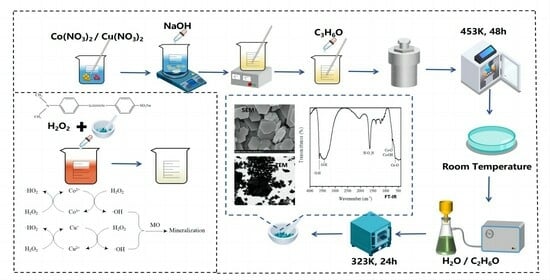
1. Introduction
In recent years, advanced oxidation processes (AOPs) [
1,
2,
3] for refractory organic pollutants have been researched, resulting in significant progress. Among the AOPs, the Fenton oxidation method [
4,
5,
6], using hydrogen peroxide decomposition to produce a hydroxyl radical (·OH) [
5,
7,
8], has a fast reaction rate. The reaction conditions are mild, the operation is simple and it has a relatively low operating cost. The reaction product of hydrogen peroxide is mainly water, which belongs to environmentally friendly reagents, thus attracting more and more attention. However, homogeneous Fenton catalysts are difficult to recover, have a narrow pH application range, and bring about secondary pollution, which is easily caused by sludge and other shortcomings [
6,
7,
8,
9]. This eventually limits their practical application in water treatment. Therefore, in the development of high catalytic activity, a wide range of pH values applies, and good-stability Fenton-like catalysts have become a hot topic [
10,
11,
12]. The outer electron orbital of transition metals can provide or accept electrons during the reaction, which creates room for redox reactions and promotes the generation of hydroxyl radicals. Thus, adding transition metals can enhance the catalytic activity of the catalyst. Research has shown that copper ions exhibited somewhat Fenton-like behaviors [
13,
14]:
Lee et al. [
15] and Zhang, X.Y. et al. [
16] have proved this. Other studies also showed that copper ions could be the Fenton-like catalyst or enhance the catalytic properties of the Fenton-like catalyst, such as nano-Cu
2O/MWNTs [
17], Cu
2O–CuO/Sr
3BiO
5.4 [
18], FeCu/Cu
2O [
19], mixed Fe-, Cu-, Al-clays [
20], and CuFeZSM-5 zeolite catalyst [
21].
In addition, cobalt ions also exhibit similar Fenton-like behaviors:
Zhong, Y.H. et al. [
22] found that Co(II) can improve the ·OH generation rate and accordingly enhance the Fenton catalytic activity of magnetite. Cai, C. et al. [
23] prepared a bimetallic Fe–Co/SBA-15 catalyst for the degradation of Orange II in water, which showed high activity. The research by Yao, Y.G. et al. [
24], Chen, Q.K. et al. [
25], and Tong, S.P. et al. [
26] also proved it.
In this study, we investigated the degradation of methyl orange simulated wastewater by a Fenton-like process using a novel catalyst that only contains cobalt and copper, which are two transition metal elements. The catalyst was prepared with a modified hydrothermal method and characterized by scanning electron microscopy (SEM), transmission electron microscopy (TEM), X-ray powder diffraction (XRD), and a Fourier transform infrared spectrometer (FT-IR). Using such cobalt and copper composite Fenton-like catalyst, the degradation of methyl orange simulated wastewater was studied.
2. Materials and Methods
2.1. Materials
Cobalt nitrate (Co(NO3)2·3H2O), cupric nitrate (Cu(NO3)2·6H2O), sodium hydroxide, sulfuric acid, propionaldehyde, ethanol, 30% (w/w) H2O, and methyl orange were provided by Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). All reagents were analytically graded and were used as received. Distilled water was used in all experiments.
2.2. Preparation of Catalyst
The molar ratios of cobalt nitrate and copper nitrate were 1:0, 1:2, 1:1, 3:2, 2:1, 5:2, 3:1, 0:1, and the required amount of cobalt nitrate and copper nitrate were weighed and dissolved in 15 mL of distilled water. Then, a 60 mL solution of 3.3 mol/L sodium hydroxide was added dropwise to the metal precursor solution under vigorous stirring, followed by sonication for 15 min and further stirring for 15 min to obtain a colloidal suspension. A total of 1 mL of propionaldehyde was added into the suspension as a reducing agent. After that, the suspension was transferred to a 100 mL Teflon-lined stainless steel autoclave, sealed, and placed in an oven at 453 K for 48 h. After the reaction, the autoclave was cooled naturally to room temperature. The solid products were collected by filtrating, then washed with distilled water and ethanol several times until the pH value of the filtrate reached about 7. Finally, the solid product was dried in a vacuum oven at 323 K for 24 h.
2.3. Characterization of Catalyst
The surface morphology of the catalyst was captured using scanning electron microscopy (SEM, FEI, Eindhoven, The Netherlands) and transmission electron microscopy (TEM Tecnai G2 20, FEI Company, Eindhoven, The Netherlands), which were operated at an acceleration voltage of 150 kv. The crystal structure and composition of the catalyst were measured by powder X-ray diffraction patterns (XRD) using an XPert PROX diffractometer (PANalytical B.V., Almelo, The Netherlands) with monochromatic Cu Kα radiation (PANalytical B.V., 40 kv and 40 mA) and Fourier transform infrared spectrometer (FT-IR NICOLET NEXUS 470, Thermo Fisher Scientific, Waltham, MA, USA).
2.4. Catalytic Degradation Experiment
The catalytic activity of the Co–Cu composite heterogeneous Fenton-like catalyst was estimated by the oxidation of methyl orange solution in a ZD-85 gas bath thermostatic oscillator (Jintan Branch Analysis Instrument Co., Ltd., Changzhou, China). A 100 mL solution of 250 mg/L of methyl orange in an Erlenmeyer flask was taken, with sulfuric acid and sodium hydroxide to adjust the pH, while adding a certain amount of catalyst and sonicating for 10 min. Next, this was transferred to a constant temperature shaker, stirring for 20 min at 200 rpm to achieve the adsorption/desorption equilibrium between the methyl orange solution and the catalyst. Then, a specified amount of H2O2 was added into the solution to initiate the degradation and to account for the time of the start of the reaction, a solution sample (5 mL) at given time intervals was taken, with Na2SO3 solution added immediately to stop the continuing Fenton reaction, and this was centrifuged at 20,000 rpm for 5 min on a 80/800 Series desktop electric centrifuge (Jintan Branch Analysis Instrument Co., Ltd., China) to remove catalyst particles. Then, the COD of the supernatant was measured.
To explore the effect of different experimental conditions on the catalyst degradation, we changed the course of the experiment under different reaction conditions, including pH, the dosage of catalyst, the dosage of hydrogen peroxide, and reaction temperature. In addition, the reusability of the catalyst was being studied. The catalyst was collected at the end of each catalytic degradation reaction and filtered with water and ethanol until it was neutral, and the filter cake was dried as the catalyst for the next catalytic degradation test. To explore the degradation pathways of methyl orange, the reaction liquid at the optimum condition was UV scanned.
2.5. Analysis Method of Degradation Effect
2.5.1. Method of Determination of Decolorization Rate
The catalytic degradation ability of the catalyst on the pollutants is based on the decolorization rate of the methyl orange simulated wastewater. In the reaction process, a certain amount of reaction solution is taken at every certain time interval after treatment to measure the absorbance by spectrophotometry, and the decolorization rate (D) is obtained according to the change in absorbance before and after the reaction. The calculation formula is as follows:
where, A
0 was the absorbance value of the original solution before the reaction and A
i was the absorbance value measured at different times.
2.5.2. Method of Determining the COD Degradation Rate
The concentration of methyl orange solution was measured at its maximum absorption peak of 464 nm using a UV–Vis spectrophotometer (WFZ UV-2000, Unico Instruments Co., Ltd., Shanghai, China). The chemical oxygen demand value of methyl orange solution was determined by a 5B-3B (V8) multi-parameter water analyzer (Lianhua Technology Co., Ltd., Shanghai, China). The COD degradation rate (R) was calculated using the following equation:
where C
0 was the initial COD of methyl orange solution and C
i was the COD of methyl orange solution at different times.
3. Results and Discussion
3.1. Characterization and Effect of Different Co/Cu
3.1.1. Morphology Analysis
On the whole, under the condition of adding two active elements at the same time, the dispersibility of the prepared catalyst particles gradually becomes better with the increase in the amount of cobalt element added, and the clarity of the catalyst particles gradually increases. Among them, b and c clearly show that the catalyst particles are not formed, the edges are smooth without edges and corners, the agglomeration phenomenon is more obvious, and the particles are fused together to form a whole, indicating that the dispersion is very poor. The particles in
Figure 1d–g all exhibit an irregular hexagonal crystal structure, with good dispersion and voids between the particles, but the edges of the particles of g change to form a multi-faceted side structure. A is the catalyst prepared when only cobalt is added. It can be seen that the catalyst is composed of many irregular hexagonal particles with voids between the particles.
Figure 1h is the catalyst prepared when only cobalt is added. The catalyst particles show a rectangular structure, the particle dispersion is good, the surface is smooth, and there is a gap between the particles.
3.1.2. XRD Analysis
Figure 2 shows that the types of crystals generated by adding different amounts of active elements are different. Among them, a detailed XRD analysis of the catalysts prepared when only cobalt was added and only copper was added as the active element found that when the molar ratio of cobalt to copper was 1:0, the peaks at 2θ values at 19.02, 32.40, 37.86, 38.66, and 51.32 corresponded to the (001) (100) (101) (002) (102) crystal plane of the standard card cobalt hydroxide (JCPDS 30-0443), respectively. In addition, there are no other peaks, indicating that the generated material is cobalt hydroxide and has a high purity. When the molar ratio of cobalt to copper is 0:1, the catalyst components are copper oxide and cuprous oxide. Because the peaks of 2θ values at 29.48, 35.65, 38.63, and 61.34 correspond to the (110) (11-1) (111) (11-3) crystal plane of standard card copper oxide (JCPDS 48-1548), and the peaks at 36.36, 42.35, and 61.50 correspond to the (111) (200) (220) crystal plane of Cu
2O (JCPDS 05-0667), there are no other peaks, indicating that the generated substances are copper oxide and cuprous oxide.
In the catalyst with two active elements of copper and cobalt, the position and shape of the peak are changed with different amounts of cobalt and copper, indicating that there is an interaction between cobalt and copper. With the increase in the cobalt element addition ratio, the peak intensity of the main peak position corresponding to cobalt hydroxide gradually increases, while the peak intensity corresponding to the characteristic peak position of copper oxide gradually decreases. However, when the ratio of cobalt to copper exceeds 5:2, the peak intensity corresponding to the main peak of cobalt hydroxide gradually decreases. This shows that the amount of active elements not only affects the shape of the crystal but also affects the crystal structure of the catalyst.
Figure 2.
The XRD patterns of catalyst under different active element content preparation conditions: (a) Co:Cu = 1:0; (b) Co:Cu = 1:2; (c) Co:Cu = 1:1; (d) Co:Cu = 3:2; (e) Co:Cu = 2:1; (f) Co:Cu = 5:2; (g) Co:Cu = 3:1; (h) Co:Cu = 0:1.
Figure 2.
The XRD patterns of catalyst under different active element content preparation conditions: (a) Co:Cu = 1:0; (b) Co:Cu = 1:2; (c) Co:Cu = 1:1; (d) Co:Cu = 3:2; (e) Co:Cu = 2:1; (f) Co:Cu = 5:2; (g) Co:Cu = 3:1; (h) Co:Cu = 0:1.
3.1.3. Effect Analysis of Different Co/Cu
In order to explore the effect of the active element addition on the catalytic performance of the catalyst, the same experimental conditions were controlled, and the methyl orange simulated wastewater was used as the treatment object to compare the effects of the catalyst prepared under different active element addition conditions on the decolorization rate of methyl orange. The specific test steps were as follows: 100 mL of methyl orange simulated organic wastewater with a concentration of 50 mg/L was taken in 8 conical flasks, the pH value was adjusted to 6.5, and 0.05 g of the catalyst prepared under different cobalt–copper ratios was added, respectively. After 10 min of ultrasound, it was transferred to a constant temperature shaker at a speed of 200 rpm for 20 min to achieve the adsorption/desorption equilibrium between the methyl orange solution and the catalyst. Then, 4 mL of hydrogen peroxide was added, and the initial time of the degradation reaction was calculated. A certain amount of reaction solution was taken every 10 min, and the sodium sulfite solution was immediately added dropwise to stop the reaction. After centrifugation, the supernatant was taken and the absorbance was measured.
It is clearly seen in
Figure 3 that the decolorization rate of methyl orange solution by the catalyst prepared under the condition of different cobalt and copper additions increases with the progress of the reaction, and it can be seen that there is a certain catalytic effect. In the system of only adding the copper element, the catalytic effect of the catalyst is relatively poor. After 60 min of reaction, the decolorization rate is 70.80%, while the decolorization rate can reach 98.90% when only the active element cobalt is added. It shows that the catalytic performance of the cobalt ion is much higher than that of the copper ion. When the ratio of cobalt to copper is 1:2, the catalyst has the lowest decolorization efficiency of methyl orange, only 19.60%. With the increase in the cobalt element in the catalyst, the decolorization rate gradually increases. When the ratio of cobalt to copper is 5:2, the catalyst prepared under this condition has the best catalytic performance, and the decolorization rate reaches 99.87%. After that, the decolorization rate decreases slightly with the increase in the proportion of cobalt ions.
This shows that in the system where the two active elements exist at the same time, with the different amounts of cobalt and copper added, different effects occur between cobalt ions and copper ions. When the cobalt–copper ratio is 1:2, the decolorization rate of the prepared catalyst for methyl orange is much lower than that of the catalyst prepared with only copper or only cobalt, indicating that under this condition, an antagonistic effect occurs between cobalt ions and copper ions. However, the catalytic performance of the catalyst prepared under other cobalt–copper ratio conditions is higher than that of the catalyst only added with cobalt or only added with copper, indicating that a synergistic effect occurs between cobalt ions and copper ions. That is to say, under the appropriate proportion of active element additions, the interaction between the cobalt element and copper element can promote the smooth and rapid progress of some reactions on the catalyst surface, thus improving the performance of the catalyst.
3.2. Characterization of the Catalyst with Co/Cu Content of 5:2
3.2.1. Morphology Analysis
In order to understand the surface morphology of the catalyst with a Co/Cu content of 5:2, SEM and TEM were used. The results are shown in
Figure 1a,b.
Figure 4a presents an SEM micrograph of the Co–Cu catalyst. The sample consists of hexagonally crystallized particles with sizes in the range of 0.5–5 μm and has voids between these particles. The morphology and microstructure of the Cu–Co catalyst were further investigated by transmission electron microscopy (TEM). It can be seen from
Figure 4b that the sample has a smooth surface and uneven sizes.
3.2.2. XRD Analysis
The crystal structure of the Co–Cu catalyst was analyzed by XRD. As shown in
Figure 5, all the peaks are clearly distinguished. The peaks with 2θ values of 19.02, 32.40, 37.86, and 38.66 correspond to (001) (100) (101) (002) crystal planes of Co(OH)
2 with a cubic phase, respectively (JCPDS 30-0443). The peaks at values of 36.36, 42.35, and 61.50 correspond to the characteristic peaks of (111) (200) (220) of Cu
2O, respectively (JCPDS 05-0667). In addition, there are no other peaks so it can be concluded that there are Co(OH)
2 and Cu
2O in the obtained Co–Cu Fenton-like catalyst.
3.2.3. FT-IR Analysis
The components were determined with the use of infrared analysis. They can be seen from the bending vibrations of water molecules H–O…H, which means there is lattice water present. A sharp peak at 3629 cm
−1 is the O–H O–H vibration absorption of Co(OH)
2, and the characteristic absorption peak at 742 cm
−1 is the bending vibration of Co–O and stretching vibration of Co–OH in the Co(OH)
2. The characteristic absorption peak at 610 cm
−1 corresponds to the Cu–O stretching vibration of cuprous oxide, and the characteristic peaks at 489 cm
−1 also correspond to the characteristic peaks of cuprous oxide. In addition, there are no other peaks appearing, indicating that the final product is Co(OH)
2 and Cu
2O, which is consistent with the XRD results. In
Figure 6, the broad peak at 3435 cm
−1 is the O–H stretching vibration, and the absorption peak is at 1636 cm
−1.
3.3. Catalytic Oxidation Test
3.3.1. Effect of pH Value on COD Degradation of Methyl Orange
Under the conditions of catalyst dosage for 2 g/L, there is a dosage of hydrogen peroxide that is 40 mL/L, a reaction temperature of 313 K, and a reaction time of 50 min. The COD degradation rate of methyl orange solution at different original pH levels is shown in
Figure 7.
It can be obtained from the
Figure 7 and
Figure 8 that COD degradation rates gradually increase under different conditions of pH. As the reaction proceeds after 50 min, in the pH range of 2–10, COD degradation rates all reached more than 80%. However, when the pH value is 11, the degradation shows poor performance. This is because the higher pH value will generate invalid decomposition of hydrogen peroxide and the cobalt and copper ion flocculation and sedimentation, which eventually halt the performance of the anticipated reaction, thereby reducing the catalytic efficiency. From the experimental results, it can be concluded that the catalyst has better catalytic performances at pH values in the range of 2 to 10, significantly expanding the scope of application of heterogeneous catalysts. Since the wastewater is mostly acidic or alkaline, we select the pH value of 7 in the following experiments.
3.3.2. Effect of Catalyst Dosage on the COD Degradation Rate of Methyl Orange Solution
In order to research the effect of the catalyst dosage on the COD degradation rate, we set the experimental conditions to the original pH value of 7, a dosage of hydrogen peroxide as 40 mL/L, the reaction temperature as 313 K, and a reaction time of 50 min. The COD degradation rate of methyl orange solution at different catalyst dosages is shown in
Figure 9.
As shown in
Figure 9, when the dosage of the catalyst is less than 3 g/L, the COD degradation rate increases with increasing catalyst dosage, but when the dosage of the catalyst exceeds 3 g/L, catalytic efficiency is reduced. This is because the amount of the catalyst directly affects the rate and number of generation of hydroxyl radicals. As the catalyst dosage increases, the rate and number of hydroxyl radicals produced increases per unit of time. The reaction rate also increases, so that it can achieve a better high degradation rate of COD. When the dosage of catalyst exceeds 3 g/L, an excessive dosage of catalysts accelerates the rate of decomposition of hydrogen peroxide, and hydroxyl radicals produced do not react with methyl orange but the metal ions of the excess catalysts, making the COD degradation rate not high.
3.3.3. Effect of Dosage of Hydrogen Peroxide on the Degradation of COD
Since hydrogen peroxide is the major player in the Fenton reaction, the dosage of hydrogen peroxide has a certain influence on the degradation. With the following test conditions: pH = 7, T = 313 K, t = 50 min, and catalyst dosage of 3 g/L, the COD degradation rate changes at different dosages of hydrogen peroxide. This is shown in
Figure 10.
Figure 10 shows that with an increasing dosage of hydrogen peroxide, the COD degradation rate increases after 30 min, and the reaction almost reaches its equilibrium in the systems of hydrogen peroxide at a dosage of 50–70 mL/L. When the amount of hydrogen peroxide is relatively small, it could not produce enough hydroxyl radicals to support the degradation of COD. Hydroxyl radical production increases with the increasing hydrogen peroxide, which makes the reaction system obtain enough hydroxyl radicals in degrading organic matter, thus, the COD is reduced. From the experimental results, after 50 min in reaction, with the dosages of hydrogen peroxide in the system of 50 mL/L, 60 mL/L, and 70 mL/L, the COD degradation rates all reached more than 93%. Taking into account the economy, 50 mL/L is chosen as the best dosage of hydrogen peroxide in the following experiments.
3.3.4. Effect of Reaction Temperature on the Degradation of COD
In order to study the effect of reaction temperature on COD degradation rate, we made experiments under the conditions of an original pH of 7, a catalyst dosage of 3 g/L, a hydrogen peroxide dosage of 50 mL/L, and an oxidation time of 50 min. The COD degradation at different reaction temperatures is shown in
Figure 11.
As shown in
Figure 11, with the increase in reaction temperature, the COD degradation rate increases. This is because heating up can increase the kinetic energy of the reactant molecules, making the reactant more easily activated, thereby increasing the reaction rate. At the same time, appropriate heating can also promote the formation of active centers on the surface of the catalyst and enhance the catalytic activity of the catalyst. Therefore, increasing the temperature can enhance the catalytic activity of the catalyst. However, it is not difficult to see that in the reaction system at 313 K, 323 K, and 333 K, the final COD degradation rate is almost the same. The temperature rises to speed up the reaction within the range of 313–333 K, but not much improvement for the final degradation rate is seen.
3.3.5. Reusability of the Catalyst
After the catalytic decomposition reaction, the catalyst was filtered after washing with distilled water and ethanol, and it was then recovered after drying for the next degradation reactions.
Table 1 clearly depicts that the catalyst can be reused three times to achieve a certain degradation effect, showing that the catalyst has a certain recyclability. With the increase in the number of times of use, the catalytic effect also gradually decreased, which may be due to the dissolution of cobalt and copper elements in the catalyst into the solution. In comparing catalysts that are easily recycled, a simple preparation, good catalytic effect, less pollution, good production, and life in the future can be widely applied.
3.3.6. Degradation of Methyl Orange Solution of UV–Vis Spectroscopy
The solution was adjusted to an original pH of 7, a catalyst dosage of 3 g/L, and hydrogen peroxide concentration of 50 mL/L, under different reaction times, UV–Vis spectral changes during the degradation of methyl orange simulated wastewater are shown in
Figure 9.
From
Figure 12, it can be seen that the characteristic peaks of methyl orange with two bands has its main band in a visible region at a maximum absorption wavelength of 464 nm, which constitutes the azo characteristic peak of methyl orange. During the degradation process, the azo characteristic absorption peak in the visible region vanished after a twenty min reaction, which explained that the azo bond of methyl orange was oxidized and the color group destroyed. The ultraviolet absorption peak position changed, indicating that the aromatic ring structure of dye molecules was destroyed and converted into other structures. With increasing the reaction time, the structures that were converted gradually degraded.
3.3.7. Methyl Orange Degradation Mechanism Analysis
As can be seen from the above studies, as opposed to the traditional Fenton catalyst, the cobalt–copper composite Fenton-like catalyst has good catalytic performance and a wider scope of pH application, probably because the cobalt ions and copper ions occur similarly as in the Fenton reaction, and their redox potentials are higher than iron ions, which improves catalytic performance to some extent (Cu
2+/Cu
+ = 0.17 V, Co
3+/Co
2+ = 1.81 V, Fe
3+/Fe
2+ = 0.77 V). Cobalt ion and copper ion regeneration is another important reason to improve the catalytic properties. This can also be interpreted as the synergy between ions. In addition, the precipitation range of cobalt and copper are wider than iron, and do not form colloids, thus, the catalyst has a relatively broad pH range (precipitation range of copper and cadmium ions are in the pH values between 9 and 11; iron precipitation range is for pH values between 7 and 9). Conclusively, the catalyst can produce more effective hydroxyl radicals to degrade methyl orange. The methyl orange degradation mechanism is shown in
Figure 13 below.
4. Conclusions
In this study, a novel Co–Cu composite heterogeneous Fenton-like catalyst was successfully synthesized and characterized by SEM, TEM, XRD, and FT-IR, which revealed that the catalyst consists of Co(OH)2 and Cu2O particles and a performance with irregular hexagons of varying sizes (0.5–5 μm).
The catalyst was used as a heterogeneous Fenton-like catalyst, which exhibited much higher catalytic activity towards the degradation of methyl orange solution in the pH range of 2–10. The optimal dosage of the catalyst is 3 g/L; increasing the dosage of hydrogen peroxide or elevated temperatures could promote the reaction processes. The catalyst also has some reuse value.
Possible degradation mechanism of methyl orange: cobalt ions and copper ions can occur within a similar Fenton reaction, and their redox potentials are higher than iron ions; cobalt ion and copper ion regeneration is another important reason to improve the catalytic properties; in addition, the precipitation ranges of cobalt and copper are wider than iron, and do not form colloids, so the catalyst has a relatively broad pH range. Therefore, the catalyst can produce more effective hydroxyl radicals to degrade methyl orange.
This study provides a useful route to develop novel types of Co–Cu composite Fenton-like catalysts for the degradation of organic pollutants. However, the stability of the catalyst is poor, and the reuse needs improvement, so further research is needed to improve the modifications and loads of this catalyst.
Author Contributions
Proposed research ideas, designed and developed research methods, writing—original draft preparation, B.Z.; investigation, experimental analysis, Y.Y.; data analysis, S.C.; data curation, supervision, academic guidance, X.X.; resource provision, C.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Collaborative Education Project of the Ministry of Education (China, No. 220602392242101) and Changzhou Sci&Tech Program (China, No. CJ20220025).
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Korpe, S.; Rao, P.V.; Sonawane, S.H. Performance evaluation of hydrodynamic cavitation in combination with AOPs for degradation of tannery wastewater. J. Environ. Chem. Eng. 2023, 11, 109731. [Google Scholar] [CrossRef]
- Dong, C.; Fang, W.; Yi, Q.; Zhang, J. A comprehensive review on reactive oxygen species (ROS) in advanced oxidation processes (AOPs). Chemosphere 2022, 308, 136205. [Google Scholar] [PubMed]
- Chinthala, M.; KAshwathanarayanaiah, B.; Kulkarni, S.; Udayakishore, Y.; Halyal, A.; Chavan, A. Intensification of advanced oxidation processes (AOPs) for the degradation of bisphenol-A. Int. J. Chem. React. Eng. 2021, 19, 605–614. [Google Scholar]
- Bracamontes-Ruelas, A.R.; Reyes-Vidal, Y.; Irigoyen-Campuzano, J.R.; Reynoso-Cuevas, L. Simultaneous Oxidation of Emerging Pollutants in Real Wastewater by the Advanced Fenton Oxidation Process. Catalysts 2023, 13, 748. [Google Scholar]
- Ni, Y.; Zhou, C.; Xing, M.; Zhou, Y. Oxidation of Emerging Organic Contaminants by In-Situ H2O2 Fenton System. Green Energy Environ. 2023, in press. [Google Scholar] [CrossRef]
- Cheng, X.; Liang, L.; Ye, J.; Li, N.; Yan, B.; Chen, G. Influence and mechanism of water matrices on H2O2-based Fenton-like oxidation processes: A review. Sci. Total Environ. 2023, 888, 164086. [Google Scholar]
- Yu, H.; Ji, J.; Yan, Q.; Xing, M. Transition metal phosphides for heterogeneous Fenton-like oxidation of contaminants in water. Chem. Eng. J. 2022, 449, 137856. [Google Scholar]
- Scaria, J.; Nidheesh, P.V. Pre-treatment of real pharmaceutical wastewater by heterogeneous Fenton and persulfate oxidation processes. Environ. Res. 2023, 217, 114786. [Google Scholar]
- Jeong, W.G.; Kim, J.G.; Baek, K. Removal of 1,2-dichloroethane in groundwater using Fenton oxidation. J. Hazard. Mater. 2022, 428, 128253. [Google Scholar]
- Liu, T.; Xiao, S.; Li, N.; Chen, J.; Zhou, X.; Qian, Y.; Huang, C.-H.; Zhang, Y. Water decontamination via nonradical process by nanoconfined Fenton-like catalysts. Nat. Commun. 2023, 14, 2881. [Google Scholar] [PubMed]
- Zhuang, S.; Wang, J. Magnetic COFs as catalyst for Fenton-like degradation of sulfamethazine. Chemosphere 2021, 264, 128561. [Google Scholar] [CrossRef]
- Assila, O.; Barros, Ó.; Fonseca, A.M.F.; Parpot, P.; Soares, O.S.G.P.; Pereira, M.F.R.; Zerrouq, F.; Kherbeche, A.; Rombi, E.; Tavares, T.; et al. Degradation of pollutants in water by Fenton-like oxidation over LaFe-catalysts: Optimization by experimental design. Microporous Mesoporous Mater. 2023, 349, 112422. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, G.; Xian, G.; Zhang, N.; Li, J. A High-Efficiency CuO/CeO2 Catalyst for Diclofenac Degradation in Fenton-Like System. Front. Chem. 2019, 7, 796. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Hu, C.; Wang, X.; Lyu, L.; Sheng, G. Enhanced degradation of organic pollutants over Cu-doped LaAlO3 perovskite through heterogeneous Fenton-like reactions. Chem. Eng. J. 2018, 332, 572–581. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, H.; Lee, C. Degradation of diclofenac and carbamazepine by the copper(II)-catalyzed dark and photo-assisted Fenton-like systems. Chem. Eng. J. 2014, 245, 258–264. [Google Scholar] [CrossRef]
- Zhang, X.; Ding, Y.; Tang, H.; Han, X.; Zhu, L.; Wang, N. Degradation of bisphenol A by hydrogen peroxide activated with CuFeO2 microparticles as a heterogeneous Fenton-like catalyst: Efficiency, stability and mechanism. Chem. Eng. J. 2014, 236, 251–262. [Google Scholar] [CrossRef]
- Zhang, L.; Li, J.; Chen, Z.; Tang, Y.; Yu, Y. Preparation of Fenton reagent with H2O2 generated by solar light-illuminated nano-Cu2O/MWNTs composites. Appl. Catal. A Gen. 2006, 299, 292–297. [Google Scholar] [CrossRef]
- Zhao, W.; Wong, K.H.; Hu, C.; Jimmy, C.Y.; Chan, C.Y.; Qi, T.; Wong, P.K. Synthesize of Cu2O-CuO/Sr3BiO5.4 and its photocatalytic activity. Appl. Surf. Sci. 2012, 258, 5955–5959. [Google Scholar] [CrossRef]
- An, J.; Zhou, Q.X. Degradation of some typical pharmaceuticals and personal care products with copper-plating iron doped Cu2O under visible light irradiation. J. Environ. Sci. 2012, 24, 827–833. [Google Scholar] [CrossRef]
- Timofeeva, M.; Khankhasaeva, S.; Talsi, E.; Panchenko, V.; Golovin, A.; Dashinamzhilova, E.; Tsybulya, S. The effect of Fe/Cu ratio in the synthesis of mixed Fe, Cu, Al-clays used as catalysts in phenol peroxide oxidation. Appl. Catal. B Environ. 2009, 90, 618–627. [Google Scholar] [CrossRef]
- Dükkancı, M.; Gündüz, G.; Yılmaz, S.; Yaman, Y.C.; Prikhod’Ko, R.V.; Stolyarova, I.V. Characterization and catalytic activity of CuFeZSM-5 catalysts for oxidative degradation of Rhodamine 6G in aqueous solutions. Appl. Catal. B Environ. 2010, 95, 270–278. [Google Scholar] [CrossRef]
- Zhong, Y.; Liang, X.; He, Z.; Tan, W.; Zhu, J.; Yuan, P.; Zhu, R.; He, H. The constraints of transition metal substitutions (Ti, Cr, Mn, Co and Ni) in magnetite on its catalytic activity in heterogeneous Fenton and UV/Fenton reaction: From the perspective of hydroxyl radical generation. Appl. Catal. B Environ. 2014, 150–151, 612–618. [Google Scholar] [CrossRef]
- Cai, C.; Zhang, H.; Zhong, X.; Hou, L. Ultrasound enhanced heterogeneous activation of peroxymonosulfate by a bimetallic Fe-Co/SBA-15 catalyst for the degradation of Orange II in water. J. Hazard Mater. 2015, 283, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Cai, Y.; Wu, G.; Wei, F.; Li, X.; Chen, H.; Wang, S. Sulfate radicals induced from peroxymonosulfate by cobalt manganese oxides (CoxMn3-xO4) for Fenton-Like reaction in water. J. Hazard Mater. 2015, 296, 128–137. [Google Scholar] [CrossRef]
- Chen, Q.; Ji, F.; Guo, Q.; Fan, J.; Xu, X. Combination of heterogeneous Fenton-like reaction and photocatalysis using Co–TiO2 nanocatalyst for activation of KHSO5 with visible light irradiation at ambient conditions. J. Environ. Sci. 2014, 26, 2440–2450. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.P.; Shi, R.; Zhang, H.; Ma, C.A. Kinetics of Fe3O4-CoO/Al2O3 catalytic ozonation of the herbicide 2-(2,4-dichlorophenoxy) propionic acid. J. Hazard Mater. 2011, 185, 162–167. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).