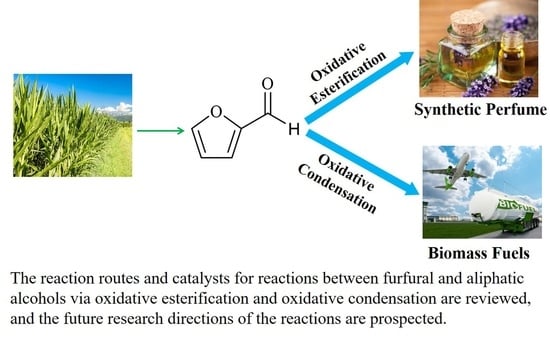
Progress of Reactions between Furfural and Aliphatic Alcohols via Catalytic Oxidation Processes: Reaction Routes, Catalysts, and Perspectives
Abstract
:1. Introduction
2. Oxidative Esterification of Furfural
2.1. Noble Metal Catalysts
2.2. Non-Noble Metal Catalysts
3. Oxidative Condensation of Furfural
3.1. Studies Using Oxygen as the Oxidant
3.2. Studies Conducted via Hydrogen Transfer Process
4. Summary and Prospect
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Delidovich, I.; Leonhard, K.; Palkovits, R. Cellulose and hemicellulose valorisation: An integrated challenge of catalysis and reaction engineering. Energy Environ. Sci. 2014, 7, 2803–2830. [Google Scholar] [CrossRef]
- Zhang, T.; Li, W.; Xiao, H.; Jin, Y.; Wu, S. Recent progress in direct production of furfural from lignocellulosic residues and hemicellulose. Bioresour. Technol. 2022, 354, 127126. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kwon, E.E.; Lee, J. Polymers derived from hemicellulosic parts of lignocellulosic biomass. Rev. Environ. Sci. Biol. 2019, 18, 317–334. [Google Scholar] [CrossRef]
- Cousin, E.; Namhaed, K.; Peres, Y.; Cognet, P.; Delmas, M.; Hermansyah, H.; Gozan, M.; Alaba, P.A.; Aroua, M.K. Towards efficient and greener processes for furfural production from biomass: A review of the recent trends. Sci. Total Environ. 2022, 847, 157599. [Google Scholar] [CrossRef]
- Jiang, Z.; Hu, D.; Zhao, Z.; Yi, Z.; Chen, Z.; Yan, K. Mini-review on the synthesis of furfural and levulinic acid from lignocellulosic biomass. Processes 2021, 9, 1234. [Google Scholar] [CrossRef]
- Mariscal, R.; Maireles-Torres, P.; Ojeda, M.; Sádaba, I.; López Granados, M. Furfural: A renewable and versatile platform molecule for the synthesis of chemicals and fuels. Energy Environ. Sci. 2016, 9, 1144–1189. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Z.; Liu, Y.N.; Meng, X.H.; Qu, J.B.; Liu, C.Y.; Qu, B. A review on the transformation of furfural residue for value-added products. Energies 2020, 13, 21. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Yin, G. Catalytic transformation of the furfural platform into bifunctionalized monomers for polymer synthesis. ACS Catal. 2021, 11, 10058–10083. [Google Scholar] [CrossRef]
- Yan, K.; Wu, G.; Lafleur, T.; Jarvis, C. Production, properties and catalytic hydrogenation of furfural to fuel additives and value−added chemicals. Renew. Sustain. Energy Rev. 2014, 38, 663–676. [Google Scholar] [CrossRef]
- Zhang, Z.; Du, B.; Quan, Z.; Da, Y.; Wang, X. Dehydration of biomass to furfural catalyzed by reusable polymer bound sulfonic acid (PEG-OSO3H) in ionic liquid. Catal. Sci. Technol. 2014, 4, 633–638. [Google Scholar] [CrossRef]
- Huo, N.; Ma, H.; Wang, X.; Wang, T.; Wang, G.; Wang, T.; Houa, L.; Gao, J.; Xu, J. High-efficiency oxidative esterification of furfural to methylfuroate with a non-precious metal Co-N-C/MgO catalyst. Chin. J. Catal. 2017, 38, 1148–1154. [Google Scholar] [CrossRef]
- Taarning, E.; Nielsen, I.S.; Egeblad, K.; Madsen, R.; Christensen, C.H. Chemicals from renewables: Aerobic oxidation of furfural and hydroxymethylfurfural over gold catalysts. ChemSusChem 2008, 1, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, I.S.; Taarning, E.; Egeblad, K.; Madsen, R.; Christensen, C.H. Direct aerobic oxidation of primary alcohols to methyl esters catalyzed by a heterogeneous gold catalyst. Catal. Lett. 2007, 116, 35–40. [Google Scholar] [CrossRef]
- Tong, X.; Liu, Z.; Yu, L.; Li, Y. A tunable process: Catalytic transformation of renewable furfural with aliphatic alcohols in the presence of molecular oxygen. Chem. Commun. 2015, 51, 3674–3677. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Tong, X.; Zhang, H. A selective oxidative valorization of biomass-derived furfural and ethanol with the supported gold catalysts. Catal. Today 2020, 355, 238–245. [Google Scholar] [CrossRef]
- Tong, X.; Yu, L.; Luo, X.; Zhuang, X.; Liao, S.; Xue, S. Efficient and selective transformation of biomass-derived furfural with aliphatic alcohols catalyzed by a binary Cu-Ce oxide. Catal. Today 2017, 298, 175–180. [Google Scholar] [CrossRef]
- Xu, B.; Liu, X.; Haubrich, J.; Friend, C.M. Vapour-phase gold-surface-mediated coupling of aldehydes with methanol. Nat. Chem. 2010, 2, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Signoretto, M.; Menegazzo, F.; Contessotto, L.; Pinna, F.; Manzoli, M.; Boccuzzi, F. Au/ZrO2: An efficient and reusable catalyst for the oxidative esterification of renewable furfural. Appl. Catal. B Environ. 2013, 129, 287–293. [Google Scholar] [CrossRef]
- Menegazzo, F.; Signoretto, M.; Pinna, F.; Manzoli, M.; Aina, V.; Cerrato, G.; Boccuzzi, F. Oxidative esterification of renewable furfural on gold-based catalysts: Which is the best support? J. Catal. 2014, 309, 241–247. [Google Scholar] [CrossRef] [Green Version]
- Manzoli, M.; Menegazzo, F.; Signoretto, M.; Cruciani, G.; Pinna, F. Effects of synthetic parameters on the catalytic performance of Au/CeO2 for furfural oxidative esterification. J. Catal. 2015, 330, 465–473. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Thiripuranthagan, S.; Devarajan, A.; Kumaravel, S.; Erusappan, E.; Kannan, K. Oxidative esterification of furfural by Au nanoparticles supported CMK-3 mesoporous catalysts. Appl. Catal. A Gen. 2017, 545, 33–43. [Google Scholar] [CrossRef]
- Jagadeesh, R.V.; Junge, H.; Pohl, M.; Radnik, J.; Brückner, A.; Beller, M. Selective oxidation of alcohols to esters using heterogeneous Co3O4-N@C catalysts under mild conditions. J. Am. Chem. Soc. 2013, 135, 10776–10782. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Song, H.; Cui, M.; Du, Y.; Fu, Y. Aerobic oxidation of hydroxymethylfurfural and furfural by using heterogeneous CoxOy-N@C catalysts. ChemSusChem 2014, 7, 3334–3340. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Ma, H.; Liu, X.; Luo, Y.; Zhang, S.; Sun, Y.; Wang, X.; Gao, J.; Xu, J. Ultrahigh-content nitrogen-doped carbon encapsulating cobalt NPs as catalyst for oxidative esterification of furfural. Chem. Asian J. 2019, 14, 1515–1522. [Google Scholar] [CrossRef]
- Liu, Z.; Tong, X.; Liu, J.; Xue, S. A smart catalyst system for the valorization of renewable furfural in aliphatic alcohols. Catal. Sci. Technol. 2016, 6, 1214–1221. [Google Scholar] [CrossRef]
- Yu, L.; Liao, S.; Ning, L.; Xue, S.; Liu, Z.; Tong, X. Sustainable and cost-effective protocol for cascade oxidative condensation of furfural with aliphatic alcohols. ACS Sustain. Chem. Eng. 2016, 4, 1894–1898. [Google Scholar] [CrossRef]
- Ning, L.; Liao, S.; Liu, X.; Yu, L.; Zhuang, X.; Tong, X. Selective transformation of renewable furfural catalyzed by diverse active species derived from 2D Co-based metal-organic frameworks. J. Catal. 2017, 352, 480–490. [Google Scholar] [CrossRef]
- Zhang, Z.; Tong, X.; Zhang, H.; Li, Y. Versatile catalysis of iron: Tunable and selective transformation of biomass-derived furfural in aliphatic alcohol. Green Chem. 2018, 20, 3092–3100. [Google Scholar] [CrossRef]
- Ning, L.; Liao, S.; Liu, X.; Guo, P.; Zhang, Z.; Zhang, H.; Tong, X. A regulatable oxidative valorization of furfural with aliphatic alcohols catalyzed by functionalized metal-organic frameworks-supported Au nanoparticles. J. Catal. 2018, 364, 1–13. [Google Scholar] [CrossRef]
- Ning, L.; Liao, S.; Cui, H.; Yu, L.; Tong, X. Selective conversion of renewable furfural with ethanol to produce furan-2-acrolein mediated by Pt@MOF-5. ACS Sustain. Chem. Eng. 2018, 6, 135–142. [Google Scholar] [CrossRef]
- Tong, X.; Zhang, Z.; Gao, Y.; Zhang, Y.; Yu, L.; Li, Y. Selective carbon-chain increasing of renewable furfural utilizing oxidative condensation reaction catalyzed by mono-dispersed palladium oxide. Mol. Catal. 2019, 477, 110545. [Google Scholar] [CrossRef]
- Cui, H.; Tong, X.; Yu, L.; Zhang, M.; Yan, Y.; Zhuang, X. A catalytic oxidative valorization of biomass-derived furfural with ethanol by copper/azodicarboxylate system. Catal. Today 2019, 319, 100–104. [Google Scholar] [CrossRef]
- Li, G.; Jiao, W.; Sun, Z.; Zhao, Y.; Shi, Z.; Yan, Y.; Feng, L.; Zhang, Y.; Tang, Y. A scalable upgrading of concentrated furfural in ethanol: Combining Meerwein–Ponndorf–Verley reduction with in situ cross aldol condensation. ACS Sustain. Chem. Eng. 2018, 6, 4316–4320. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhou, B.; Wang, L. Fe/FeOx embedded in LDH catalyzing C-C bond forming reactions of furfural with alcohols in the absence of a homogeneous base. Mol. Catal. 2020, 493, 111056. [Google Scholar] [CrossRef]









| Entry | Condition | Catalyst | Furfural Conversion (%) | MF Selectivity (%) | Ref. |
|---|---|---|---|---|---|
| 1 | Catalyst (0.25 g), furfural (4 mmol), methanol (12.65 mL), molar ratio Au/furfural/methanol = 1/300/23,500, 8% CH3ONa relative to furfural, 22 °C, 0.1 MPa O2 (bubbling conditions), 10–12 h. | Au/TiO2 + CH3ONa | ~100 | ~100 | [13] |
| 2 a | Catalyst (0.1 g), furfural (3.6 mmol), methanol (150 mL), molar ratio Au/furfural/methanol = 1/500/5 × 105, 120 °C, 0.6 MPa O2, 3 h. | AZ150 | 99 | 94 | [18] |
| AZ300 | 98 | 94 | |||
| AZ500 | 100 | 98 | |||
| AZ600 | 57 | 76 | |||
| AZ650 | 24 | 60 | |||
| 3 | Catalyst (0.1 g), furfural (3.6 mmol), methanol (150 mL), molar ratio Au/furfural/methanol = 1/500/5 × 105, 120 °C, 0.6 MPa O2, 90 min. | Au/ZrO2 | 82 | 92 | [19] |
| Au/CeO2 | 66 | ~70 | |||
| Au/TiO2 | 20 | ~90 | |||
| 4 b | Catalyst (0.1 g), furfural (3.6 mmol), methanol (150 mL), molar ratio Au/furfural/methanol = 1/500/5 × 105, 120 °C, 0.6 MPa O2, 90 min. | Ce90Au300 | 29 | 100 | [20] |
| Ce110Au300 | 29 | 100 | |||
| Ce300Au300 | 54 | 100 | |||
| Ce90Au500 | 6 | 100 | |||
| Ce110Au500 | 28 | 100 | |||
| Ce500Au500 | 74 | 100 | |||
| 5 | Catalyst (0.05 g), furfural (2.1 mmol), K2CO3 (0.05 g), methanol (15 mL), molar ratio Au/furfural/methanol = 1/160/29,000, 140 °C, 0.3 MPa O2, 4 h. | Au/FH + K2CO3 | 93 | 99 | [14] |
| 6 | Catalyst (0.05 g), furfural (3.6 mmol), methanol (20 mL), molar ratio Au/furfural/methanol = 1/300/38,900, 120 °C, 1.5 MPa O2, 3 h. | 5%Au/CMK-3 | 99.7 | 99.6 | [21] |
| Entry | Condition | Catalyst | Furfural Conversion (%) | MF Selectivity (%) | Ref. |
|---|---|---|---|---|---|
| 1 | 25 mg catalyst, 0.5 mmol furfural, 4 mL methanol, 0.2 equiv K2CO3, 0.1 MPa O2, 60 °C, 12 h. | CoxOy-N@C | 100 | 95 | [23] |
| CoxOy-2,2-diPy/C | 58 | 46 | |||
| 2 | 80 mg catalyst, 0.5 mmol furfural, 5 mL methanol, 0.5 MPa O2, 100 °C, 6 h. | Co-N-C/MgO | 89.3 | 90.1 | [11] |
| Co-N-C/MgO a | 93.0 | 98.5 | |||
| CoOx-N/C | 67.2 | 84.4 | |||
| CoNC/CaO | 22.0 | 38.8 | |||
| CoNC/NaX | 40.1 | 83.6 | |||
| CoNC/NaY | 64.0 | 85.4 | |||
| 3 | 60 mg catalyst, 1 mmol furfural, 5 mL of methanol, 0.5 MPa O2, 100 °C, 6 h. | CoOx@N-C(g) | 95.0 | 97.1 | [24] |
| Entry | Condition | Catalyst | Aliphatic Alcohols | Furfural Conversion (%) | Product Seletivity (%) | Ref. |
|---|---|---|---|---|---|---|
| 1 | 0.2 g furfural, 0.05 g catalyst, 0.05 g K2CO3, 15 mL n-propanol, 0.3 MPa O2, 140 °C, 4 h. | Au/FH + K2CO3 | n-propanol | 94 | 97 | [14] |
| 2 | 0.2 g furfural, 0.05 g catalyst, 0.05 g K2CO3, 15 mL ethanol or n-propanol, 0.3 MPa O2, 140 °C, 4 h. | Pt/FH + K2CO3 | n-propanol | 90.1 | 90.0 | [25] |
| Pt/H + K2CO3 | n-propanol | 73.0 | 90.1 | |||
| Pt/HTc + K2CO3 | n-propanol | 87.8 | 88.5 | |||
| Pt/Fe3O4 + K2CO3 | n-propanol | 80.6 | 91.1 | |||
| Pt/Al2O3 + K2CO3 | n-propanol | 81.4 | 90.4 | |||
| Pt/ZrO2 + K2CO3 | n-propanol | 31.9 | 90.0 | |||
| Pt/FH + K2CO3 | ethanol | 93.9 | 67.9 | |||
| 3 | 0.2 g furfural, 0.05 g catalyst, 0.05 g additive, 15 mL n-propanol, 0.3 MPa O2, 140 °C, 4 h. | Pt/FH + Cs2CO3 | n-propanol | 75.1 | 92.8 | [26] |
| Pt/FH + K2CO3 | 36.4 | 91.2 | ||||
| Pt/FH + Na2CO3 | 12.5 | 2.1 | ||||
| Pt/FH + Li2CO3 | 3.8 | 4.3 | ||||
| Pt/FH + CaCO3 | 25.0 | 1.0 | ||||
| Pt/FH + KOH | 82.3 | 93.2 | ||||
| Pt/FH + NaOH | 75.9 | 53.4 | ||||
| Pt/FH + H3PO4 | 49.3 | 2.1 | ||||
| 4 | 0.2 g furfural, 0.05 g catalyst, 0.05 g K2CO3, 15 mL n-propanol, 0.3 MPa O2, 140 °C, 4 h. | CuO-CeO2 + K2CO3 | n-propanol | 85.4 | 95.3 | [16] |
| ethanol | 83.2 | 92.1 | ||||
| 5 | 0.1 g furfural, 0.025 g catalyst, 0.025 g Cs2CO3, 15 mL ethanol or n-propanol, 0.3 MPa O2, 140 °C, 4 h. | ACS-I + Cs2CO3 | n-propanol | 63.4 | 99 | [27] |
| ACS-II + Cs2CO3 | n-propanol | 84.9 | 99.7 | |||
| ACS-I + Cs2CO3 | ethanol | 74.9 | 93.9 | |||
| ACS-II + Cs2CO3 | ethanol | 83.3 | 83.2 | |||
| 6 | 0.2 g furfural, 0.05 g catalyst, 0.05 g K2CO3, 15 mL aliphatic alcohol, 0.3 MPa O2, 140 °C, 4 h. | Fe@C + K2CO3 | ethanol | 84.2 | 82.7 | [28] |
| n-propanol | 86.7 | 96.2 | ||||
| i-propanol | 50.7 | 78.1 | ||||
| n-butanol | 55.6 | 53.3 | ||||
| n-pentanol | 34.9 | 47.9 | ||||
| n-hexanol | 26.8 | 36.4 | ||||
| 7 | 0.1 g furfural, 0.025 g catalyst, 0.025 g K2CO3, 15 mL ethanol, 0.3 MPa O2, 140 °C, 4 h. | Au@UiO-66-COOH + K2CO3 | ethanol | 66.7 | 84.1 | [29] |
| 8 | 0.1 g furfural, 0.025 g catalyst, 0.025 g K2CO3, 15 mL ethanol, 0.3 MPa O2, 140 °C, 4 h. | Pt@MOF-5 + K2CO3 | ethanol | 84.1 | 90.1 | [30] |
| 9 | 0.2 g furfural, 0.05 g catalyst, 0.05 g K2CO3, 15 mL ethanol or n-propanol, 0.3 MPa O2, 140 °C, 4 h. | PdO@TiO2 + K2CO3 | n-propanol | 77.8 | 89.2 | [31] |
| Ethanol | 88.4 | 85.7 | ||||
| 10 | 0.2 g furfural, the initial ratio of azo dicarboxylates and K2CO3 is 7.5:10, 7.25% mol CuI, 7.25% mol Phen, 17.4mol% DBAD, 15 mL ethanol, 0.3 MPa O2, 160 °C, 4 h. | CuI and o-phenanthroline + K2CO3 | Ethanol | 82.5 | 87.7 | [32] |
| 11 | 0.2 g furfural, 0.1 g catalyst, 15 mL ethanol, 0.3 MPa O2, 140 °C, 4 h. | Au@CaO | Ethanol | 85.9 | 81.8 | [15] |
| Entry | Gas Atmosphere | Furfural Conversion (%) | Product Seletivity (%) |
|---|---|---|---|
| 1 | O2 | 20.8 | ~99 |
| 2 | Ar | 71.3 | 87.1 |
| 3 | mixture gas of Ar (95%) and H2 (5%) | 71.4 | ~99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, J.; Cheng, X.; Fan, Y.; Fang, W.; Dong, P.; Li, G.; Guo, Y. Progress of Reactions between Furfural and Aliphatic Alcohols via Catalytic Oxidation Processes: Reaction Routes, Catalysts, and Perspectives. Processes 2023, 11, 640. https://doi.org/10.3390/pr11020640
Tian J, Cheng X, Fan Y, Fang W, Dong P, Li G, Guo Y. Progress of Reactions between Furfural and Aliphatic Alcohols via Catalytic Oxidation Processes: Reaction Routes, Catalysts, and Perspectives. Processes. 2023; 11(2):640. https://doi.org/10.3390/pr11020640
Chicago/Turabian StyleTian, Junying, Xiaowei Cheng, Yingying Fan, Weiguo Fang, Peng Dong, Guixian Li, and Yongle Guo. 2023. "Progress of Reactions between Furfural and Aliphatic Alcohols via Catalytic Oxidation Processes: Reaction Routes, Catalysts, and Perspectives" Processes 11, no. 2: 640. https://doi.org/10.3390/pr11020640