The Effect of Electro-Induced Multi-Gas Modification on Polymer Substrates’ Surface Structure for Additive Manufacturing
Abstract
:1. Introduction
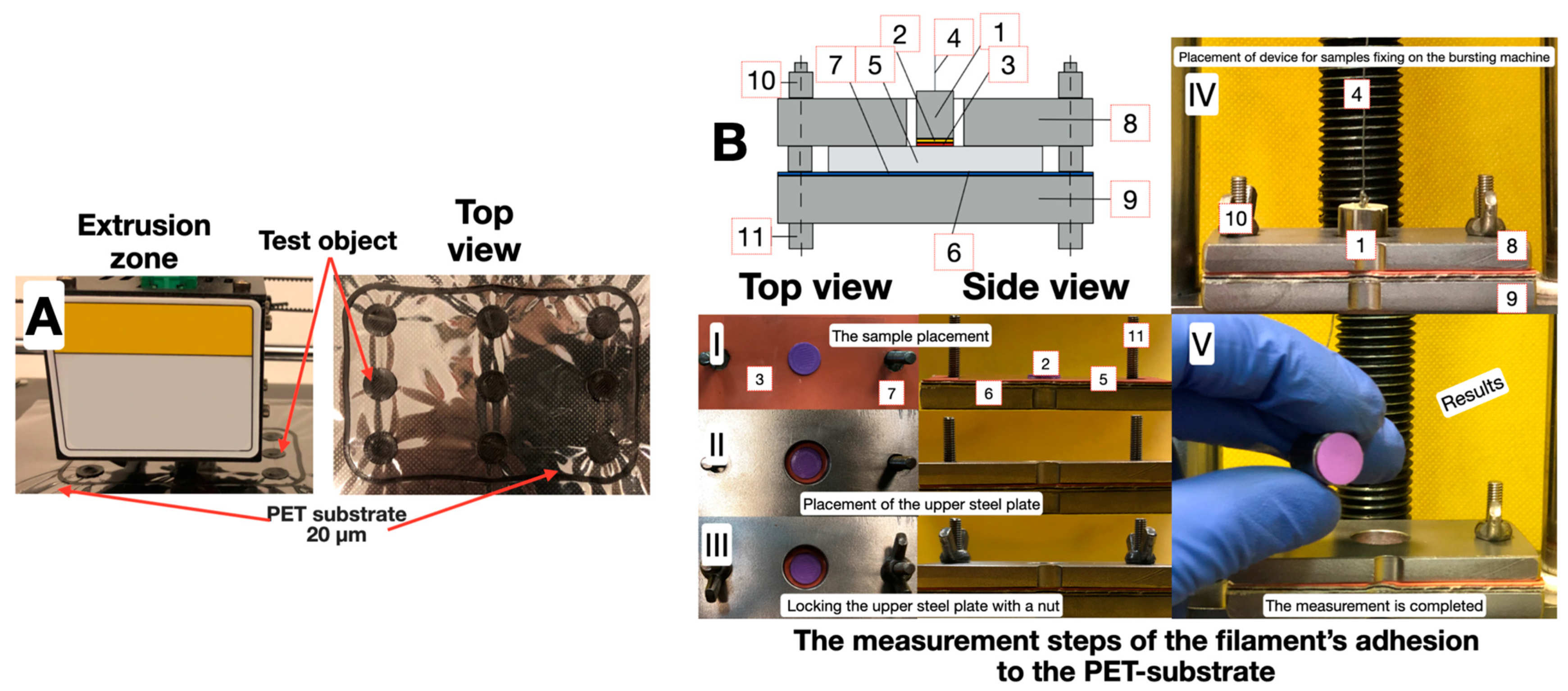
2. Materials and Methods
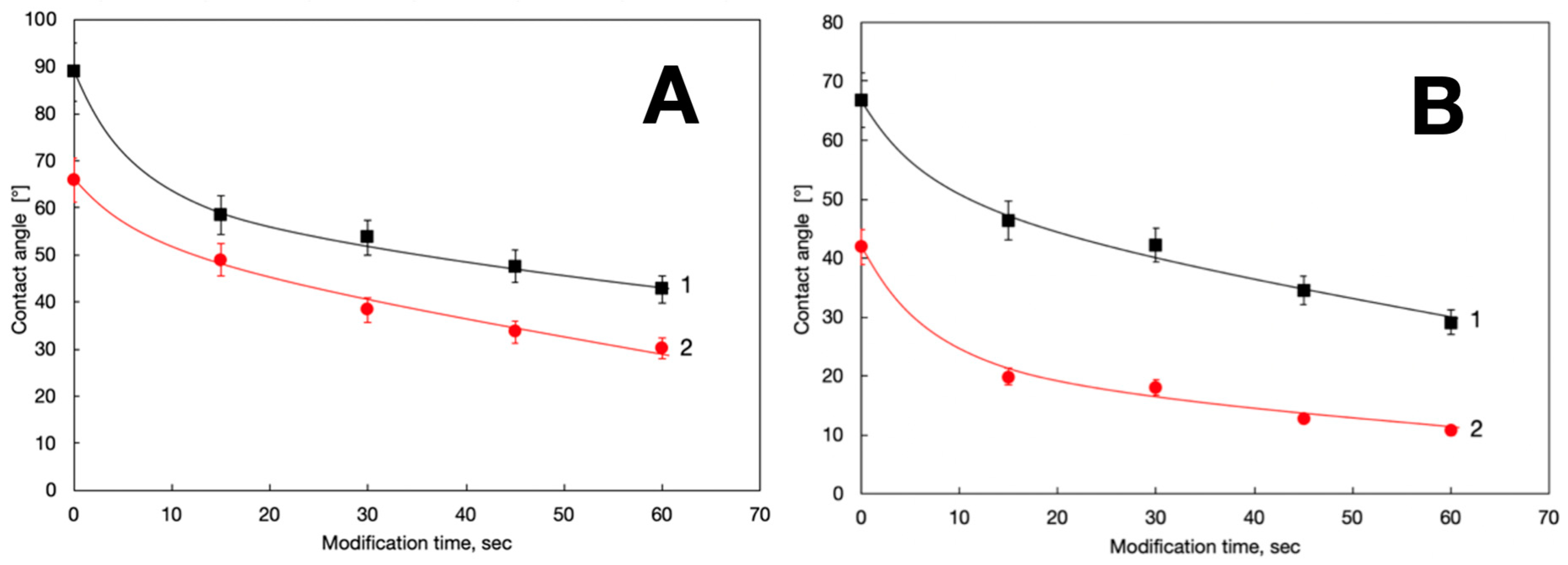
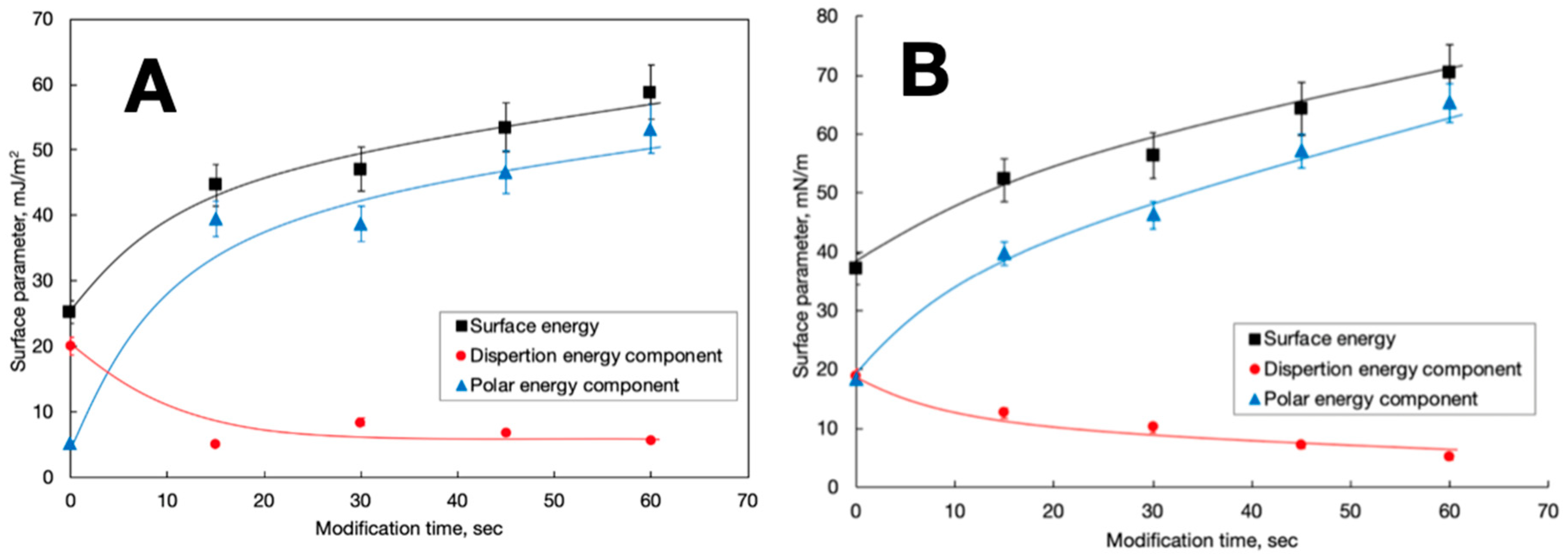
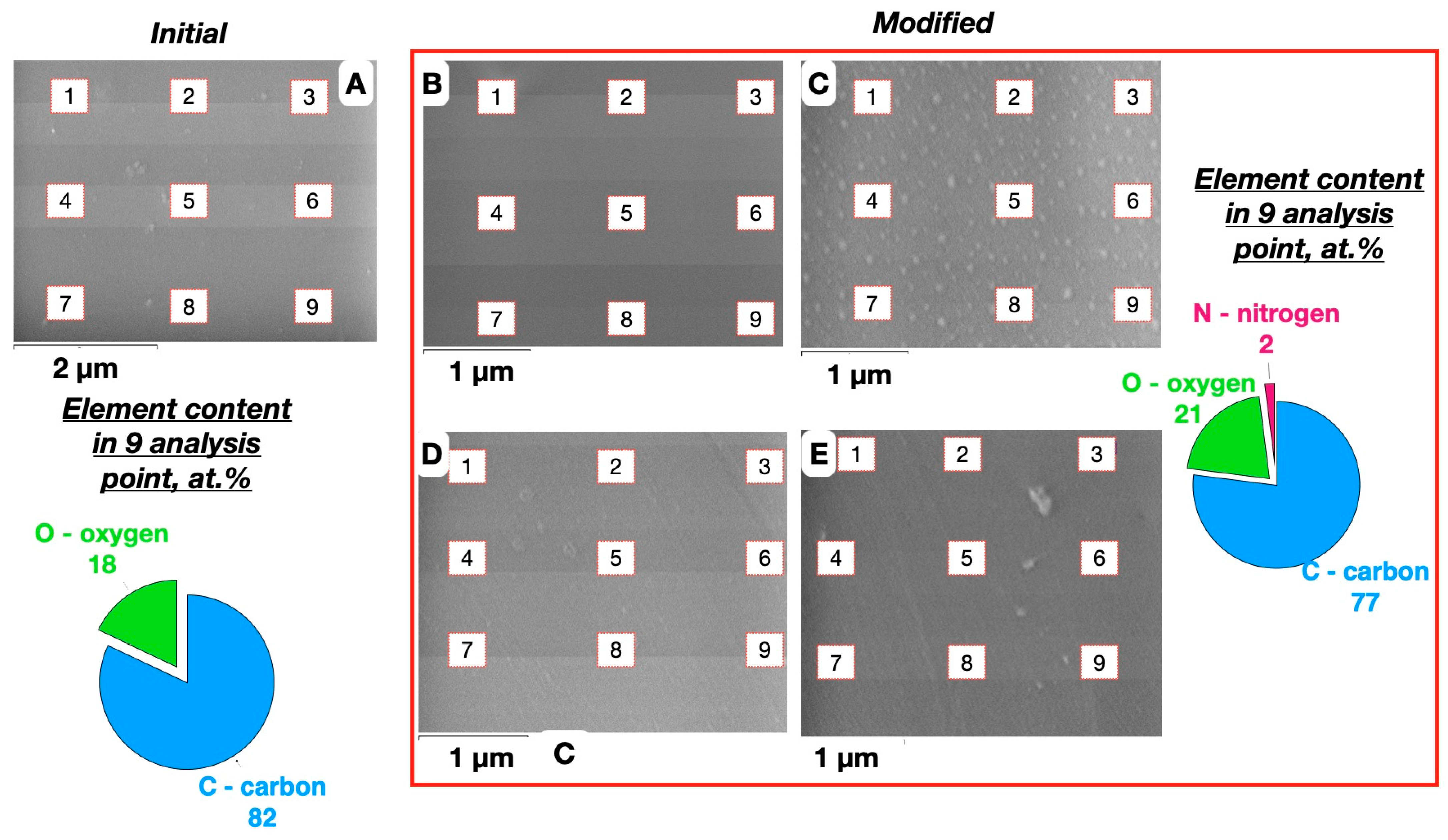
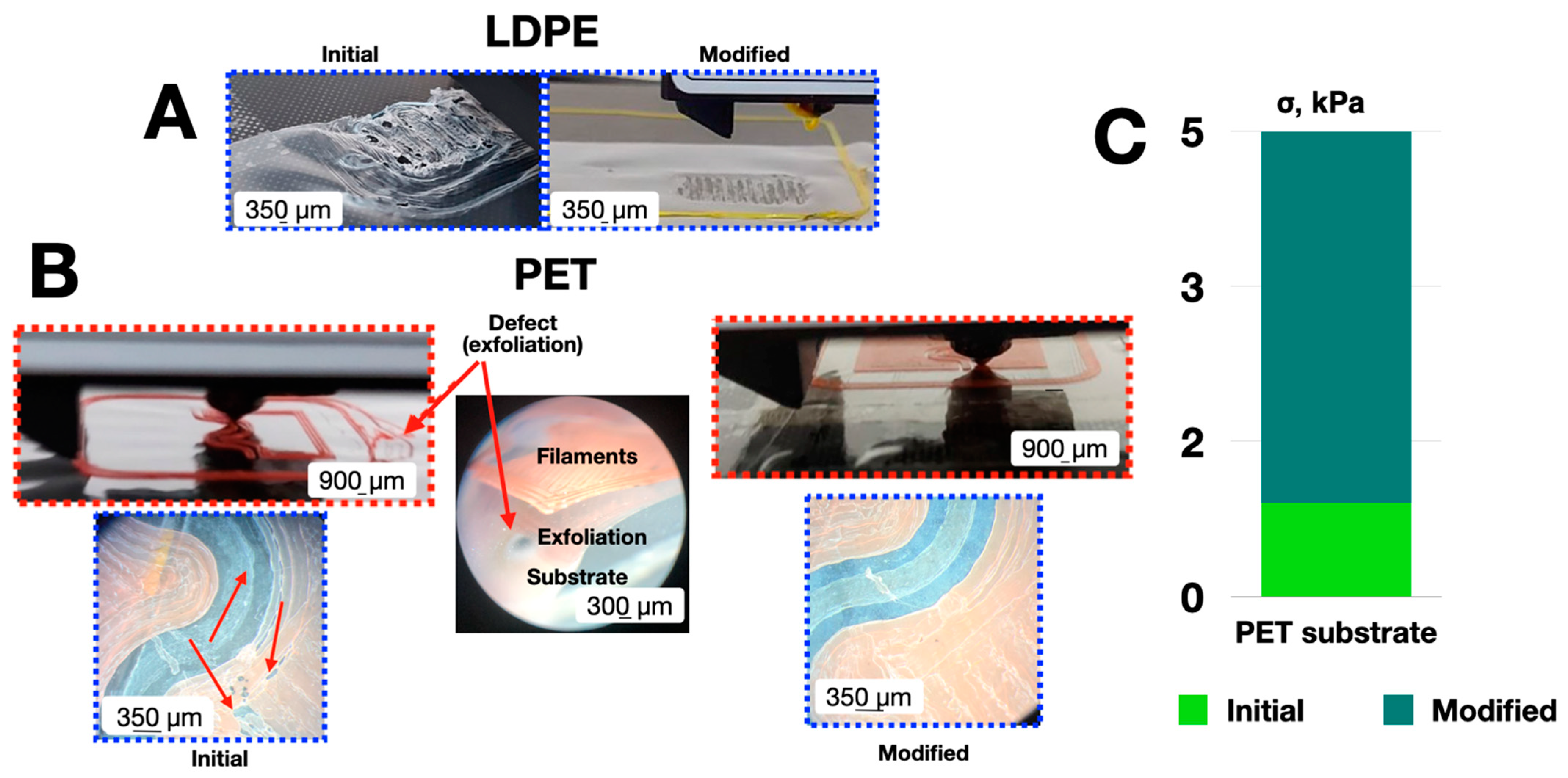
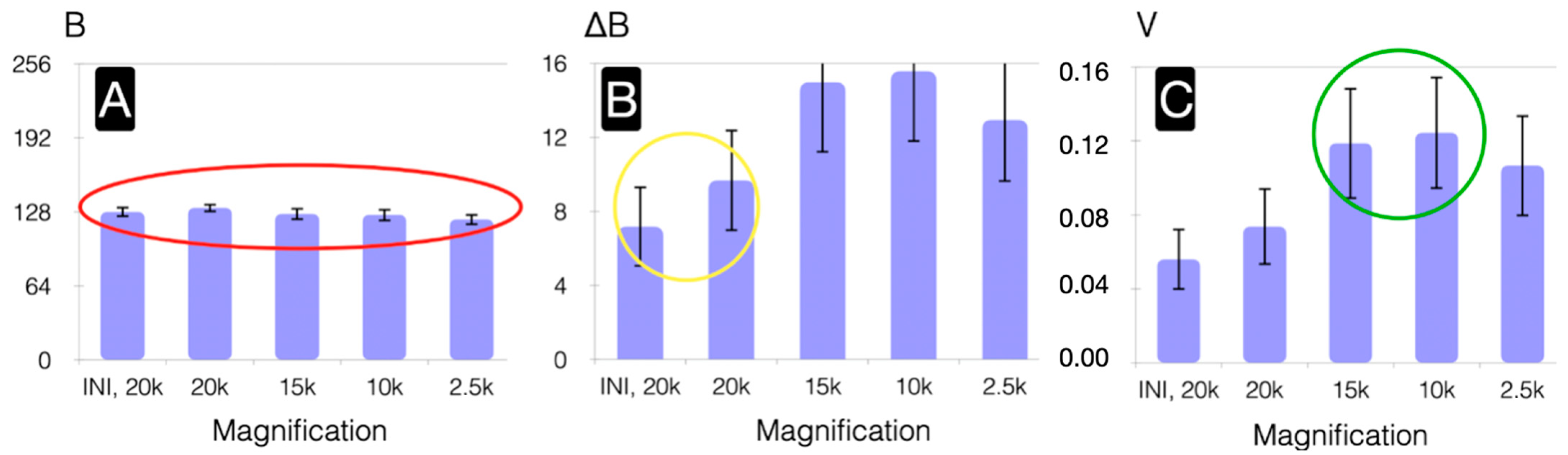
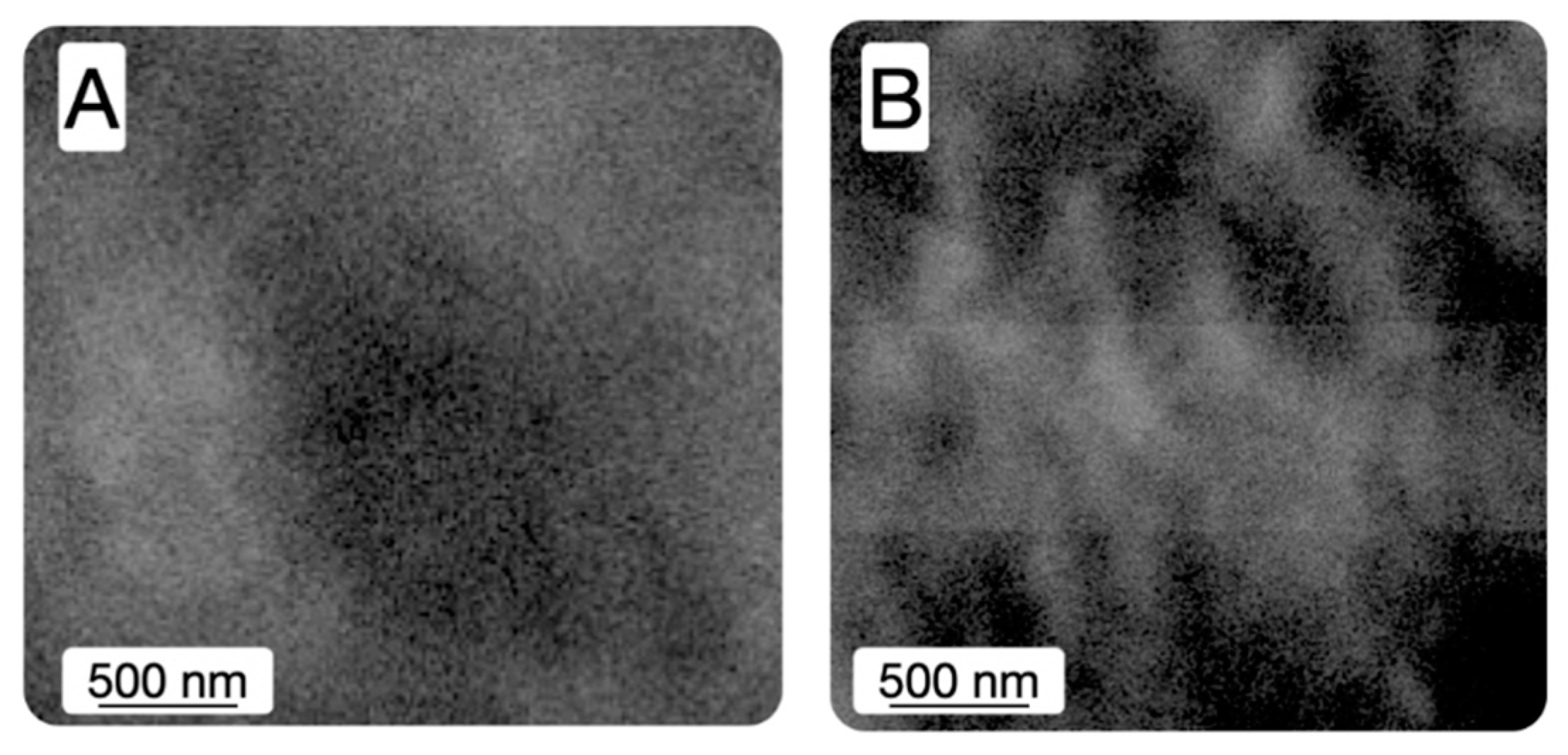
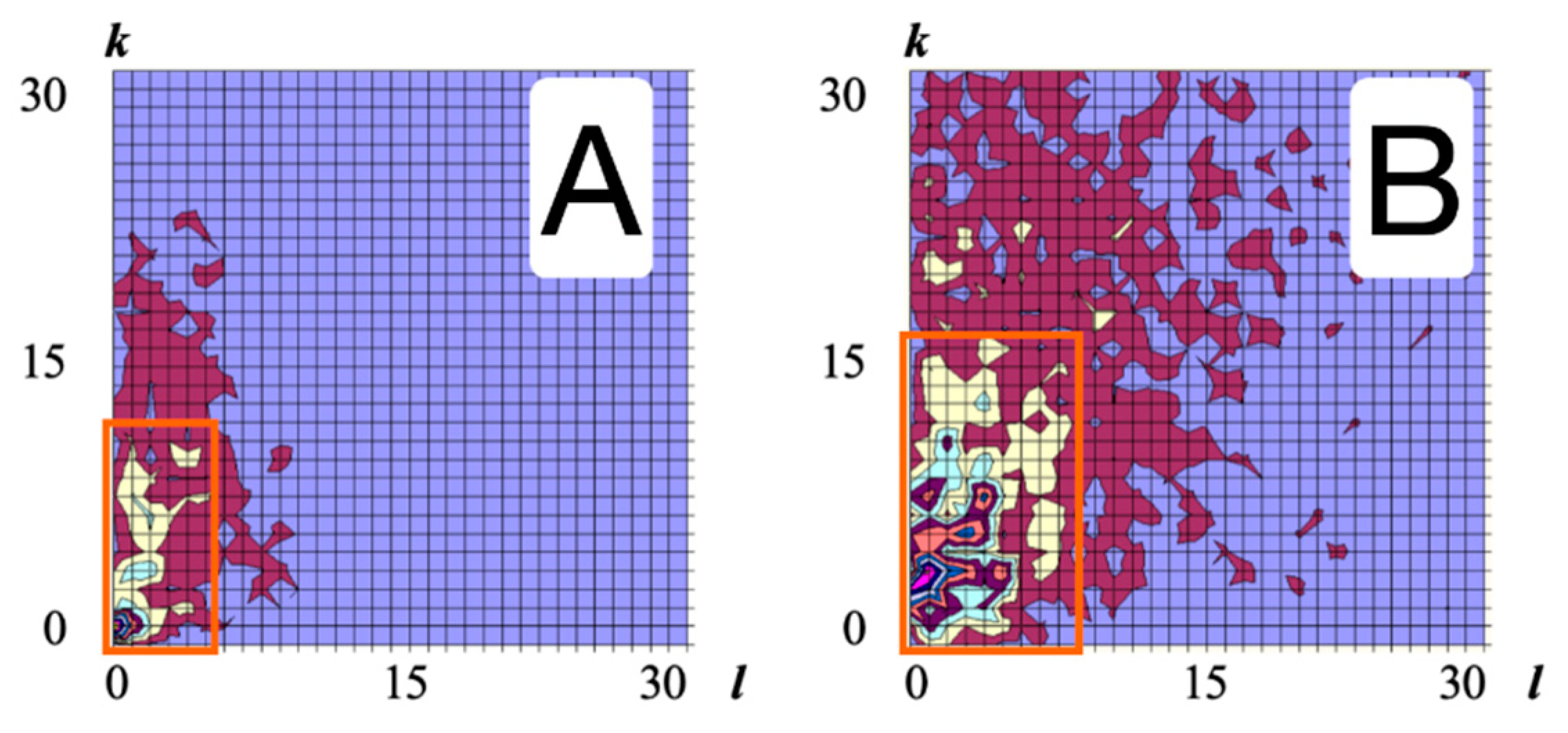
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bressers, H.J.L.; van Driel, W.D.; Jansen, K.M.B.; Ernst, L.J.; Zhang, G.Q. Correlation between chemistry of polymer building blocks and microelectronics reliability. Microelectron. Reliab. 2007, 47, 290–294. [Google Scholar] [CrossRef]
- Krebs, F.C. Pad printing as a film forming technique for polymer solar cells. Sol. Energy Mater. Sol. Cells 2009, 93, 484–490. [Google Scholar] [CrossRef]
- Matyushin, V.; Schalkhammer, T.; Rauter, H.; Alguel, Y.; Pittner, F. Nanotechnology for smart polymer optical devices. Macromol. Symp. 2004, 217, 109–133. [Google Scholar]
- Zhang, T.; Amin, I.; Sheng, W.; Jordan, R.; Rodriguez, R.D.; Gasiorowski, J.; Rahaman, M.; Kalbacova, J.; Sheremet, E.; Zahn, D.R.T. Bottom-up fabrication of graphene-based conductive polymer carpets for optoelectronics. J. Mater. Chem. C 2018, 6, 4919–4927. [Google Scholar] [CrossRef]
- Herrero, J.; Guillén, C. Transparent films on polymers for photovoltaic applications. Vacuum 2002, 67, 611–616. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, T.; Wen, L.; Zhang, A. Fabricating metallic circuit patterns on polymer substrates through laser and selective metallization. ACS Appl. Mater. Interfaces 2016, 8, 33999–34007. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Zhao, X.; Li, J.; Lin, S.; Zhu, H. Recent developments in graphene conductive ink: Preparation, printing technology and application. Chin. Sci. Bull. 2017, 62, 3217–3235. [Google Scholar] [CrossRef]
- Kravtsova, V.D.; Umerzakova, M.B.; Korobova, N.E.; Vertyanov, D.V. Copper-containing compositions based on alicyclic polyimide for microelectronic applications. Russ. Microelectron. 2018, 47, 455–459. [Google Scholar] [CrossRef]
- Molji, C.; Renjith, S.; Sudha, J.D.; Jinesh, K.B. Macroscopically oriented (3-pentadecyl phenol) dangled fluorene based conductive polymer through side chain engineering for microelectronics. Express Polym. Lett. 2019, 13, 1027–1040. [Google Scholar] [CrossRef]
- Mukhin, N.; Sokolova, I.; Chigirev, D.; Schmidt, M.-P.; Rudaja, L.; Lebedeva, G.; Bol’shakov, M.; Kastro, R.; Hirsch, S. Composite ferroelectric coatings based on a heat-resistant polybenzoxazole polymer matrix. Coatings 2020, 10, 286. [Google Scholar] [CrossRef] [Green Version]
- Ivanov, A.A. Polymer composite materials for semiconductor optoelectronics and microelectronics. Biosci. Biotechnol. Res. Asia 2015, 12, 239–245. [Google Scholar] [CrossRef]
- Agrawal, A.; Satapathy, A. Thermal and dielectric behaviour of polypropylene composites reinforced with ceramic fillers. J. Mater. Sci. Mater. Electron. 2014, 26, 103–112. [Google Scholar] [CrossRef]
- Lee, J.-W.; Kil Mun, K.; Yoo, Y.T. A comparative study on roll-to-roll gravure printing on PET and BOPP webs with aqueous ink. Prog. Org. Coatings 2009, 64, 98–108. [Google Scholar] [CrossRef]
- Siau, S.; Vervaet, A.; Van Calster, A.; Van Vaeck, L.; Schacht, E.; Demeter, U. Adhesion strength of the epoxy polymer/copper interface for use in microelectronics. J. Electrochem. Soc. 2005, 152, C442–C455. [Google Scholar] [CrossRef]
- Czvikovszky, T. Expected and unexpected achievements and trends in radiation processing of polymers. Radiat. Phys. Chem. 2003, 67, 437–440. [Google Scholar] [CrossRef]
- Nazarov, V.G.; Кoндратoв, A.P.; Stolyarov, V.P.; Evlampieva, L.A.; Baranov, V.A.; Gagarin, M.V. Morphology of the surface layer of polymers modified by gaseous fluorine. Polym. Sci. Ser. A 2006, 48, 1164–1170. [Google Scholar] [CrossRef]
- Kumar, A.; Jang, S.Y.; Padilla, J.; Otero, T.F.; Sotzing, G.A. Photopatterned electrochromic conjugated polymer films via precursor approach. Polymer 2008, 49, 3686–3692. [Google Scholar] [CrossRef]
- Nazarov, V.G. Multiple surface structures in polyolefins formed by modification methods. J. Appl. Polym. Sci. 2005, 95, 1198–1208. [Google Scholar] [CrossRef]
- Nazarov, V.G. Structure and composition of the surface layer in polymers modified by elemental fluorine. J. Appl. Polym. Sci. 2005, 95, 897–902. [Google Scholar] [CrossRef]
- Nazarov, V.; Stolyarov, V.; Gagarin, M. Simulation of chemical modification of polymer surface. J. Fluor. Chem. 2014, 161, 120–127. [Google Scholar] [CrossRef]
- Nazarov, V.G.; Doronin, F.A.; Evdokimov, A.G.; Rytikov, G.O.; Stolyarov, V.P. Oxyfluorination-Controlled Variations in the Wettability of Polymer Film Surfaces. Colloid J. 2019, 81, 146–157. [Google Scholar] [CrossRef]
- Nazarov, V.G.; Stolyarov, V.P.; Doronin, F.A.; Evdokimov, A.G.; Rytikov, G.O.; Brevnov, P.N.; Zabolotnov, A.S.; Novokshonova, L.A.; Berlin, A.A. Comparison of the Effects of Some Modification Methods on the Characteristics of Ultrahigh-Molecular-Weight Polyethylene and Composites on Its Basis. Polym. Sci. Ser. A 2019, 61, 325–333. [Google Scholar] [CrossRef]
- Wang, W.; Meng, Q.; Li, Q.; Liu, J.; Jin, Z.; Zhou, M.; Zhao, K. Chitosan derivatives and their application in biomedicine. Int. J. Mol. Sci. 2020, 21, 487. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, S.; Jain, N.; Nagaich, U. Nanodiamonds with powerful ability for drug delivery and biomedical applications: Recent updates on in vivo study and patents. J. Pharm. Anal. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Elskens, J.P.; Elskens, J.M.; Madder, A. Chemical modification of aptamers for increased binding affinity in diagnostic applications: Current status and future prospects. Int. J. Mol. Sci. 2020, 21, 4522. [Google Scholar] [CrossRef] [PubMed]
- Shum, K.T.; Tanner, J.A. Differential inhibitory activities and stabilisation of DNA aptamers against the SARS coronavirus helicase. Chembiochem 2008, 9, 3037–3045. [Google Scholar] [CrossRef]
- Holt, K.B. Diamond at the nanoscale: Applications of diamond nanoparticles from cellular biomarkers to quantum computing. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2007, 365, 2845–2861. [Google Scholar] [CrossRef]
- Raty, E.J.-Y.; Galli, G. Ultradispersity of diamond at the nanoscale. Nat. Mater. 2003, 2, 792–795. [Google Scholar] [CrossRef]
- Mostovoy, A.S.; Vikulova, M.A.; Lopukhova, M.I. Reinforcing effects of aminosilane-functionalized h-bn on the physicochemical and mechanical behaviors of epoxy nanocomposites. Sci. Rep. 2020, 10, 10676. [Google Scholar] [CrossRef]
- Lee, H.; Lim, S.; Birajdar, M.; Lee, S.-H.; Park, H. Fabrication of FGF-2 immobilized electrospun gelatin nanofibers for tissue engineering. Int. J. Biol. Macromol. 2016, 93, 1559–1566. [Google Scholar] [CrossRef] [PubMed]
- Chahal, S.; Hussain, F.S.J.; Kumar, A.; Yusoff, M.M.; Rasad, M.S.B.A. Electrospun hydroxyethyl cellulose nanofibers functionalized with calcium phosphate coating for bone tissue engineering. RSC Adv. 2015, 5, 29497–29504. [Google Scholar] [CrossRef] [Green Version]
- Rezvani, Z.; Venugopal, J.R.; Urbanska, A.M.; Mills, D.K.; Ramakrishna, S.; Mozafari, M. A bird’s eye view on the use of electrospun nanofibrous scaffolds for bone tissue engineering: Current state-of-the-art, emerging directions and future trends. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 2181–2200. [Google Scholar] [CrossRef] [PubMed]
- Sakdaronnarong, C.; Sangjan, A.; Boonsith, S.; Kim, D.C.; Shin, H.S. Recent developments in synthesis and photocatalytic applications of carbon dots. Catalysts 2020, 10, 320. [Google Scholar] [CrossRef] [Green Version]
- Castro-Muñoz, R.; Ahmad, M.Z.; Fíla, V. Tuning of nano-based materials for embedding into low-permeability polyimides for a featured gas separation. Front. Chem. 2020, 7, 897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lonergan, M.C.; Cheng, C.H.; Langsdorf, B.L.; Zhou, X. Electrochemical characterization of polyacetylene ionomers and polyelectrolyte-mediated electrochemistry toward interfaces between dissimilarly doped conjugated polymers. J. Am. Chem. Soc. 2002, 124, 690–701. [Google Scholar] [CrossRef] [PubMed]
- Maex, K.; Shamiryan, D.; Iacopi, F.; Brongersma, S.H.; Baklanov, M.R.; Yanovitskaya, Z.S. Low dielectric constant materials for microelectronics. J. Appl. Phys. 2003, 93, 8793–8841. [Google Scholar] [CrossRef]
- Menegatti, E.; Berardi, D.; Messina, M.; Giachino, O.; Restagno, G.; Roccatello, D.; Ferrante, I.; Spagnolo, B.; Cognolato, L. Lab-on-a-chip: Emerging analytical platforms for immune-mediated diseases. Autoimmun. Rev. 2013, 12, 814–820. [Google Scholar] [CrossRef]
- Junkar, I. Plasma treatment of amorphous and semicrystalline polymers for improved biocompatibility. Vacuum 2013, 98, 111–115. [Google Scholar] [CrossRef]
- Popelka, A.; Kronek, J.; Novák, I.; Kleinová, A.; Mičušík, M.; Špírková, M.; Omastová, M. Surface modification of low-density polyethylene with poly(2-ethyl-2-oxazoline) using a low-pressure plasma treatment. Vacuum 2014, 100, 53–56. [Google Scholar] [CrossRef]
- Louzi, V.C.; Campos, J.S.D.C. Corona treatment applied to synthetic polymeric monofilaments (PP, PET, and PA-6). Surf. Interfaces 2019, 14, 98–107. [Google Scholar] [CrossRef]
- Dai, L.; Xu, D. Polyethylene surface enhancement by corona and chemical co-treatment. Tetrahedron Lett. 2019, 60, 1005–1010. [Google Scholar] [CrossRef]
- Udomluck, N.; Park, H.; Koh, W.-G.; Lim, D.-J. Recent developments in nanofiber fabrication and modification for bone tissue engineering. Int. J. Mol. Sci. 2020, 21, 99. [Google Scholar] [CrossRef] [Green Version]
- Siow, K.S.; Britcher, L.; Kumar, S.; Griesser, H.J. Plasma methods for the generation of chemically reactive surfaces for biomolecule immobilization and cell colonization—A review. Plasma Process. Polym. 2006, 3, 392–418. [Google Scholar] [CrossRef]
- Chen, J.-P.; Su, C.-H. Surface modification of electrospun PLLA nanofibers by plasma treatment and cationized gelatin immobilization for cartilage tissue engineering. Acta Biomater. 2011, 7, 234–243. [Google Scholar] [CrossRef]
- Ho, M.-H.; Hou, L.-T.; Tu, C.-Y.; Hsieh, H.-J.; Lai, J.-Y.; Chen, W.-J.; Wang, D.-M. Promotion of cell affinity of porous PLLA scaffolds by immobilization of RGD peptides via plasma treatment. Macromol. Biosci. 2006, 6, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Sanders, J.E.; Lamont, S.E.; Karchin, A.; Golledge, S.L.; Ratner, B.D. Fibro-porous meshes made from polyurethane micro-fibers: Effects of surface charge on tissue response. Biomaterials 2005, 26, 813–818. [Google Scholar] [CrossRef]
- Manakhov, A.; Kedronová, E.; Medalová, J.; Cernochová, P.; Obrusník, A.; Michlícek, M.; Shtansky, D.V.; Zajíčková, L. Carboxyl-anhydride and amine plasma coating of PCL nanofibers to improve their bioactivity. Mater. Des. 2017, 132, 257–265. [Google Scholar] [CrossRef]
- Vesel, A.; Primc, G.; Zaplotnik, R.; Mozetič, M. Applications of highly non-equilibrium low-pressure oxygen plasma for treatment of polymers and polymer composites on an industrial scale. Plasma Phys. Control Fusion 2020, 62, 024008. [Google Scholar] [CrossRef]
- Karakassides, A.; Ganguly, A.; Papakonstantinou, P.; Tsirka, K.; Paipetis, A.S. Radi-ally grown graphene nanoflakes on carbon fibers as reinforcing interface for polymer composites. ACS Appl. Nano Mater. 2020, 3, 2402–2413. [Google Scholar] [CrossRef] [Green Version]
- Lapenna, A.; Fracassi, F.; Armenise, V.; Angarano, V.; Palazzo, G.; Fanelli, F.; Mal-lardi, A. Direct exposure of dry enzymes to atmospheric pressure non-equilibrium plas-mas: The case of tyrosinase. Materials 2020, 13, 2181. [Google Scholar] [CrossRef]
- Pagliusi, P.; Audia, B.; Provenzano, C.; Cipparrone, G.; Piñol, M.; Oriol, L. Tunable surface patterning of azopolymer by vectorial holography: The role of photoanisotropies in the driving force. ACS Appl. Mater. Interfaces 2019, 11, 34471–34477. [Google Scholar] [CrossRef] [PubMed]
- Sochol, R.D.; Sweet, E.; Glick, C.C.; Wu, S.Y.; Yang, C.; Restaino, M.; Lin, L. 3D printed microfluidics and microelectronics. Microelectron. Eng. 2018, 189, 52–68. [Google Scholar] [CrossRef]
- Choong, Y.Y.C.; Tan, H.W.; Patel, D.C.; Choong, W.T.N.; Chen, C.H.; Low, H.Y.; Tan, M.J.; Patel, C.D.; Chua, C.K. The global rise of 3D printing during the COVID-19 pandemic. Nat. Rev. Mater. 2020, 5, 637–639. [Google Scholar] [CrossRef] [PubMed]
- Ward, K.; Fan, Z.H. Mixing in microfluidic devices and enhancement methods. J. Micromech. Microeng. 2015, 25, 094001. [Google Scholar] [CrossRef] [Green Version]
- Waheed, S.; Cabot, J.M.; Macdonald, N.P.; Paull, B.; Breadmore, M.C.; Lewis, T.; Guijt, R.M. 3D printed microfluidic devices: Enablers and barriers. Lab A Chip Miniat. Chem. Biol. 2016, 16, 1993–2013. [Google Scholar] [CrossRef] [Green Version]
- Razavi Bazaz, S.; Rouhi, O.; Raoufi, M.A.; Ejeian, F.; Ebrahimi Warkiani, M.; Asadnia, M.; Jin, D. 3D printing of inertial microfluidic devices. Sci. Rep. 2020, 10, 5929. [Google Scholar] [CrossRef] [Green Version]
- Saghir, N.; Aggarwal, A.; Soneji, N.; Valencia, V.; Rodgers, G.; Kurian, T. A comparison of manual electrocardiographic interval and waveform analysis in lead 1 of 12-lead ECG and Apple Watch ECG: A validation study. Cardiovasc. Digit. Health J. 2020, 1, 30–36. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, B.; Hojaiji, H.; Wang, Z.; Lin, S.; Yeung, C.; Lin, H.; Nguyen, P.; Chiu, K.; Salahi, K.; et al. A wearable freestanding electrochemical sensing system. Sci. Adv. 2020, 6, eaaz0007. [Google Scholar] [CrossRef] [Green Version]
- Tison, G.H.; Sanchez, J.M.; Ballinger, B.; Singh, A.; Olgin, J.E.; Pletcher, M.J.; Vittinghoff, E.; Lee, E.S.; Fan, S.M.; Gladstone, R.A.; et al. Passive detection of atrial fibrillation using a commercially available smart watch. JAMA Cardiol. 2018, 3, 409–416. [Google Scholar] [CrossRef] [Green Version]
- Sequeira, N.; D’Souza, D.; Angaran, P.; Aves, T.; Dorian, P. Common wearable devices demonstrate variable accuracy in measuring heart rate during supraventricular tachycardia. Heart Rhythm. 2020, 17, 854–859. [Google Scholar] [CrossRef]
- Kang, Z.; Zhang, Y.; Zhou, M. AgNPs@CNTs/Ag hybrid films on thiolated PET substrate for flexible electronics. Chem. Eng. J. 2019, 368, 223–234. [Google Scholar] [CrossRef]
- Kondratov, A.P.; Nagornova, I.V.; Varepo, L.G. Tenso-resistive printed sensors forf lexible elements of systems and mechanisms. J. Phys. Conf. Ser. 2019, 1210, 012067. [Google Scholar] [CrossRef]
- Deganello, D.; Cherry, J.A.; Gethin, D.T.; Claypole, T.C. Impact of metered ink volume on reel-to-reel flexographic printed conductive networks for enhanced thin film conductivity. Thin Solid Film. 2012, 520, 2233–2237. [Google Scholar] [CrossRef]
- Deganello, D.; Cherry, J.A.; Gethin, D.T.; Claypole, T.C. Patterning of micro-scale conductive networks using reel-to-reel flexographic printing. Thin Solid Film. 2010, 518, 6113–6116. [Google Scholar] [CrossRef]
- Núñez, C.G.; Navaraj, W.T.; Polat, E.O.; Dahiya, R. Energy-autonomous, flexible, and transparent tactile skin. Adv. Funct. Mater. 2017, 27, 1606287. [Google Scholar] [CrossRef] [Green Version]
- Walker, B.W.; Annabi, N.; Portillo Lara, R.; Hsiang Yu, C.; Kimball, W.; Mogadam, E. Rational design of microfabricated electroconductive hydrogels for biomedical applications. Prog. Polym. Sci. 2019, 92, 135–157. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.; Ali, S.; Bermak, A. Recent developments in printing flexible and wearable sensing electronics for healthcare applications. Sensors 2019, 19, 1230. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://pinshape.com/items/40493-3d-printed-lesson-of-design-of-microfluidics-channels (accessed on 18 December 2022).
- El Moumen, A.; Tarfaoui, M.; Lafdi, K. Modelling of the temperature and residual stress fields during 3D printing of polymer composites. Int. J. Adv. Manuf. Technol. 2019, 104, 1661–1676. [Google Scholar] [CrossRef]
- Hilberman, M.; Hogan, J.S.; Peters, R.M. The effects of carbon dioxide on pulmonary mechanics in hyperventilating, normal volunteers. J. Thorac. Cardiovasc. Surg. 1976, 71, 268–273. [Google Scholar] [CrossRef]
- Kerolli-Mustafa, M.; Çadraku, H.; Musliu, A. Surface characterization of glas fibers made from jarosite waste using contact angle measurements. IFAC-PapersOnLine 2016, 49, 196–199. [Google Scholar] [CrossRef]
- Doronin, F.; Rudyak, Y.; Rytikov, G.; Evdokimov, A.; Nazarov, V. 3D-printed planar microfluidic device on oxyfluorinated PET-substrate. Polym. Test. 2021, 99, 107209. [Google Scholar] [CrossRef]
- Drozdov, S.; Nazarov, V.; Nozdrachev, S.; Rudyak, Y.; Rytikov, G. The polymer composites’ morphological structure simulation. Nanosyst. Phys. Chem. Math. 2017, 8, 137–145. [Google Scholar] [CrossRef] [Green Version]
- Petrushin, V.N.; Rudyak, Y.V.; Rytikov, G.O. The representativeness of the statistical data frame in the quantitative image analysis. ITM Web Conf. 2018, 18, 01007. [Google Scholar] [CrossRef] [Green Version]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doronin, F.; Rytikov, G.; Evdokimov, A.; Rudyak, Y.; Taranets, I.; Nazarov, V. The Effect of Electro-Induced Multi-Gas Modification on Polymer Substrates’ Surface Structure for Additive Manufacturing. Processes 2023, 11, 774. https://doi.org/10.3390/pr11030774
Doronin F, Rytikov G, Evdokimov A, Rudyak Y, Taranets I, Nazarov V. The Effect of Electro-Induced Multi-Gas Modification on Polymer Substrates’ Surface Structure for Additive Manufacturing. Processes. 2023; 11(3):774. https://doi.org/10.3390/pr11030774
Chicago/Turabian StyleDoronin, Fedor, Georgy Rytikov, Andrey Evdokimov, Yury Rudyak, Irina Taranets, and Victor Nazarov. 2023. "The Effect of Electro-Induced Multi-Gas Modification on Polymer Substrates’ Surface Structure for Additive Manufacturing" Processes 11, no. 3: 774. https://doi.org/10.3390/pr11030774




