; Hu, T.; Chu, R.; Mo, F.; Hu, D.; Liu, C.; Li, B. A comprehensive review on cultivation and harvesting of microalgae for biodiesel production: Environmental pollution control and future directions. Bioresour. Technol. 2020, 301, 122804. [Google Scholar] [CrossRef]
- Demirbas, M.F. Biofuels from algae for sustainable development. Appl. Energy 2011, 88, 3473–3480. [Google Scholar] [CrossRef]
- Ajala, E.O.; Ajala, M.A.; Akinpelu, G.S.; Akubude, V.C. Cultivation and Processing of Microalgae for Its Sustainability as a Feedstock for Biodiesel Production. Niger. J. Technol. Dev. 2021, 18, 322–343. [Google Scholar] [CrossRef]
- Naveena, B.; Armshaw, P.; Tony Pembroke, J. Ultrasonic intensification as a tool for enhanced microbial biofuel yields. Biotechnol. Biofuels 2015, 8, 140. [Google Scholar] [CrossRef]
- Luo, J.; Fang, Z.; Smith, R.L. Ultrasound-enhanced conversion of biomass to biofuels. Prog. Energy Combust. Sci. 2014, 41, 56–93. [Google Scholar] [CrossRef]
- Singh, N.; Kumar, K.; Goyal, A.; Moholkar, V.S. Ultrasound-assisted biodiesel synthesis by in–situ transesterification of microalgal biomass: Optimization and kinetic analysis. Algal Res. 2022, 61, 102582. [Google Scholar] [CrossRef]
- Keris-Sen, U.D.; Sen, U.; Soydemir, G.; Gurol, M.D. An investigation of ultrasound effect on microalgal cell integrity and lipid extraction efficiency. Bioresour. Technol. 2014, 152, 407–413. [Google Scholar] [CrossRef]
- Yamamoto, K.; King, P.M.; Wu, X.; Mason, T.J.; Joyce, E.M. Effect of ultrasonic frequency and power on the disruption of algal cells. Ultrason. Sonochem. 2015, 24, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Adam, F.; Abert-Vian, M.; Peltier, G.; Chemat, F. “Solvent-free” ultrasound-assisted extraction of lipids from fresh microalgae cells: A green, clean and scalable process. Bioresour. Technol. 2012, 114, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.F.; Dias, A.P.S.; Silva, C.M.; Costa, M. Effect of low frequency ultrasound on microalgae solvent extraction: Analysis of products, energy consumption and emissions. Algal Res. 2016, 14, 9–16. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, S.; Tyagi, R.D.; Drogui, P.; Surampalli, R.Y. Ultrasonication assisted lipid extraction from oleaginous microorganisms. Bioresour. Technol. 2014, 158, 253–261. [Google Scholar] [CrossRef]
- Natarajan, R.; Ang, W.M.R.; Chen, X.; Voigtmann, M.; Lau, R. Lipid releasing characteristics of microalgae species through continuous ultrasonication. Bioresour. Technol. 2014, 158, 7–11. [Google Scholar] [CrossRef]
- Kurokawa, M.; King, P.M.; Wu, X.; Joyce, E.M.; Mason, T.J.; Yamamoto, K. Effect of sonication frequency on the disruption of algae. Ultrason. Sonochem. 2016, 31, 157–162. [Google Scholar] [CrossRef]
- Tay, W.H.; Lau, K.K.; Shariff, A.M. High frequency ultrasonic-assisted chemical absorption of CO2 using monoethanolamine (MEA). Sep. Purif. Technol. 2017, 183, 136–144. [Google Scholar] [CrossRef]
- Bhown, A.S.; Freeman, B.C. Analysis and Status of Post-Combustion Carbon Dioxide Capture Technologies. Environ. Sci. Technol. 2011, 45, 8624–8632. [Google Scholar] [CrossRef] [PubMed]
- Duraccio, V.; Gnoni, M.G.; Elia, V. Carbon capture and reuse in an industrial district: A technical and economic feasibility study. J. CO2 Util. 2015, 10, 23–29. [Google Scholar] [CrossRef]
- Baker, R.W.; Lokhandwala, K. Natural Gas Processing with Membranes: An Overview. Ind. Eng. Chem. Res. 2008, 47, 2109–2121. [Google Scholar] [CrossRef]
- Tan, L.S.; Shariff, A.M.; Lau, K.K.; Bustam, M.A. Factors affecting CO2 absorption efficiency in packed column: A review. J. Ind. Eng. Chem. 2012, 18, 1874–1883. [Google Scholar] [CrossRef]
- Rangwala, H.A. Absorption of carbon dioxide into aqueous solutions using hollow fiber membrane contactors. J. Membr. Sci. 1996, 112, 229–240. [Google Scholar] [CrossRef]
- Elhajj, J.; Al-Hindi, M.; Azizi, F. A Review of the Absorption and Desorption Processes of Carbon Dioxide in Water Systems. Ind. Eng. Chem. Res. 2014, 53, 2–22. [Google Scholar] [CrossRef]
- Luis, P.; Van Gerven, T.; Van der Bruggen, B. Recent developments in membrane-based technologies for CO2 capture. Prog. Energy Combust. Sci. 2012, 38, 419–448. [Google Scholar] [CrossRef]
- Tay, W.H.; Lau, K.K.; Shariff, A.M. High frequency ultrasonic-assisted CO2 absorption in a high pressure water batch system. Ultrason. Sonochem. 2016, 33, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Cheremisinoff, N.P.; Cheremisinoff, P.N. Water Treatment and Waste Recovery: Advanced Technology and Applications; Prentice-Hall, Inc.: Hoboken, NY, USA, 1993. [Google Scholar]
- Gao, S.; Hemar, Y.; Ashokkumar, M.; Paturel, S.; Lewis, G.D. Inactivation of bacteria and yeast using high-frequency ultrasound treatment. Water Res. 2014, 60, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Earnshaw, R.G.; Appleyard, J.; Hurst, R.M. Understanding physical inactivation processes: Combined preservation opportunities using heat, ultrasound and pressure. Int. J. Food Microbiol. 1995, 28, 197–219. [Google Scholar] [CrossRef] [PubMed]
- Butz, P.; Tauscher, B. Emerging technologies: Chemical aspects. Food Res. Int. 2002, 35, 279–284. [Google Scholar] [CrossRef]
- Drakopoulou, S.; Terzakis, S.; Fountoulakis, M.S.; Mantzavinos, D.; Manios, T. Ultrasound-induced inactivation of gram-negative and gram-positive bacteria in secondary treated municipal wastewater. Ultrason. Sonochem. 2009, 16, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Olvera, M.; Eguía, A.; Rodríguez, O.; Chong, E.; Pillai, S.D.; Ilangovan, K. Inactivation of Cryptosporidium parvum oocysts in water using ultrasonic treatment. Bioresour. Technol. 2008, 99, 2046–2049. [Google Scholar] [CrossRef] [PubMed]
- Capodaglio, A.G. Critical Perspective on Advanced Treatment Processes for Water and Wastewater: AOPs, ARPs, and AORPs. Appl. Sci. 2020, 10, 4549. [Google Scholar] [CrossRef]
- Brayman, A.A.; MacConaghy, B.E.; Wang, Y.-N.; Chan, K.T.; Monsky, W.L.; Chernikov, V.P.; Buravkov, S.V.; Khokhlova, V.A.; Matula, T.J. Inactivation of Planktonic Escherichia coli by Focused 1-MHz Ultrasound Pulses with Shocks: Efficacy and Kinetics Upon Volume Scale-Up. Ultrasound Med. Biol. 2018, 44, 1996–2008. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Yasuda, K.; Liu, X. Simulation of the formation and characteristics of ultrasonic fountain. Ultrason. Sonochem. 2016, 32, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Kim, M.; Kim, J. Analysis of an Acoustic Fountain Generated by Using an Ultrasonic Plane Wave for Different Water Depths. J. Korean Phys. Soc. 2019, 74, 336–339. [Google Scholar] [CrossRef]
- Fang, Y.; Yamamoto, T.; Komarov, S. Cavitation and acoustic streaming generated by different sonotrode tips. Ultrason. Sonochem. 2018, 48, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Nanzai, B.; Okitsu, K.; Takenaka, N.; Bandow, H.; Tajima, N.; Maeda, Y. Effect of reaction vessel diameter on sonochemical efficiency and cavitation dynamics. Ultrason. Sonochem. 2009, 16, 163–168. [Google Scholar] [CrossRef]
- Sancheti, S.V.; Gogate, P.R. A review of engineering aspects of intensification of chemical synthesis using ultrasound. Ultrason. Sonochem. 2017, 36, 527–543. [Google Scholar] [CrossRef]
- Merouani, S.; Hamdaoui, O.; Rezgui, Y.; Guemini, M. Effects of ultrasound frequency and acoustic amplitude on the size of sonochemically active bubbles—Theoretical study. Ultrason. Sonochem. 2013, 20, 815–819. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, X.; Cui, Y.; Yuan, W. Ultrasound for microalgal cell disruption and product extraction: A review. Ultrason. Sonochem. 2022, 87, 106054. [Google Scholar] [CrossRef] [PubMed]
- Gude, V.G.; Grant, G.E. Biodiesel from waste cooking oils via direct sonication. Appl. Energy 2013, 109, 135–144. [Google Scholar] [CrossRef]
- Moyano, D.B.; Paraiso, D.A.; González-Lezcano, R.A. Possible Effects on Health of Ultrasound Exposure, Risk Factors in the Work Environment and Occupational Safety Review. Healthcare 2022, 10, 423. [Google Scholar] [CrossRef]
- Yu, D.; Tian, L.; Wu, H.; Wang, S.; Wang, Y.; Ma, D.; Fang, X. Ultrasonic irradiation with vibration for biodiesel production from soybean oil by Novozym 435. Process Biochem. 2010, 45, 519–525. [Google Scholar] [CrossRef]


| Authors | |||
|---|---|---|---|
| Oliveira et al. [31] | Mahamuni and Adewuyi [18] | Aghbashlo et al. [35] | |
| Feed | Soybean oil | Waste cooking oil (WCO) | |
| Ultrasonic Reactor Type | Transducer | Piezoelectric-based ultrasonic reactor | |
| Catalyst | Potassium hydroxide (KOH) | ||
| Alcohol | Methanol | ||
| Conditions | Study was conducted at the frequencies of 1 MHz and 3 MHz with alcohol-to-oil molar ratios of 6:1 and 8:1, respectively, at a reaction time of 40 min (without external heating or mechanical stirring). | Study was conducted at the frequencies of 323 kHz, 581 kHz, 611 kHz, and 1.3 MHz with an alcohol-to-oil molar ratio of 6:1 and a power between 12 and 223 W, with a reaction time ranging from 60 min to 180 min. | Study was conducted at the frequency of 1.7 MHz with alcohol-to-oil molar ratios of 4:1, 6:1, and 8:1 and a power of 31 W, with the reaction times of 6, 8, and 10 min. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chit, V.; Tan, L.S.; Kiew, P.L.; Tsuji, T.; Funazukuri, T.; Lock, S.S.M. Advancing Process Intensification with High-Frequency Ultrasound: A Mini-Review of Applications in Biofuel Production and Beyond. Processes 2023, 11, 1236. https://doi.org/10.3390/pr11041236
Chit V, Tan LS, Kiew PL, Tsuji T, Funazukuri T, Lock SSM. Advancing Process Intensification with High-Frequency Ultrasound: A Mini-Review of Applications in Biofuel Production and Beyond. Processes. 2023; 11(4):1236. https://doi.org/10.3390/pr11041236
Chicago/Turabian StyleChit, Viesuieda, Lian See Tan, Peck Loo Kiew, Tomoya Tsuji, Toshitaka Funazukuri, and Serene Sow Mun Lock. 2023. "Advancing Process Intensification with High-Frequency Ultrasound: A Mini-Review of Applications in Biofuel Production and Beyond" Processes 11, no. 4: 1236. https://doi.org/10.3390/pr11041236






