Modelling and Optimization of Methylene Blue Adsorption Process on Leonurus cardiaca L. Biomass Powder
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. SEM and Color Analyzes for Adsorbent Material
3.2. Dye Adsorption Process Optimization Using Taguchi Methods
3.3. Equilibrium Isotherms
3.4. Adsorption Kinetic
3.5. Thermodynamic Study
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hamad, H.N.; Idrus, S. Recent Developments in the Application of Bio-Waste-Derived Adsorbents for the Removal of Methylene Blue from Wastewater: A Review. Polymers 2022, 14, 783. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Syakir Ishak, M.I.; Bhawani, S.A.; Umar, K. Various Natural and Anthropogenic Factors Responsible for Water Quality Degradation: A Review. Water 2021, 13, 2660. [Google Scholar] [CrossRef]
- Beagan, A.; Alshammari, R.; Alotaibi, L.; Albarrak, H.; Alotaibi, K.; Alswieleh, A. High-Efficient Anionic Dyes Removal from Water by Cationic Polymer Brush Functionalized Magnetic Mesoporous Silica Nanoparticles. Processes 2022, 10, 1565. [Google Scholar] [CrossRef]
- Salzano de Luna, M.; Greco, F.; Pastore, R.; Mensitieri, G.; Filippone, G.; Aprea, P.; Mallamace, D.; Mallamace, F.; Chen, S.-H. Tailoring Chitosan/LTA Zeolite Hybrid Aerogels for Anionic and Cationic Dye Adsorption. Int. J. Mol. Sci. 2021, 22, 5535. [Google Scholar] [CrossRef] [PubMed]
- Valério Filho, A.; Xavaré Kulman, R.; Vaz Tholozan, L.; Felkl de Almeida, A.R.; Silveira da Rosa, G. Preparation and Characterization of Activated Carbon Obtained from Water Treatment Plant Sludge for Removal of Cationic Dye from Wastewater. Processes 2020, 8, 1549. [Google Scholar] [CrossRef]
- Alghamdi, W.M.; El Mannoubi, I. Investigation of Seeds and Peels of Citrullus colocynthis as Efficient Natural Adsorbent for Methylene Blue Dye. Processes 2021, 9, 1279. [Google Scholar] [CrossRef]
- Iwuozor, K.O.; Ighalo, J.O.; Ogunfowora, L.A.; Adeniyi, A.G.; Igwegbe, C.A. An empirical literature analysis of adsorbent performance for methylene blue uptake from aqueous media. J. Environ. Chem. Eng. 2021, 9, 105658. [Google Scholar] [CrossRef]
- Ge, M.; Xi, Z.; Zhu, C.; Liang, G.; Yang, Y.; Hu, G.; Jamal, L.; Jahangir Alam, S.M. Adsorption Process and Properties Analyses of a Pure Magadiite and a Modified Magadiite on Rhodamine-B from an Aqueous Solution. Processes 2019, 7, 565. [Google Scholar] [CrossRef]
- Shakoor, S.; Nasar, A. Removal of methylene blue dye from artificially contaminated water using citrus limetta peel waste as a very low cost adsorbent. J. Taiwan Inst. Chem. Eng. 2016, 66, 154–163. [Google Scholar] [CrossRef]
- Setiabudi, H.D.; Jusoh, R.; Suhaimi, S.F.R.M.; Masrur, S.F. Adsorption of methylene blue onto oil palm (Elaeis guineensis) leaves: Process optimization, isotherm, kinetics and thermodynamic studies. J. Taiwan Inst. Chem. Eng. 2016, 63, 363–370. [Google Scholar] [CrossRef]
- Mitrogiannis, D.; Markou, G.; Çelekli, A.; Bozkurt, H. Biosorption of methylene blue onto Arthrospira platensis biomass: Kinetic, equilibrium and thermodynamic studies. J. Environ. Chem. Eng. 2015, 3, 670–680. [Google Scholar] [CrossRef]
- Mosoarca, G.; Vancea, C.; Popa, S.; Dan, M.; Boran, S. The Use of Bilberry Leaves (Vaccinium myrtillus L.) as an Efficient Adsorbent for Cationic Dye Removal from Aqueous Solutions. Polymers 2022, 14, 978. [Google Scholar] [CrossRef] [PubMed]
- Khodabandehloo, A.; Rahbar-Kelishami, A.; Shayesteh, H. Methylene blue removal using Salix babylonica (Weeping willow) leaves powder as a low-cost biosorbent in batch mode: Kinetic, equilibrium, and thermodynamic studies. J. Mol. Liq. 2017, 244, 540–548. [Google Scholar] [CrossRef]
- Weng, C.H.; Lin, Y.T.; Tzeng, T.W. Removal of methylene blue from aqueous solution by adsorption onto pineapple leaf powder. J. Hazard. Mater. 2009, 170, 417–424. [Google Scholar] [CrossRef]
- Bulgariu, L.; Escudero, L.B.; Bello, O.S.; Iqbal, M.; Nisar, J.; Adegoke, K.A.; Alakhras, F.; Kornaros, M.; Anastopoulos, I. The utilization of leaf-based adsorbents for dyes removal: A review. J. Mol. Liq. 2019, 276, 728–747. [Google Scholar] [CrossRef]
- Peydayesh, M.; Rahbar-Kelishami, A. Adsorption of methylene blue onto Platanus orientalis leaf powder: Kinetic, equilibrium and thermodynamic studies. J. Ind. Eng. Chem. 2015, 21, 1014–1019. [Google Scholar] [CrossRef]
- Mosoarca, G.; Popa, S.; Vancea, C.; Boran, S. Optimization, Equilibrium and Kinetic Modeling of Methylene Blue Removal from Aqueous Solutions Using Dry Bean Pods Husks Powder. Materials 2021, 14, 5673. [Google Scholar] [CrossRef]
- Shojaei, S.; Shojaei, S.; Band, S.S.; Farizhandi, A.A.K.; Ghoroqi, M.; Mosavi, A. Application of Taguchi method and response surface methodology into the removal of malachite green and auramine-O by NaX nanozeolites. Sci. Rep. 2021, 11, 16054. [Google Scholar] [CrossRef]
- Korake, S.R.; Jadhao, P.D. Investigation of Taguchi optimization, equilibrium isotherms, and kinetic modeling for cadmium adsorption onto deposited silt. Heliyon 2020, 6, e05755. [Google Scholar] [CrossRef]
- Fernandez-Lopez, J.A.; Angosto, J.M.; Roca, M.J.; Doval Minarro, M. Taguchi design-based enhancement of heavy metals bioremoval by agroindustrial waste biomass from artichoke. Sci. Total Environ. 2019, 653, 55–63. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. J. Chem. Eng. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Piccin, J.S.; Cadaval, T.R.S.; de Pinto, L.A.A.; Dotto, G.L. Adsorption isotherms in liquid phase: Experimental, modeling, and interpretations. In Adsorption Processes for Water Treatment and Purification; Bonilla-Petriciolet, A., Mendoza-Castillo, D., Reynel-Avila, H., Eds.; Springer: Cham, Switzerland, 2017; pp. 19–51. [Google Scholar]
- Yadav, M.; Thakore, S.; Jadeja, R. Removal of organic dyes using Fucus vesiculosus seaweed bioadsorbent an ecofriendly approach: Equilibrium, kinetics and thermodynamic studies. J. Environ. Chem. Ecotoxicol. 2022, 4, 67–77. [Google Scholar] [CrossRef]
- Sobolčiak, P.; Popelka, A.; Tanvir, A.; Al-Maadeed, M.A.; Adham, S.; Krupa, I. Some Theoretical Aspects of Tertiary Treatment of Water/Oil Emulsions by Adsorption and Coalescence Mechanisms: A Review. Water 2021, 13, 652. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef] [PubMed]
- Dotto, G.L.; Salau, N.P.G.; Piccin, J.S.; Cadaval, T.R.S.; de Pinto, L.A.A. Adsorption kinetics in liquid phase: Modeling for discontinuous and continuous systems. In Adsorption Processes for Water Treatment and Purification; Bonilla-Petriciolet, A., Mendoza-Castillo, D., Reynel-Avila, H., Eds.; Springer: Cham, Switzerland, 2017; pp. 53–76. [Google Scholar]
- Wanga, J.; Guo, X. Adsorption kinetic models: Physical meanings, applications, and solving methods. J. Hazard. Mater. 2020, 390, 122156. [Google Scholar] [CrossRef] [PubMed]
- Googerdchian, F.; Moheb, A.; Emadi, R.; Asgari, M. Optimization of Pb(II) ions adsorption on nanohydroxyapatite adsorbents by applying Taguchi method. J. Hazard. Mater. 2018, 349, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Zolgharnein, J.; Rastgordani, M. Optimization of simultaneous removal of binary mixture of indigo carmine and methyl orange dyes by cobalt hydroxide nano-particles through Taguchi method. J. Mol. Liq. 2018, 262, 405–414. [Google Scholar] [CrossRef]
- Razmi, B.; Ghasemi-Fasaei, R. Investigation of Taguchi optimization, equilibrium isotherms, and kinetic modeling for phosphorus adsorption onto natural zeolite of clinoptilolite type. Adsorp. Sci. Technol. 2018, 36, 1470–1483. [Google Scholar] [CrossRef]
- Ghorbani, F.; Kamari, S. Application of response surface methodology for optimization of methyl orange adsorption by Fe-grafting sugar beet bagasse. Adsorp. Sci. Technol. 2017, 35, 317–338. [Google Scholar] [CrossRef]
- Madan, S.S.; Wasewar, K.L. Optimization for benzeneacetic acid removal from aqueous solution using CaO2 nanoparticles based on Taguchi method. J. Appl. Res. Technol. 2017, 15, 332–339. [Google Scholar] [CrossRef]
- Mosoarca, G.; Vancea, C.; Popa, S.; Dan, M.; Boran, S. Crystal Violet Adsorption on Eco-Friendly Lignocellulosic Material Obtained from Motherwort (Leonurus cardiaca L.) Biomass. Polymers 2022, 14, 3825. [Google Scholar] [CrossRef]
- Mosoarca, G.; Popa, S.; Vancea, C.; Dan, M.; Boran, S. Removal of Methylene Blue from Aqueous Solutions Using a New Natural Lignocellulosic Adsorbent—Raspberry (Rubus idaeus) Leaves Powder. Polymers 2022, 14, 1966. [Google Scholar] [CrossRef] [PubMed]
- Vasanth Kumar, K.; Porkodi, K. Relation between some two- and three-parameter isotherm models for the sorption of methylene blue onto lemon peel. J. Hazard. Mater. 2006, 138, 633–635. [Google Scholar] [CrossRef] [PubMed]
- Balarak, D.; Jaafari, J.; Hassani, G.; Mahdavi, Y.; Tyagi, I.; Agarwal, S.; Gupta, V.K. The use of low-cost adsorbent (Canola residues) for the adsorption of methylene blue from aqueous solution: Isotherm, kinetic and thermodynamic studies. Colloids Interface Sci. Commun. 2015, 7, 16–19. [Google Scholar] [CrossRef]
- Ahmed, M.J.; Dhedan, S.K. Equilibrium isotherms and kinetics modeling of methylene blue adsorption on agricultural wastes-based activated carbons. Fluid Ph. Equilibria 2012, 317, 9–14. [Google Scholar] [CrossRef]
- Shahryari, Z.; Goharrizi, A.S.; Azadi, M. Experimental study of methylene blue adsorption from aqueous solutions onto carbon nano tubes. Int. J. Water Res. Environ. Eng. 2010, 2, 16–28. [Google Scholar]
- Fiaz, R.; Hafeez, M.; Mahmood, R. Ficcus palmata leaves as a low-cost biosorbent for methylene blue: Thermodynamic and kinetic studies. Water Environ. Res. 2019, 91, 689–699. [Google Scholar] [CrossRef]
- Bhattacharyya, K.G.; Sharma, A. Kinetics and thermodynamics of Methylene Blue adsorption on Neem (Azadirachta indica) leaf powder. Dyes Pigm. 2005, 65, 51–59. [Google Scholar] [CrossRef]
- Singh, R.; Singh, T.S.; Odiyo, J.O.; Smith, J.A.; Edokpayi, J.N. Evaluation of methylene blue sorption onto low-cost biosorbents: Equilibrium, kinetics, and thermodynamics. J. Chem. 2020, 2020, 8318049. [Google Scholar] [CrossRef]
- Gupta, N.; Kushwaha, A.K.; Chattopadhyaya, M.C. Application of potato (Solanum tuberosum) plant wastes for the removal of methylene blue and malachite green dye from aqueous solution. Arab. J. Chem. 2016, 9, S707–S716. [Google Scholar] [CrossRef]
- Kushwaha, A.K.; Gupta, N.; Chattopadhyaya, M.C. Removal of cationic methylene blue and malachite green dyes from aqueous solution by waste materials of Daucus carota. J. Saudi Chem. Soc. 2014, 18, 200–207. [Google Scholar] [CrossRef]
- Han, R.; Zou, W.; Yu, W.; Cheng, S.; Wang, Y.; Shi, J. Biosorption of methylene blue from aqueous solution by fallen phoenix tree’s leaves. J. Hazard. Mater. 2007, 141, 156–162. [Google Scholar] [CrossRef]
- Zolgharnein, J.; Bagtash, M. Hybrid central composite design optimization for removal of Methylene blue by Acer tree leaves: Characterization of adsorption. Desalin. Water Treat. 2015, 54, 2601–2610. [Google Scholar] [CrossRef]
- Krishni, R.R.; Foo, K.Y.; Hameed, B.H. Adsorptive removal of methylene blue using the natural adsorbent-banana leaves. Desalin. Water Treat. 2014, 52, 6104–6112. [Google Scholar] [CrossRef]
- Dotto, G.L.; Santos, J.M.N.; Rodrigues, I.L.; Rosa, R.; Pavan, F.A.; Lima, E.C. Adsorption of Methylene Blue by ultrasonic surface modified chitin. J. Colloid Interface Sci. 2015, 446, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Diehl, M.; Silva, L.F.O.; Schnorr, C.; Netto, M.S.; Bruckmann, F.S.; Dotto, G.L. Cassava bagasse as an alternative biosorbent to uptake methylene blue environmental pollutant from water. Environ. Sci. Pollut. Res. 2023, 30, 51920–51931. [Google Scholar] [CrossRef] [PubMed]
- Schadeck Netto, M.; da Silva, N.F.; Mallmann, E.S.; Dotto, G.L.; Foletto, E.L. Effect of Salinity on the Adsorption Behavior of Methylene Blue onto Comminuted Raw Avocado Residue: CCD-RSM Design. Water Air Soil Pollut. 2019, 230, 187. [Google Scholar] [CrossRef]
- Hernandes, P.T.; Oliveira, M.L.S.; Georgin, J.; Franco, D.S.P.; Allasia, D.; Dotto, G.L. Adsorptive decontamination of wastewater containing methylene blue dye using golden trumpet tree bark (Handroanthus albus). Environ. Sci. Pollut. Res. 2019, 26, 31924–31933. [Google Scholar] [CrossRef]
- Wakkel, M.; Khiari, B.; Zagrouba, F. Textile wastewater treatment by agro-industrial waste: Equilibrium modelling, thermodynamics and mass transfer mechanisms of cationic dyes adsorption onto low-cost lignocellulosic adsorbent. J. Taiwan Inst. Chem. Eng. 2019, 96, 439–452. [Google Scholar] [CrossRef]





| Parameters | Equation |
|---|---|
| Dye removal percentage | |
| Adsorption capacity |
| Equilibrium Isotherm | Equation |
|---|---|
| Langmuir | |
| Freundlich | |
| Temkin | |
| Redlich–Peterson | |
| Sips |
| Kinetic Models | Equation |
|---|---|
| Pseudo-first-order | |
| Pseudo-second-order | |
| Elovich | |
| Avrami | |
| General order |
| Error Parameter | Equation |
|---|---|
| R2 | |
| R2adj | |
| SSE | |
| χ2 | |
| ARE | |
| HYBRID |
| Thermodynamic Parameters | Equation |
|---|---|
| Standard Gibbs free energy change | |
| Standard enthalpy change | |
| Standard entropy change |
| Factor | Level 1 | Level 2 | Level 3 |
|---|---|---|---|
| pH | 2 | 6 | 10 |
| Time (min) | 5 | 30 | 60 |
| Adsorbent dose (g·L−1) | 1 | 3 | 5 |
| Initial dye concentration (mg·L−1) | 25 | 100 | 200 |
| Temperature (K) | 281 | 291 | 305 |
| Ionic strength (mol L−1) | 0 | 0.15 | 0.25 |
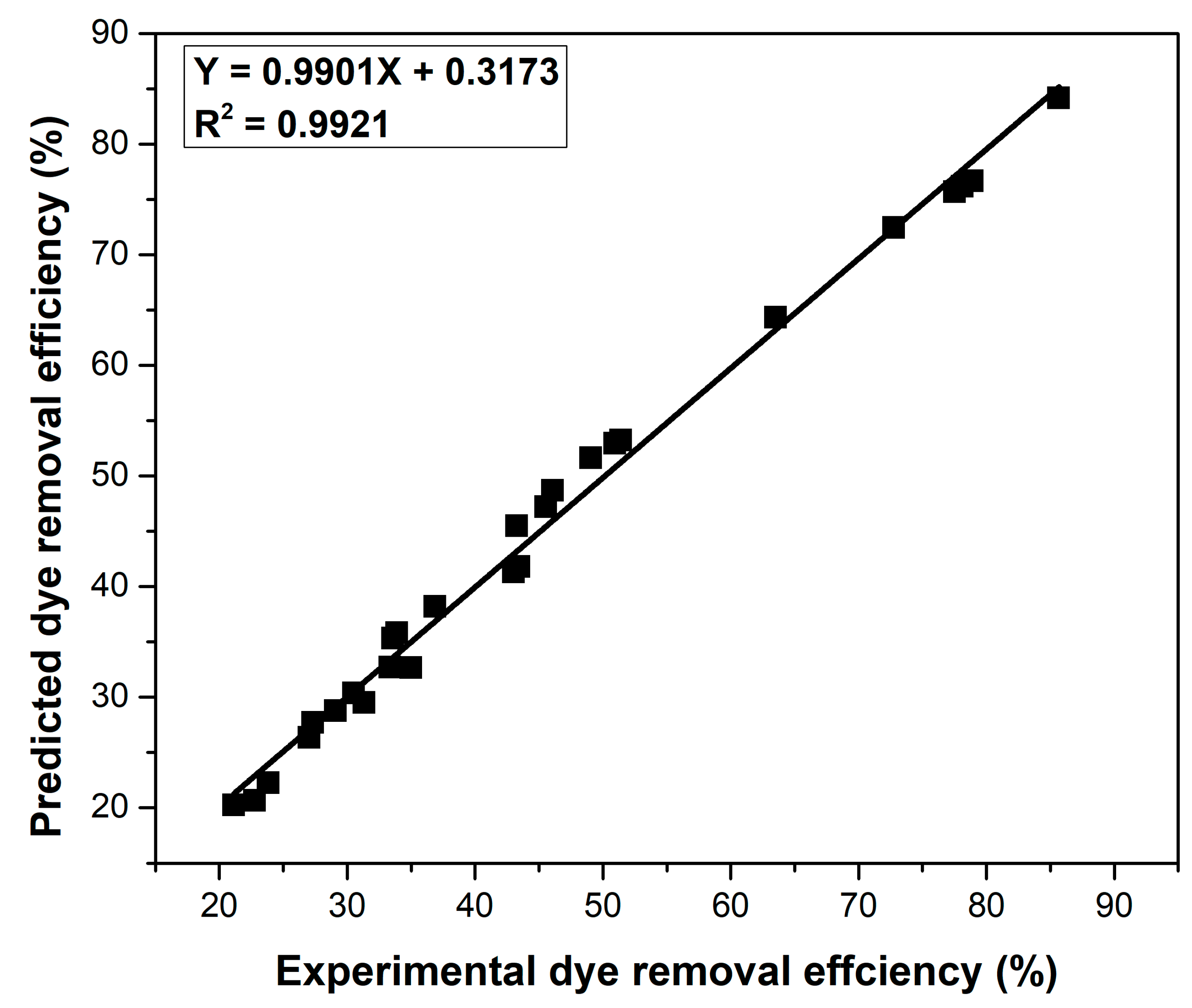
| pH | Time (min) | Adsorbent Dose (g L−1) | Initial Dye Concentration (mg L−1) | Temperature (K) | Ionic Strength (mol L−1) | Dye Removal Efficiency (%) | S/N Ratio |
|---|---|---|---|---|---|---|---|
| 2 | 5 | 1 | 25 | 281 | 0.00 | 45.56 | 33.17 |
| 2 | 5 | 1 | 25 | 291 | 0.15 | 27.04 | 28.64 |
| 2 | 5 | 1 | 25 | 305 | 0.25 | 21.14 | 26.50 |
| 2 | 30 | 3 | 100 | 281 | 0.00 | 51.39 | 34.21 |
| 2 | 30 | 3 | 100 | 291 | 0.15 | 30.50 | 29.68 |
| 2 | 30 | 3 | 100 | 305 | 0.25 | 23.85 | 27.55 |
| 2 | 60 | 5 | 200 | 281 | 0.00 | 49.05 | 33.81 |
| 2 | 60 | 5 | 200 | 291 | 0.15 | 29.11 | 29.28 |
| 2 | 60 | 5 | 200 | 305 | 0.25 | 22.76 | 27.14 |
| 6 | 5 | 3 | 200 | 281 | 0.15 | 34.98 | 30.87 |
| 6 | 5 | 3 | 200 | 291 | 0.25 | 27.30 | 28.72 |
| 6 | 5 | 3 | 200 | 305 | 0.00 | 63.54 | 36.06 |
| 6 | 30 | 5 | 25 | 281 | 0.15 | 43.43 | 32.75 |
| 6 | 30 | 5 | 25 | 291 | 0.25 | 33.90 | 30.60 |
| 6 | 30 | 5 | 25 | 305 | 0.00 | 78.90 | 37.94 |
| 6 | 60 | 1 | 100 | 281 | 0.15 | 43.01 | 32.67 |
| 6 | 60 | 1 | 100 | 291 | 0.25 | 33.57 | 30.52 |
| 6 | 60 | 1 | 100 | 305 | 0.00 | 78.12 | 37.85 |
| 10 | 5 | 5 | 100 | 281 | 0.25 | 33.37 | 30.46 |
| 10 | 5 | 5 | 100 | 291 | 0.00 | 77.52 | 37.78 |
| 10 | 5 | 5 | 100 | 305 | 0.15 | 46.09 | 33.27 |
| 10 | 30 | 1 | 200 | 281 | 0.25 | 31.32 | 29.91 |
| 10 | 30 | 1 | 200 | 291 | 0.00 | 72.76 | 37.23 |
| 10 | 30 | 1 | 200 | 305 | 0.15 | 43.26 | 32.72 |
| 10 | 60 | 3 | 25 | 281 | 0.25 | 36.87 | 31.33 |
| 10 | 60 | 3 | 25 | 291 | 0.00 | 85.67 | 38.65 |
| 10 | 60 | 3 | 25 | 305 | 0.15 | 50.93 | 34.14 |
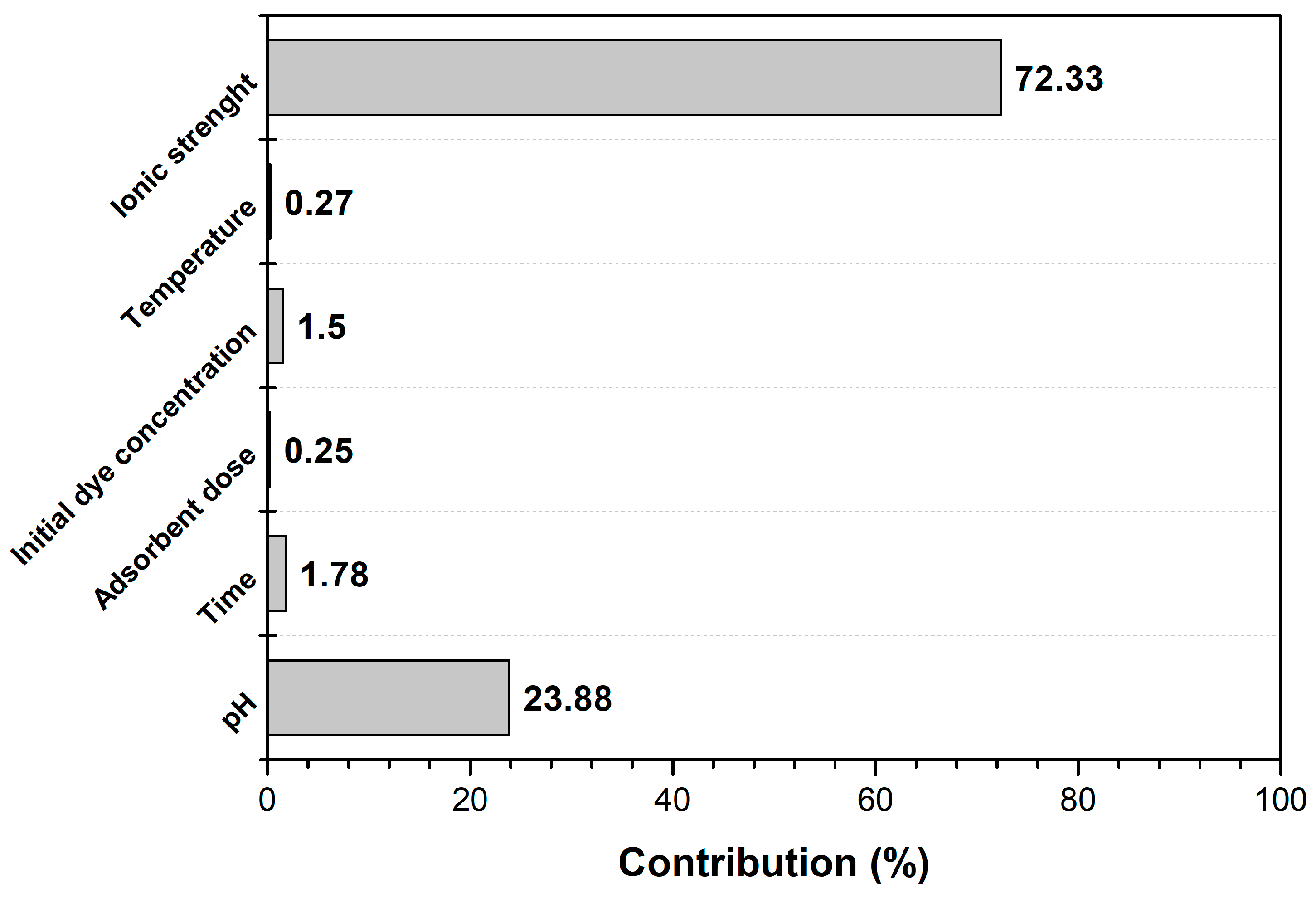
| Level | pH | Time | Adsorbent Dose | Initial Dye Concentration | Temperature | Ionic Strength |
|---|---|---|---|---|---|---|
| 1 | 30.00 | 31.72 | 32.14 | 32.64 | 32.14 | 36.31 |
| 2 | 33.11 | 32.52 | 32.36 | 32.67 | 32.35 | 31.56 |
| 3 | 33.95 | 32.82 | 32.56 | 31.75 | 32.58 | 29.20 |
| Delta | 3.95 | 1.10 | 0.43 | 0.92 | 0.44 | 7.11 |
| Rank | 2 | 3 | 6 | 4 | 5 | 1 |
| Isotherm Model | Parameters | Value |
|---|---|---|
| Langmuir | KL (L mg−1) | 0.023 ± 0.003 |
| qmax (mg g−1) | 109.5 ± 5.24 | |
| R2 | 0.9916 | |
| Radj2 | 0.9891 | |
| SSE | 81.96 | |
| χ2 | 2.59 | |
| ARE (%) | 7.66 | |
| HYBRID | 37.06 | |
| Freundlich | Kf (mg g−1) | 9.37 ± 1.84 |
| 1/n | 0.44 ± 0.03 | |
| R2 | 0.9424 | |
| Radj2 | 0.9381 | |
| SSE | 513.45 | |
| χ2 | 13.86 | |
| ARE (%) | 15.40 | |
| HYBRID | 190.97 | |
| Temkin | KT (L mg−1) | 0.227 ± 0.029 |
| b (kJ g−1) | 100.96 ± 5.13 | |
| R2 | 0.9877 | |
| Radj2 | 0.9860 | |
| SSE | 106.02 | |
| χ2 | 2.54 | |
| ARE (%) | 8.36 | |
| HYBRID | 36.37 | |
| Redlich–Peterson | KRP (L g−1) | 2.59 ± 0.18 |
| aRP (L mg−1) | 0.024 ± 0.006 | |
| βRP | 0.93 ± 0.05 | |
| R2 | 0.9915 | |
| Radj2 | 0.9972 | |
| SSE | 82.45 | |
| χ2 | 2.62 | |
| ARE (%) | 7.72 | |
| HYBRID | 37.56 | |
| Sips | Qsat (mg g−1) | 103.2 ± 4.21 |
| KS (L mg−1) | 0.014 ± 0.003 | |
| n | 1.17 | |
| R2 | 0.9992 | |
| Radj2 | 0.9990 | |
| SSE | 6.99 | |
| χ2 | 0.24 | |
| ARE (%) | 2.46 | |
| HYBRID | 2.82 |
| Kinetic Model | Parameters | Value |
|---|---|---|
| Pseudo-first order | k1 (min−1) | 0.461 ± 0.041 |
| qe,calc (mg g−1) | 18.67 ± 0.32 | |
| R2 | 0.9919 | |
| Radj2 | 0.9858 | |
| SSE | 2.46 | |
| χ2 | 0.13 | |
| ARE (%) | 13.44 | |
| HYBRID | 1.90 | |
| Pseudo-second order | k2 (min−1) | 0.065 ± 0.007 |
| qe,calc (g mg−1 min−1) | 19.31 ± 0.16 | |
| R2 | 0.9979 | |
| Radj2 | 0.9973 | |
| SSE | 0.62 | |
| χ2 | 0.03 | |
| ARE (%) | 12.43 | |
| HYBRID | 0.52 | |
| Elovich | a (g mg−1) | 0.641 ± 0.052 |
| b (mg g−1 min−1) | 9407 ± 374 | |
| R2 | 0.9932 | |
| Radj2 | 0.9914 | |
| SSE | 2.28 | |
| χ2 | 0.83 | |
| ARE (%) | 13.01 | |
| HYBRID | 11.94 | |
| Avrami | kAV (min−1) | 0.819 ± 0.078 |
| qAV (mg g−1) | 18.67 ± 0.25 | |
| nAV | 0.56 ± 0.07 | |
| R2 | 0.9919 | |
| Radj2 | 0.9893 | |
| SSE | 2.46 | |
| χ2 | 0.13 | |
| ARE (%) | 2.33 | |
| HYBRID | 1.89 | |
| General order | kN (min−1 (g mg−1)n–1) | 0.065 ± 0.012 |
| qn (mg g−1) | 19.31 ± 0.18 | |
| n | 2.01 ± 0.16 | |
| R2 | 0.9992 | |
| Radj2 | 0.9987 | |
| SSE | 0.21 | |
| χ2 | 0.01 | |
| ARE (%) | 0.70 | |
| HYBRID | 0.17 |
| ΔG0 (kJ mol−1) | ΔH0 (kJ mol−1) | ΔS0 (J mol−1 K−1) | ||
|---|---|---|---|---|
| 281 K | 291 K | 305 K | ||
| −20.05 | −20.85 | −21.97 | 0.29 | 9.62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mosoarca, G.; Popa, S.; Vancea, C.; Dan, M.; Boran, S. Modelling and Optimization of Methylene Blue Adsorption Process on Leonurus cardiaca L. Biomass Powder. Processes 2023, 11, 3385. https://doi.org/10.3390/pr11123385
Mosoarca G, Popa S, Vancea C, Dan M, Boran S. Modelling and Optimization of Methylene Blue Adsorption Process on Leonurus cardiaca L. Biomass Powder. Processes. 2023; 11(12):3385. https://doi.org/10.3390/pr11123385
Chicago/Turabian StyleMosoarca, Giannin, Simona Popa, Cosmin Vancea, Mircea Dan, and Sorina Boran. 2023. "Modelling and Optimization of Methylene Blue Adsorption Process on Leonurus cardiaca L. Biomass Powder" Processes 11, no. 12: 3385. https://doi.org/10.3390/pr11123385






