Neuroprotective Activities of New Monoterpenoid Indole Alkaloid from Nauclea officinalis
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Procedures
2.2. Plant Materials
2.3. Extraction, Isolation and Purification of Compound
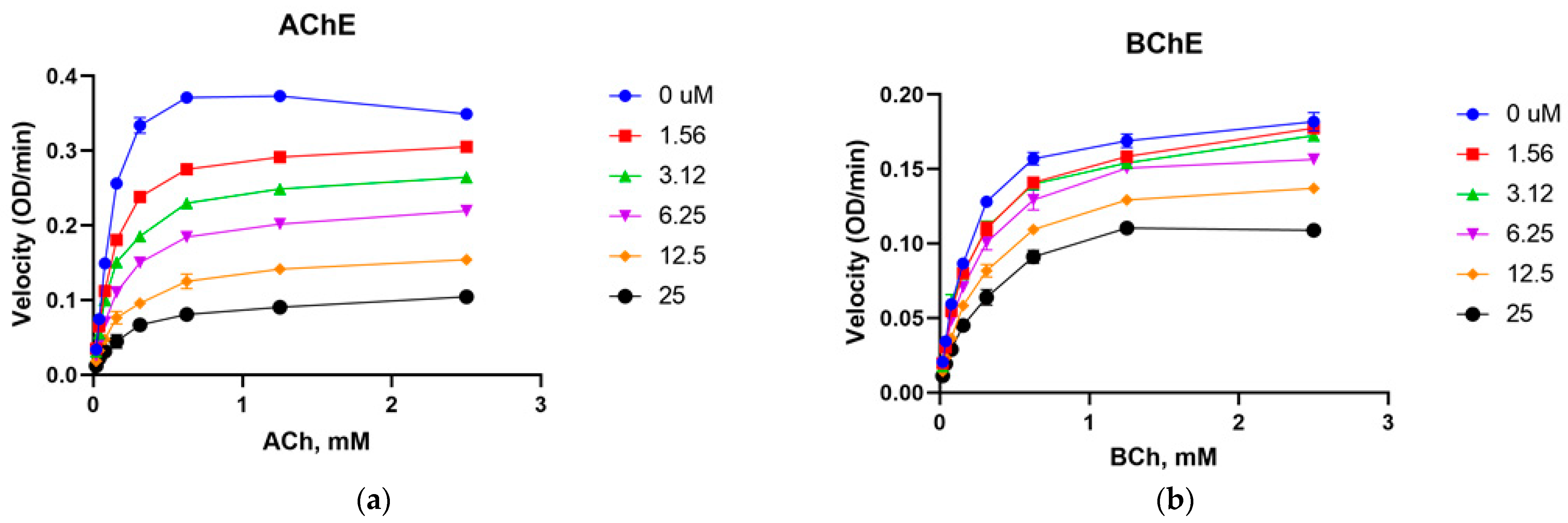
2.4. Cholinesterase Enzyme Inhibitory Activity
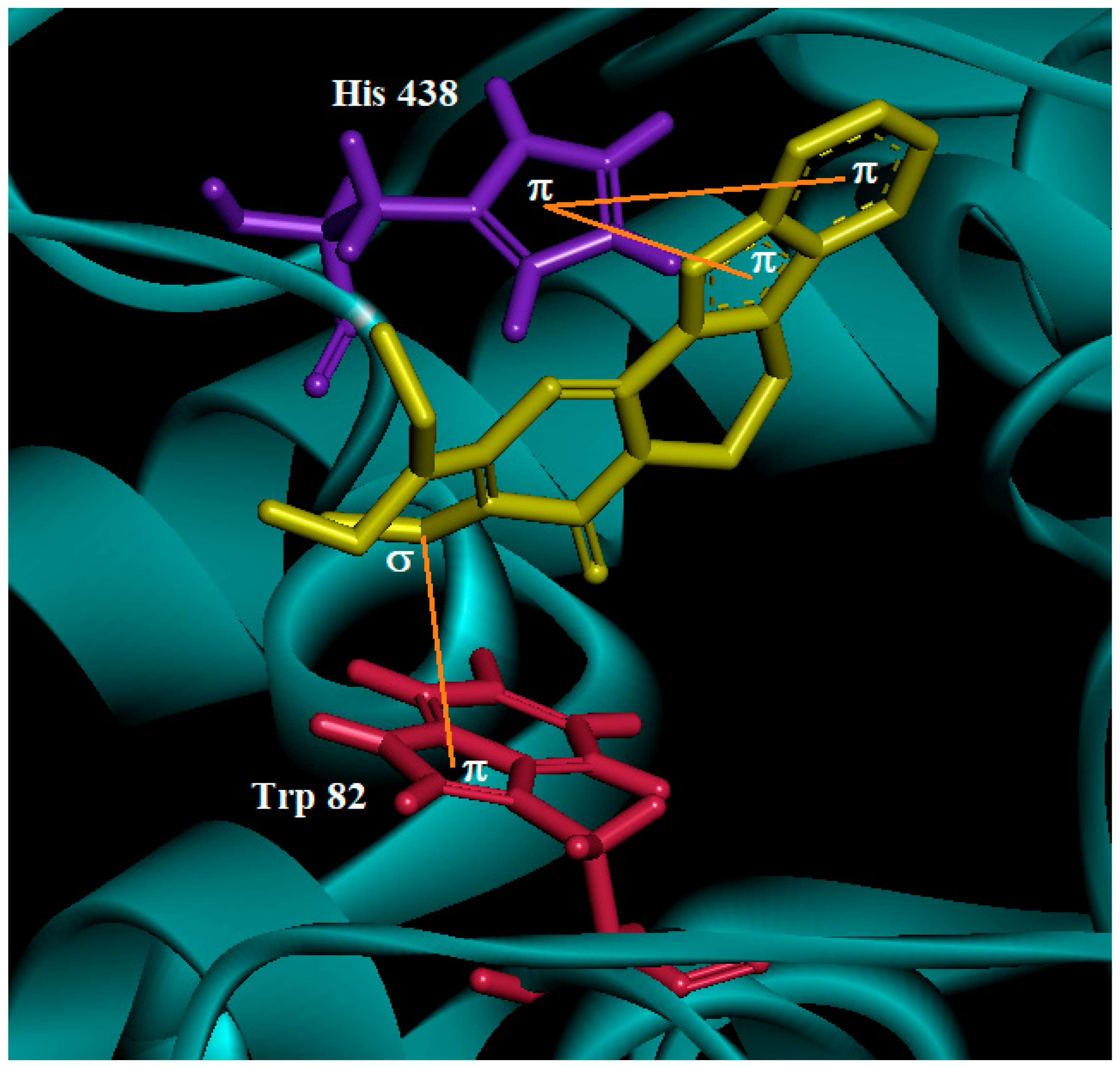
2.5. Molecular Docking
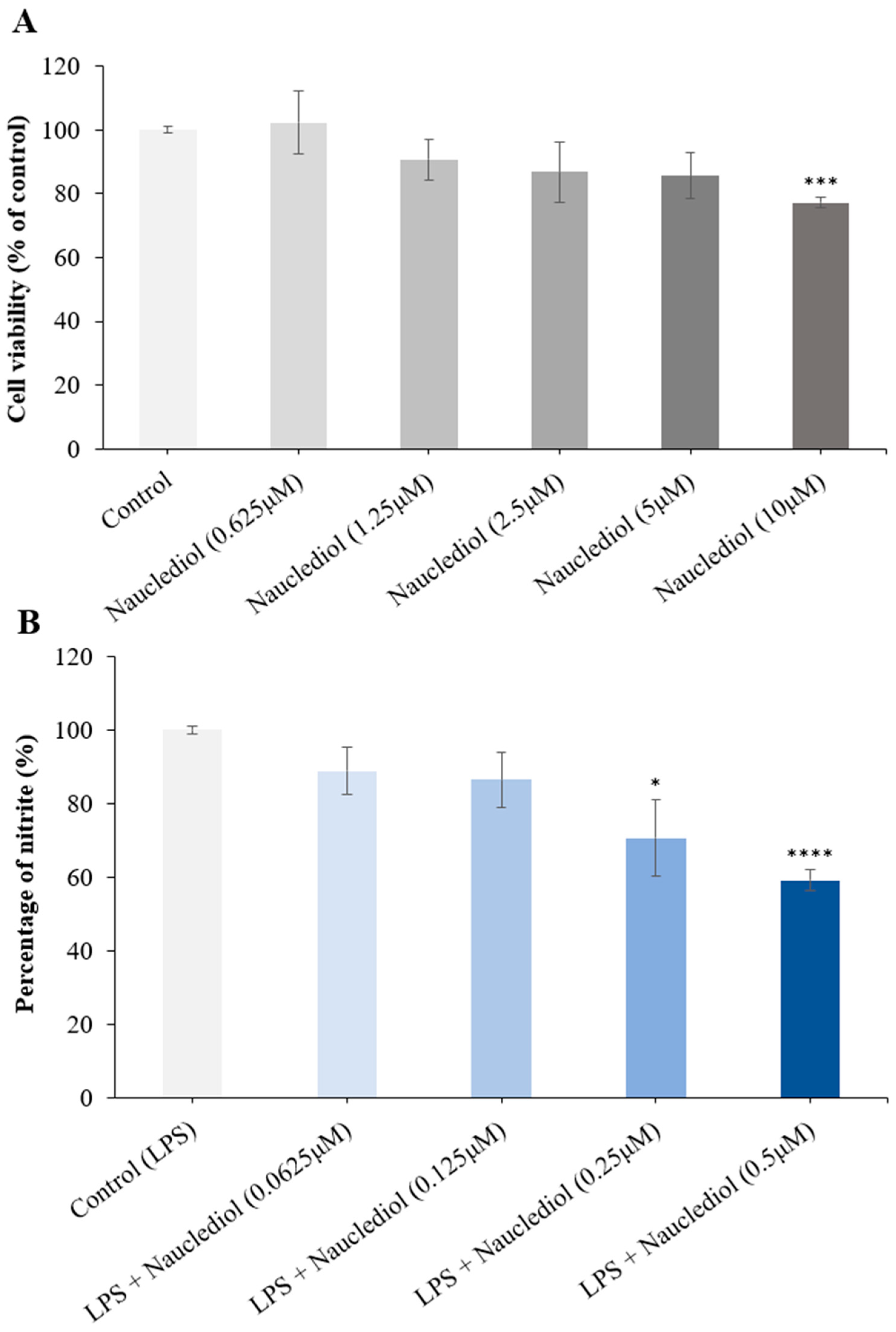
2.6. Neuroprotective Potential of Nauclediol on Aβ-(1-42)-Induced Toxicity on SH-SY5Y Cells
2.6.1. Cell Culture and Cell Viability Assay
2.6.2. Neuroprotective Assay against Beta Amyloid-Induced Toxicity
2.7. Anti-Neuroinflammatory Effect of Nauclediol on LPS-Induced Neuro-Inflammation in BV2 Cells
2.7.1. Cell Culture and Cell Viability Assay
2.7.2. Neuroprotective Assay against LPS-Induced Inflammation
2.7.3. Statistical Analysis
3. Results and Discussion
3.1. Structural Elucidation of Nauclediol
3.2. Cholinesterase Inhibition of Nauclediol
3.3. Molecular Docking of Nauclediol
3.4. Nauclediol Reduces Aβ-(1-42)-Induced Toxicity on SH-SY5Y Cells
3.5. Nauclediol Reduces LPS-Induced Neuroinflammation in BV2 Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wee, S.A.; Nhu, D.T.; Khaw, Y.K.; Tang, S.K.; Yeong, Y.K. Linking Diabetes to Alzheimer’s Disease: Potential Roles of Glucose Metabolism and Alpha-Glucosidase. Curr. Neuropharmacol. 2023. ahead of print. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Kumar, V.; Mal, M.; Houghton, P.J. Acetylcholinesterase inhibitors from plants. Phytomedicine 2007, 14, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Iqubal, A.; Rahman, S.O.; Ahmed, M.; Bansal, P.; Haider, M.R.; Iqubal, M.K.; Najmi, A.K.; Pottoo, F.H.; Haque, S.E. Current Quest in Natural Bioactive Compounds for Alzheimer’s Disease: Multi-Targeted-Designed-Ligand Based Approach with Preclinical and Clinical Based Evidence. Curr. Drug Targets 2021, 22, 685–720. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.R.; Tay, K.C.; Su, Y.X.; Wong, C.K.; Tan, W.N.; Khaw, K.Y. Potential of Naturally Derived Alkaloids as Multi-Targeted Therapeutic Agents for Neurodegenerative Diseases. Molecules 2021, 26, 728. [Google Scholar] [CrossRef]
- Kong, Y.R.; Jong, Y.X.; Balakrishnan, M.; Bok, Z.K.; Weng, J.K.K.; Tay, K.C.; Goh, B.H.; Ong, Y.S.; Chan, K.G.; Lee, L.H.; et al. Beneficial Role of Carica papaya Extracts and Phytochemicals on Oxidative Stress and Related Diseases: A Mini Review. Biology 2021, 10, 287. [Google Scholar] [CrossRef]
- Williams, D.H.; Stone, M.J.; Hauck, P.R.; Rahman, S.K. Why Are Secondary Metabolites (Natural Products) Biosynthesized? J. Nat. Prod. 1989, 52, 1189–1208. [Google Scholar] [CrossRef]
- Croteau, R.; Kutchan, T.M.; Lewis, N.G. Natural Products (Secondary Metabolites); American Society of Plant Physiologists: Rockville, MD, USA, 2000; Volume 24, pp. 1250–1318. [Google Scholar]
- Calixto, J.B.; Otuki, M.F.; Santos, A.R.S. Anti-Inflammatory Compounds of Plant Origin. Part I. Action on Arachidonic Acid Pathway, Nitric Oxide and Nuclear Factor κ B (NF-κB). Planta Med. 2003, 69, 973–983. [Google Scholar] [CrossRef]
- Mcconnell, O.J.; Saucy, G.; Jacobs, R. Use for Topsentin Compounds and Pharmaceutical Compositions Containing Same. U.S. Patent US5290777A, March 1994. [Google Scholar]
- Sun, J.Y.; Lou, H.X.; Dai, S.J.; Xu, H.; Zhao, F.; Liu, K. Indole alkoloids from Nauclea officinalis with weak antimalarial activity. Phytochemistry 2008, 69, 1405–1410. [Google Scholar] [CrossRef]
- Sun, J.Y.; Lou, H.X.; Xu, H.; Dai, S.J.; Liu, K. Two new indole alkaloids from Nauclea officinalis. Chin. Chem. Lett. 2007, 18, 1084–1086. [Google Scholar] [CrossRef]
- Su, K.; Gong, M.; Zhou, J.; Deng, S. Study of Chemical Composition of Nauclea Officinalis Leaves. Int. J. Chem. 2009, 1, 77–81. [Google Scholar] [CrossRef] [Green Version]
- Song, L.-L.; Mu, Y.-L.; Zhang, H.-C.; Wu, G.-Y.; Sun, J.-Y. A new indole alkaloid with anti-inflammatory from the branches of Nauclea officinalis. Nat. Prod. Res. 2018, 34, 2283–2288. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Liu, P.; Wang, L.; Li, D.; Fan, H.; Chen, D.; Zhao, F. In vitro anti-inflammatory activities of naucleoffieine H as a natural alkaloid from Nauclea officinalis Pierrc ex Pitard, through inhibition of the iNOS pathway in LPS-activated RAW 264.7 macrophages. Nat. Prod. Res. 2020, 34, 2694–2697. [Google Scholar] [CrossRef] [PubMed]
- Liew, S.Y.; Looi, C.Y.; Paydar, M.; Cheah, F.K.; Leong, K.H.; Wong, W.F.; Mustafa, M.R.; Litaudon, M.; Awang, K. Subditine, a New Monoterpenoid Indole Alkaloid from Bark of Nauclea subdita (Korth.) Steud. Induces Apoptosis in Human Prostate Cancer Cells. PLoS ONE 2014, 9, e87286. [Google Scholar] [CrossRef]
- Qureshi, A.K.; Mukhtar, M.R.; Hirasawa, Y.; Hosoya, T.; Nugroho, A.E.; Morita, H.; Shirota, O.; Mohamad, K.; Hadi, A.H.A.; Litaudon, M.; et al. Neolamarckines A and B, New Indole Alkaloids from Neolamarckia Cadamba. Chem. Pharm. Bull. 2011, 59, 291–293. [Google Scholar] [CrossRef] [Green Version]
- Mukhtar, M.R.; Osman, N.; Awang, K.; Hazni, H.; Qureshi, A.K.; Hadi, A.H.A.; Zaima, K.; Morita, H.; Litaudon, M. Neonaucline, a New Indole Alkaloid from the Leaves of Ochreinauclea maingayii (Hook. f.) Ridsd. (Rubiaceae). Molecules 2011, 17, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Liew, S.Y.; Mukhtar, M.R.; Hadi, A.H.A.; Awang, K.; Mustafa, M.R.; Zaima, K.; Morita, H.; Litaudon, M. Naucline, a New Indole Alkaloid from the Bark of Nauclea officinalis. Molecules 2012, 17, 4028–4036. [Google Scholar] [CrossRef] [Green Version]
- Liew, S.Y.; Khaw, K.Y.; Murugaiyah, V.; Looi, C.Y.; Wong, Y.L.; Mustafa, M.R.; Litaudon, M.; Awang, K. Natural indole butyrylcholinesterase inhibitors from Nauclea officinalis. Phytomedicine 2015, 22, 45–48. [Google Scholar] [CrossRef]
- Khaw, K.Y.; Chong, C.W.; Murugaiyah, V. LC-QTOF-MS analysis of xanthone content in different parts of Garcinia mangostana and its influence on cholinesterase inhibition. J. Enzyme Inhib. Med. Chem. 2020, 35, 1433–1441. [Google Scholar] [CrossRef]
- Walsh, R. Comparing enzyme activity modifier equations through the development of global data fitting templates in Excel. PeerJ 2018, 6, e6082. [Google Scholar] [CrossRef]
- Abdul Wahab, S.M.; Sivasothy, Y.; Liew, S.Y.; Litaudon, M.; Mohamad, J.; Awang, K. Natural cholinesterase inhibitors from Myristica cinnamomea King. Bioorganic Med. Chem. Lett. 2016, 26, 3785–3792. [Google Scholar] [CrossRef]
- Morris, G.M.; Goodsell, D.S.; Halliday, R.S.; Huey, R.; Hart, W.E.; Belew, R.K.; Olson, A.J. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem. 1998, 19, 1639–1662. [Google Scholar] [CrossRef]
- Greenblatt, H.M.; Guillou, C.; Guénard, D.; Argaman, A.; Botti, S.; Badet, B.; Thal, C.; Silman, I.; Sussman, J.L. The Complex of a Bivalent Derivative of Galanthamine with Torpedo Acetylcholinesterase Displays Drastic Deformation of the Active-Site Gorge: Implications for Structure-Based Drug Design. J. Am. Chem. Soc. 2004, 126, 15405–15411. [Google Scholar] [CrossRef] [PubMed]
- Carletti, E.; Aurbek, N.; Gillon, E.; Loiodice, M.; Nicolet, Y.; Fontecilla-Camps, J.-C.; Masson, P.; Thiermann, H.; Nachon, F.; Worek, F. Structure–activity analysis of aging and reactivation of human butyrylcholinesterase inhibited by analogues of tabun. Biochem. J. 2009, 421, 97–106. [Google Scholar] [CrossRef] [Green Version]
- Chunhui, H.; Dilin, X.; Ke, Z.; Jieyi, S.; Sicheng, Y.; Dapeng, W.; Qinwen, W.; Wei, C. A11-positive β-amyloid Oligomer Preparation and Assessment Using Dot Blotting Analysis. J. Vis. Exp. 2018, 135, e57592. [Google Scholar] [CrossRef] [Green Version]
- Au, T.Y.; Cheung, H.T.; Sternhell, S. New corynanthé alkaloids from Strychnos angustiflora. J. Chem. Soc. Perkin Trans. 1 1973, 0, 13–16. [Google Scholar] [CrossRef]
- Shigemori, H.; Kagata, T.; Ishiyama, H.; Morah, F.; Ohsaki, A.; Kobayashi, J.i. Naucleamides A-E, New Monoterpene Indole Alkaloids from Nauclea latifolia. Chem. Pharm. Bull. 2003, 51, 58–61. [Google Scholar] [CrossRef] [Green Version]
- Cavalli, A.; Bolognesi, M.L.; Minarini, A.; Rosini, M.; Tumiatti, V.; Recanatini, M.; Melchiorre, C. Multi-target-directed ligands to combat neurodegenerative diseases. J. Med. Chem. 2008, 51, 347–372. [Google Scholar] [CrossRef]
- Doherty, G.H. Nitric oxide in neurodegeneration: Potential benefits of non-steroidal anti-inflammatories. Neurosci. Bull. 2011, 27, 366–382. [Google Scholar] [CrossRef]
- Sparrow, J.R. Inducible nitric oxide synthase in the central nervous system. J. Mol. Neurosci. 1994, 5, 219–229. [Google Scholar] [CrossRef]
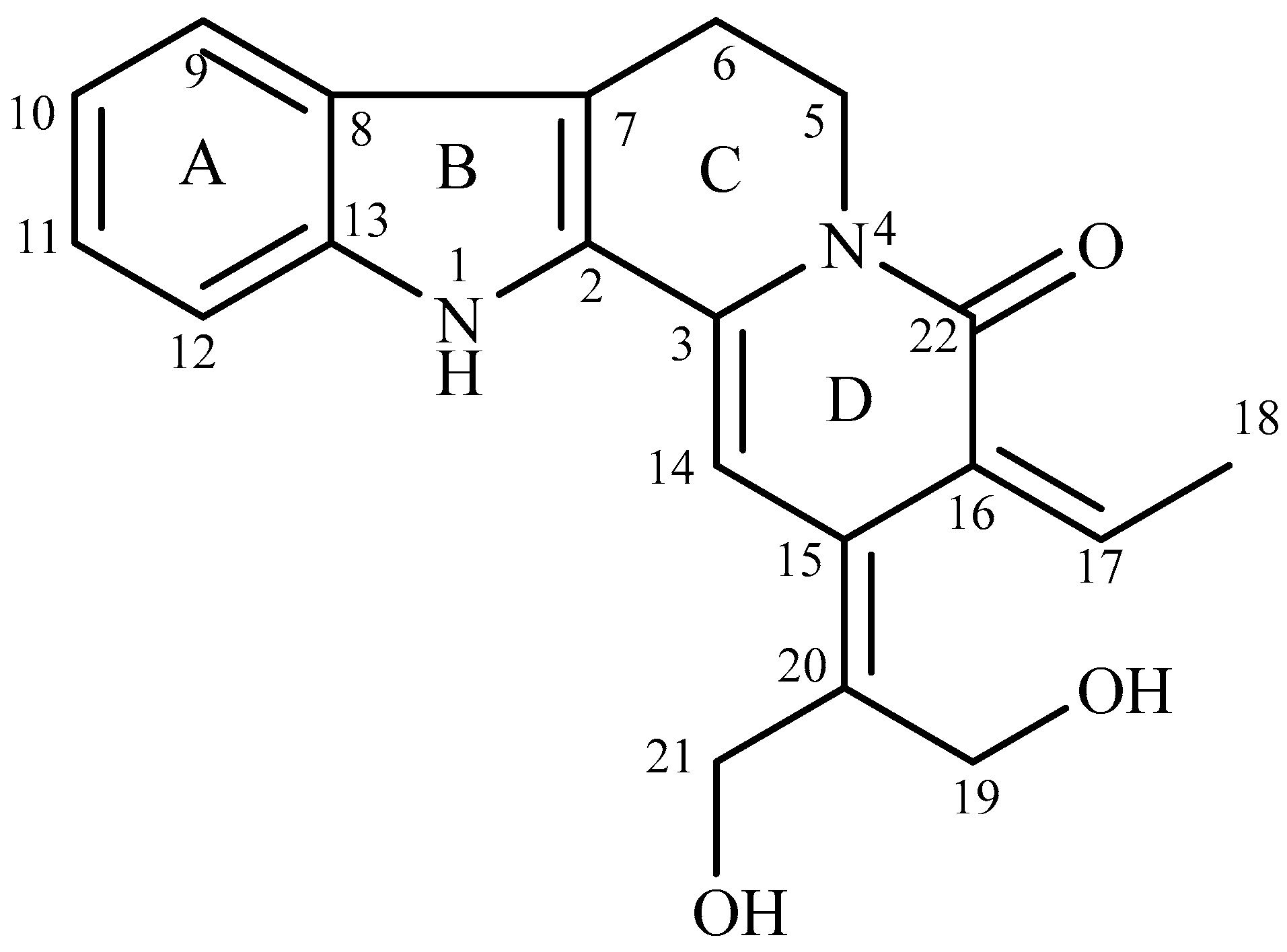
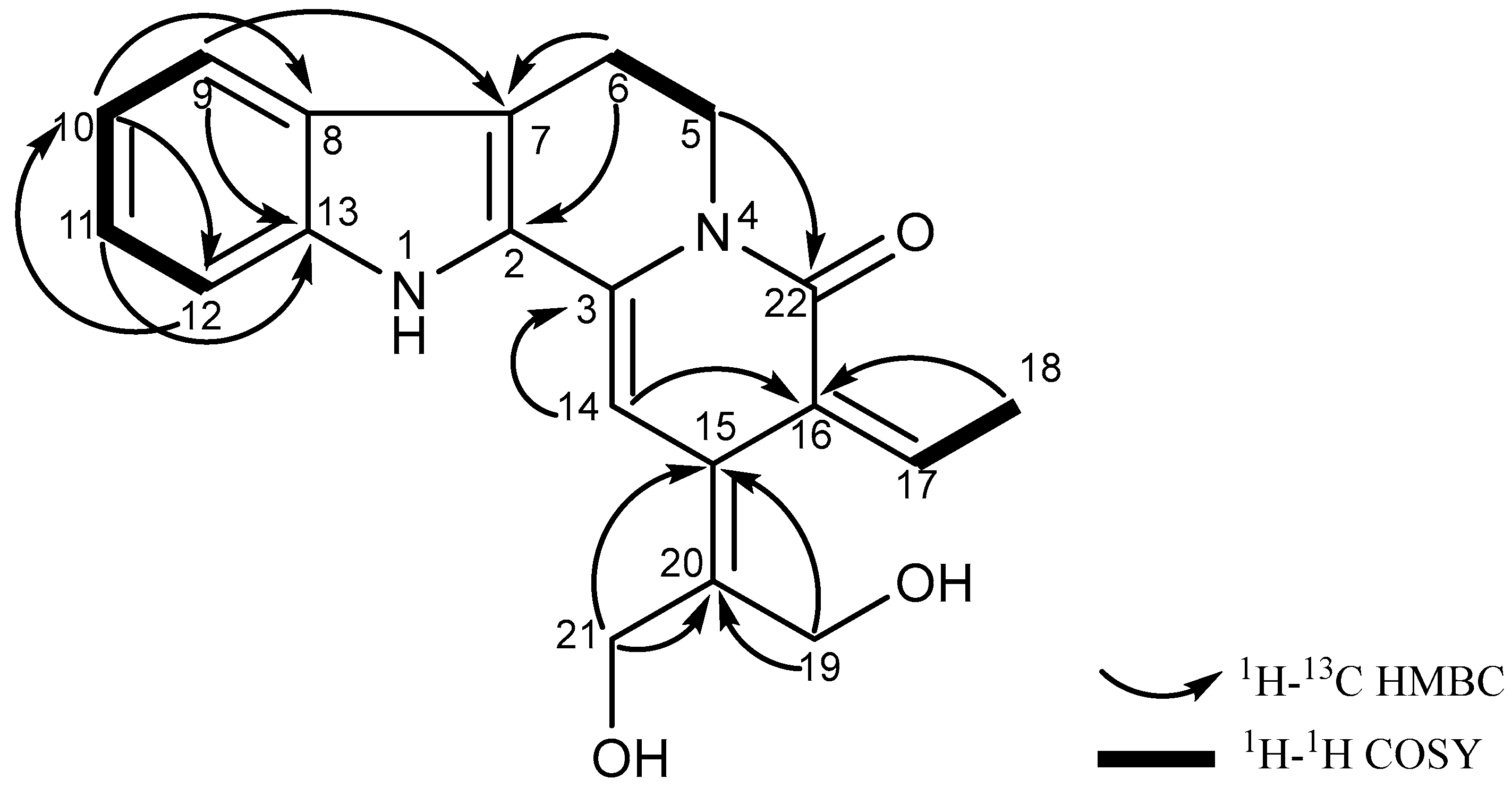
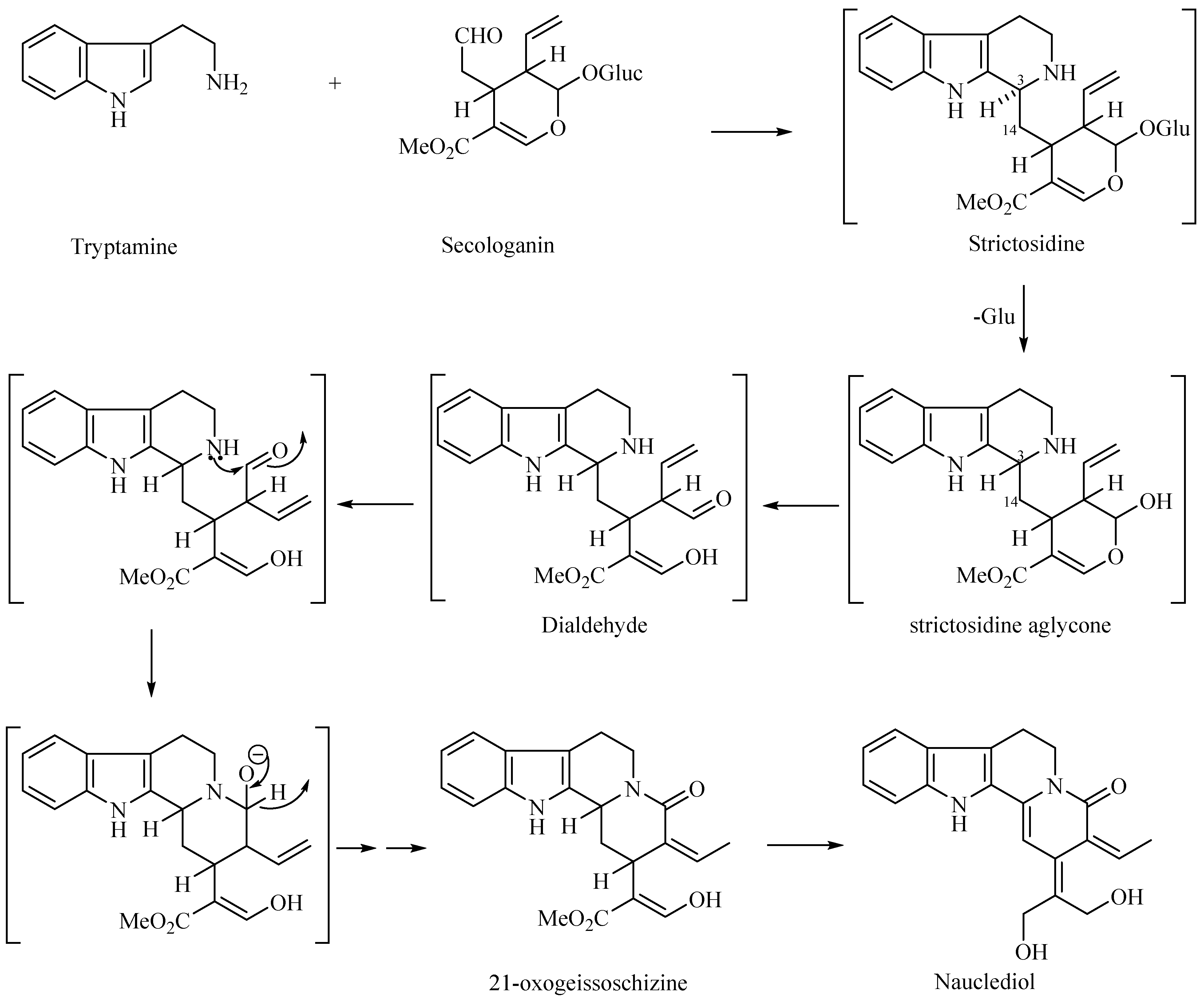
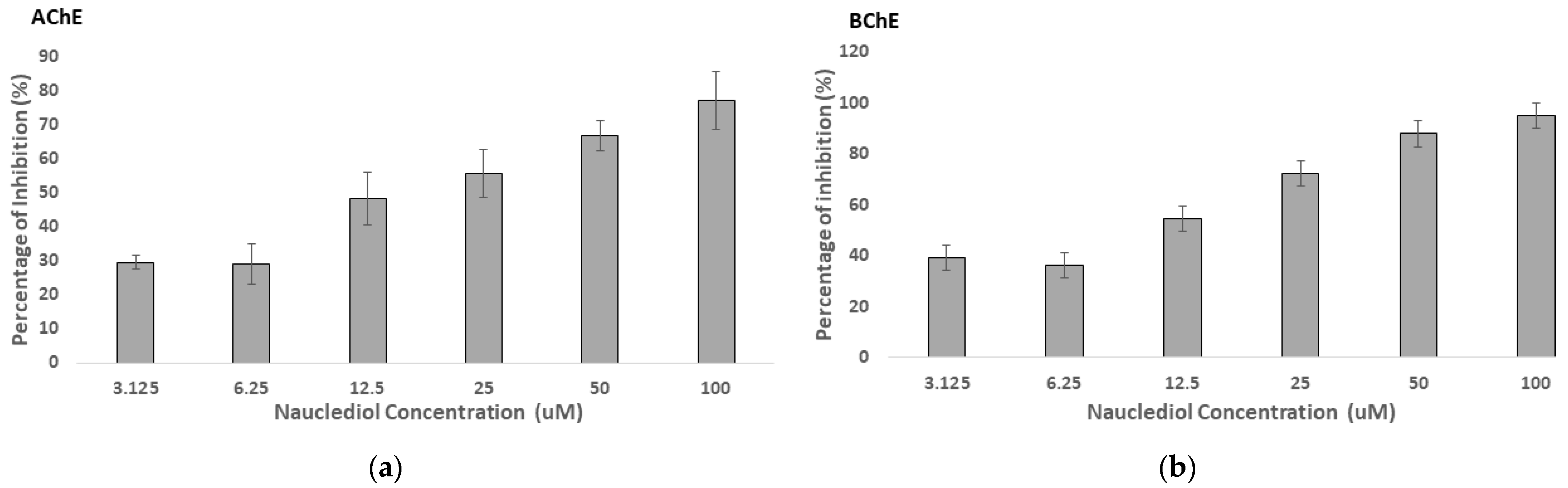


| Position | 1H, δH (Multiplicity, J in Hz) | 13C (δC) |
|---|---|---|
| 2 | - | 127.5 |
| 3 | - | 135.6 |
| 5 | 4.33 (t, 6.9) | 40.5 |
| 6 | 3.06 (t, 6.9) | 19.0 |
| 7 | - | 113.0 |
| 8 | - | 125.7 |
| 9 | 7.53 (d, 7.8) | 118.9 |
| 10 | 7.04 (t, 7.8) | 119.6 |
| 11 | 7.19 (t, 7.8) | 124.0 |
| 12 | 7.37 (d, 7.8) | 111.3 |
| 13 | - | 138.6 |
| 14 | 7.00 (s) | 95.5 |
| 15 | - | 141.2 |
| 16 | - | 120.0 |
| 17 | 6.46 (q, 6.9) | 123.8 |
| 18 | 1.86 (d, 6.9) | 12.2 |
| 19 | 4.45 (s) | 64.1 |
| 20 | - | 129.2 |
| 21 | 4.55 (s) | 64.3 |
| 22 | - | 160.9 |
| Ligand/ Compound | Enzyme | Binding Energy (kcal/mol) | Number of Conformations in the Cluster | Interacting Site | Residue | Type of Interaction | Distance (Å) | Ligand Interacting |
|---|---|---|---|---|---|---|---|---|
| Nauclediol | TcAChE | −13.01 | 64 | Catalytic triad | His 440 | Hydrogen | 2.60 | Hydroxyl group at C-19 |
| Choline binding site | Trp 84 Tyr 130 | Hydrophobic Hydrogen | - 2.26 | Ring B and D Oxygen atom of the carbonyl group C-22 | ||||
| hBChE | −12.19 | 60 | Catalytic triad | His 438 | Hydrophobic | - | Ring A and B | |
| Choline binding site | Trp 82 | Hydrophobic | - | C-17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liew, S.Y.; Mak, W.Q.; Thew, H.Y.; Khaw, K.Y.; Hazni, H.; Litaudon, M.; Awang, K. Neuroprotective Activities of New Monoterpenoid Indole Alkaloid from Nauclea officinalis. Processes 2023, 11, 646. https://doi.org/10.3390/pr11030646
Liew SY, Mak WQ, Thew HY, Khaw KY, Hazni H, Litaudon M, Awang K. Neuroprotective Activities of New Monoterpenoid Indole Alkaloid from Nauclea officinalis. Processes. 2023; 11(3):646. https://doi.org/10.3390/pr11030646
Chicago/Turabian StyleLiew, Sook Yee, Wen Qi Mak, Hin Yee Thew, Kooi Yeong Khaw, Hazrina Hazni, Marc Litaudon, and Khalijah Awang. 2023. "Neuroprotective Activities of New Monoterpenoid Indole Alkaloid from Nauclea officinalis" Processes 11, no. 3: 646. https://doi.org/10.3390/pr11030646






