Valorization of Cereal Byproducts with Supercritical Technology: The Case of Corn
Abstract
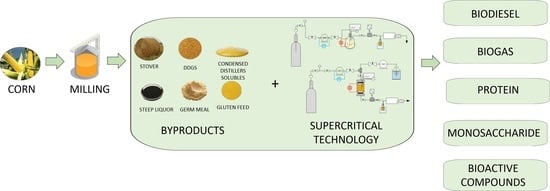
:1. Introduction
2. Dry Grinding
3. Wet Milling
4. Dry Grinding and Wet Milling Byproducts
4.1. Condensed Distillers’ Solubles (CDS)
4.2. Dried Distillers’ Grains with Solubles (DDGS)
4.3. Germ Meal
4.4. Gluten Feed and Gluten Meal
4.5. Stover, Silk, and Steep Liquor
5. Supercritical Technology
5.1. Processes
5.1.1. Supercritical Fluid Extraction (SFE) and Hydrolysis Assisted with SC-CO2
5.1.2. Subcritical Water-Assisted Hydrolysis
5.1.3. Pressurized Liquid Extraction
5.1.4. Chemical Reactions: Transesterification and Gasification
5.1.5. Micronization and Encapsulation
5.1.6. Impregnation and Extrusion
5.2. Economic Evaluation
5.3. Patents Survey
6. Current Trends and Opportunities for Future Studies
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| CDS | Condensed Distillers’ Solubles |
| DDGS | Dried Distillers’ Grains with Solubles |
| PLE | Pressurized liquid extraction |
| SC-CO2 | Supercritical carbon dioxide |
| SFE | Supercritical Fluid Extraction |
| SWE | Subcritical Water Extraction |
| SWG | Supercritical Water Gasification |
| TS | Thin Stillage |
| WDG | Wet Distillers’ Grains |
References
- USDA World Agricultural Production. Available online: https://apps.fas.usda.gov/psdonline/circulars/production.pdf (accessed on 18 December 2022).
- Khanna, M.; Paulson, N. To Harvest Stover or Not: Is It Worth It? Available online: https://farmdocdaily.illinois.edu (accessed on 10 December 2022).
- Ruan, Z.; Wang, X.; Liu, Y.; Liao, W. Corn; Integrated Processing Technologies for Food and Agricultural By-Products; Pan, Z., Zhang, R., Zicari, S., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 59–72. [Google Scholar]
- Stanford, J.P.; Keener, K.M. Cedar Rapids Food and Bioprocessors Manufacturing Report; Iowa State University: Ames, IA, USA, 2018. [Google Scholar]
- Anderson, B.; Almeida, H. Corn Dry Milling: Processes, Products, and Applications. In Corn; Serna-Saldivar, S.O., Ed.; AACC International Press: Oxford, UK, 2019; pp. 405–433. [Google Scholar]
- Rausch, K.D.; Hummel, D.; Johnson, L.A.; May, J.B. Wet Milling: The Basis for Corn Biorefineries. In Corn; Serna-Saldivar, S.O., Ed.; AACC International Press: Oxford, UK, 2019; pp. 501–535. [Google Scholar]
- Malumba, P.; Boudry, C.; Roiseux, O.; Bindelle, J.; Beckers, Y.; Béra, F. Chemical characterisation and in vitro assessment of the nutritive value of co-products yield from the corn wet-milling process. Food Chem. 2015, 166, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Cargill Corn—Advances Sustainability Across Corn Supply Chains. Available online: https://www.cargill.com/sustainability/corn/sustainable-corn (accessed on 10 December 2022).
- Bunge Milling. Available online: https://www.bunge.com/our-businesses/milling (accessed on 10 December 2022).
- Gavilon Gavilon. Available online: https://www.gavilon.com/ (accessed on 10 December 2022).
- J-Six Dry Corn Milling. Available online: https://www.jsixenterprises.com/services/dry-corn-milling/ (accessed on 10 December 2022).
- Lagerkvist, A.; Dahlén, L. Solid Wastesolid wasteGenerationsolid wastegenerationand Characterizationsolid wastecharacterization. In Encyclopedia of Sustainability Science and Technology; Meyers, R.A., Ed.; Springer: New York, NY, USA, 2012; pp. 10000–10013. ISBN 978-1-4419-0851-3. [Google Scholar]
- Qi, Y.Y.; Zhang, K.Y.; Tian, G.; Bai, S.P.; Ding, X.M.; Wang, J.P.; Peng, H.W.; LV, L.; Xuan, Y.; Zeng, Q.F. Effects of dietary corn germ meal levels on growth performance, serum biochemical parameters, meat quality, and standardized ileal digestibility of amino acids in Pekin ducks. Poult. Sci. 2022, 101, 101779. [Google Scholar] [CrossRef]
- Shin, E.-C.; Shurson, G.C.; Gallaher, D.D. Antioxidant capacity and phytochemical content of 16 sources of corn distillers dried grains with solubles (DDGS). Anim. Nutr. 2018, 4, 435–441. [Google Scholar] [CrossRef]
- Chañi-Paucar, L.O.; Santana, Á.L.; Albarelli, J.Q.; Meireles, M.A.A. Chapter 6—Extraction of polyphenols by sub/supercritical based technologies. In Technologies to Recover Polyphenols from AgroFood By-Products and Wastes; Pintado, M.E., Saraiva, J.M.A., da Cruz Alexandre, E.M., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 137–168. ISBN 978-0-323-85273-9. [Google Scholar]
- Torres, R.A.C.; Santana, Á.L.; Santos, D.T.; Albarelli, J.Q.; Meireles, M.A.A. A novel process for CO2 purification and recycling based on subcritical adsorption in oat bran. J. CO2 Util. 2019, 34, 362–374. [Google Scholar] [CrossRef]
- Attard, T.M.; McElroy, C.R.; Hunt, A.J. Economic Assessment of Supercritical CO2 Extraction of Waxes as Part of a Maize Stover Biorefinery. Int. J. Mol. Sci. 2015, 16, 17546–17564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosa, M.T.M.G.; Alvarez, V.H.; Albarelli, J.Q.; Santos, D.T.; Meireles, M.A.A.; Saldaña, M.D.A. Supercritical anti-solvent process as an alternative technology for vitamin complex encapsulation using zein as wall material: Technical-economic evaluation. J. Supercrit. Fluids 2020, 159, 104499. [Google Scholar] [CrossRef]
- Knez, Ž. Enzymatic reactions in dense gases. J. Supercrit. Fluidsupercrit. Fluids 2009, 47, 357–372. [Google Scholar] [CrossRef]
- Fărcaș, A.C.; Socaci, S.A.; Nemeș, S.A.; Salanță, L.C.; Chiș, M.S.; Pop, C.R.; Borșa, A.; Diaconeasa, Z.; Vodnar, D.C. Cereal Waste Valorization through Conventional and Current Extraction Techniques—An Up-to-Date Overview. Foods 2022, 11, 2454. [Google Scholar] [CrossRef]
- Marinho, C.; Lemos, C.; Arvelos, S.; Barrozo, M.; Hori, C.; Watanabe, E. Extraction of corn germ oil with supercritical CO2 and cosolvents. J. Food Sci. Technol. 2019, 56, 4448–4456. [Google Scholar] [CrossRef]
- Yadav, G.; Fabiano, L.A.; Soh, L.; Zimmerman, J.; Sen, R.; Seider, W.D. Supercritical CO2 Transesterification of Triolein to Methyl-Oleate in a Batch Reactor: Experimental and Simulation Results. Processes 2019, 7, 16. [Google Scholar] [CrossRef]
- Li, H.; Hu, Y.; Wang, H.; Han, X.; El-Sayed, H.; Zeng, Y.; Charles Xu, C. Supercritical water gasification of lignocellulosic biomass: Development of a general kinetic model for prediction of gas yield. Chem. Eng. J. 2022, 433, 133618. [Google Scholar] [CrossRef]
- Jayasinghe, S.; Miller, D.U.S. Corn Usage for Ethanol, Dry Mill Ethanol Co-Products Production, and Ethanol Yields Update. Available online: https://www.agmrc.org/ (accessed on 2 November 2022).
- Deshwal, G.; Alam, T.; Panjagari, N.R.; Bhardwaj, A. Utilization of Cereal Crop Residues, Cereal Milling, Sugarcane and Dairy Processing By-Products for Sustainable Packaging Solutions. J. Polym. Environ. 2021, 29, 2046–2061. [Google Scholar] [CrossRef]
- Vohra, M.; Manwar, J.; Manmode, R.; Padgilwar, S.; Patil, S. Bioethanol production: Feedstock and current technologies. J. Environ. Chem. Eng. 2014, 2, 573–584. [Google Scholar] [CrossRef]
- Reis, C.E.R.; Rajendran, A.; Hu, B. New technologies in value addition to the thin stillage from corn-to-ethanol process. Rev. Environ. Sci. Bio/Technol. 2017, 16, 175–206. [Google Scholar] [CrossRef]
- US Grains Council. Guide to Distiller’s Dried Grains with Solubles (DDGS). Available online: https://www.canr.msu.edu/uploads/236/58572/cfans_asset_417244.pdf (accessed on 12 December 2022).
- Shurson, J.; Spiehs, M.; Wilson, J.; Whitney, M. Value and Use of ‘New Generation’ Distiller’s Dried Grains with Solubles in Swine Diets. Available online: https://en.engormix.com/pig-industry/articles/dried-grains-solubles-in-swine-diets-t33444.htm (accessed on 9 December 2022).
- USDA Grain Crushings and CoProducts Production 2019 Summary. In USDA, National Agricultural Statistics Service; USDA: Washington, DC, USA, 2020; pp. 1–7. Available online: https://www.nass.usda.gov/Publications/Todays_Reports/reports/cagcan20.pdf (accessed on 14 November 2022).
- Li, Q.; Singh, V.; de Mejia, E.G.; Somavat, P. Effect of sulfur dioxide and lactic acid in steeping water on the extraction of anthocyanins and bioactives from purple corn pericarp. Cereal Chem. 2019, 96, 575–589. [Google Scholar] [CrossRef]
- Rausch, K.D.; Eckhoff, S.R. Maize: Wet Milling. In Encyclopedia of Food Grain; Wrigley, C., Corke, H., Seetharaman, K., Faubion, J., Eds.; Academic Press: Oxford, UK, 2016; pp. 467–481. [Google Scholar]
- Sharma, S.; Pradhan, R.; Manickavasagan, A.; Thimmanagari, M.; Saha, D.; Singh, S.S.; Dutta, A. Production of antioxidative protein hydrolysates from corn distillers solubles: Process optimization, antioxidant activity evaluation, and peptide analysis. Ind. Crops Prod. 2022, 184, 115107. [Google Scholar] [CrossRef]
- Sharma, S.; Pradhan, R.; Manickavasagan, A.; Tsopmo, A.; Thimmanagari, M.; Dutta, A. Corn distillers solubles by two-step proteolytic hydrolysis as a new source of plant-based protein hydrolysates with ACE and DPP4 inhibition activities. Food Chem. 2023, 401, 134120. [Google Scholar] [CrossRef]
- Purdum, S.; Hanford, K.; Kreifels, B. Short-term effects of lower oil dried distillers grains with solubles in laying hen rations. Poult. Sci. 2014, 93, 2592–2595. [Google Scholar] [CrossRef]
- Heuzé, V.; Tran, G.; Sauvant, D.; Noblet, J.; Renaudeau, D.; Bastianelli, D.; Lessire, M.; Lebas, F. Corn Distillers Grain. Available online: https://www.feedipedia.org/node/71 (accessed on 10 December 2022).
- Langemeier, M. Explaining Fluctuations in DDG Prices. Farmdoc Dly. 2022, 12, 82. [Google Scholar]
- Calendula Flower Powder Organic. Available online: https://www.starwest-botanicals.com/product/calendula-flower-powder-organic/ (accessed on 17 December 2022).
- Luthria, D.L.; Liu, K.; Memon, A.A. Phenolic Acids and Antioxidant Capacity of Distillers Dried Grains with Solubles (DDGS) as Compared with Corn. J. Am. Oil Chem. Soc. 2012, 89, 1297–1304. [Google Scholar] [CrossRef]
- Lorigooini, Z.; Jamshidi-kia, F.; Hosseini, Z. Chapter 4—Analysis of aromatic acids (phenolic acids and hydroxycinnamic acids). In Recent Advances in Natural Products Analysis; Sanches Silva, A., Nabavi, S.F., Saeedi, M., Nabavi, S.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 199–219. ISBN 978-0-12-816455-6. [Google Scholar]
- Impact of Oil Extraction on the Nutritional Value of DDGS. Available online: https://www.wengerfeeds.com/impact-of-oil-extraction-on-the-nutritional-value-of-ddgs/ (accessed on 18 December 2022).
- Masisi, K.; Diehl-Jones, W.L.; Gordon, J.; Chapman, D.; Moghadasian, M.H.; Beta, T. Carotenoids of aleurone, germ, and endosperm fractions of barley, corn and wheat differentially inhibit oxidative stress. J. Agric. Food Chem. 2015, 63, 2715–2724. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Pardo, F.A.; Savoire, R.; Subra-Paternault, P.; Harscoat-Schiavo, C. Oil and protein recovery from corn germ: Extraction yield, composition and protein functionality. Food Bioprod. Process. 2020, 120, 131–142. [Google Scholar] [CrossRef]
- Whisner, C.M.; Martin, B.R.; Nakatsu, C.H.; Story, J.A.; MacDonald-Clarke, C.J.; McCabe, L.D.; McCabe, G.P.; Weaver, C.M. Soluble Corn Fiber Increases Calcium Absorption Associated with Shifts in the Gut Microbiome: A Randomized Dose-Response Trial in Free-Living Pubertal Females. J. Nutr. 2016, 146, 1298–1306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costabile, A.; Bergillos-Meca, T.; Rasinkangas, P.; Korpela, K.; De Vos, W.M.; Gibson, G.R. Effects of soluble corn fiber alone or in synbiotic combination with lactobacillus rhamnosus GG and the pilus-deficient derivative GG-PB12 on fecal microbiota, metabolism, and markers of immune function: A randomized, double-blind, placebo-controlled, cro. Front. Immunol. 2017, 8, 1443. [Google Scholar] [CrossRef]
- Fernandes, A.C.; Vieira, N.C.; de Santana, Á.L.; de Pádua Gandra, R.L.; Rubia, C.; Castro-Gamboa, I.; Macedo, J.A.; Macedo, G.A. Peanut skin polyphenols inhibit toxicity induced by advanced glycation end-products in RAW264.7 macrophages. Food Chem. Toxicol. 2020, 145, 111619. [Google Scholar] [CrossRef]
- Zuo, G.; Song, X.; Cheng, F.; Shen, Z. Physical and structural characterization of edible bilayer films made with zein and corn-wheat starch. J. Saudi Soc. Agric. Sci. 2019, 18, 324–331. [Google Scholar] [CrossRef]
- Liu, W.; Fang, L.; Feng, X.; Li, G.; Gu, R. In vitro antioxidant and angiotensin I-converting enzyme inhibitory properties of peptides derived from corn gluten meal. Eur. Food Res. Technol. 2020, 246, 2017–2027. [Google Scholar] [CrossRef]
- Wang, L.; Ding, L.; Yu, Z.; Zhang, T.; Ma, S.; Liu, J. Intracellular ROS scavenging and antioxidant enzyme regulating capacities of corn gluten meal-derived antioxidant peptides in HepG2 cells. Food Res. Int. 2016, 90, 33–41. [Google Scholar] [CrossRef]
- Heng, L.; Zhang, H.; Xiao, J.; Xiao, R. Life Cycle Assessment of Polyol Fuel from Corn Stover via Fast Pyrolysis and Upgrading. ACS Sustain. Chem. Eng. 2018, 6, 2733–2740. [Google Scholar] [CrossRef]
- Saha, B.C.; Kennedy, G.J.; Qureshi, N.; Cotta, M.A. Biological pretreatment of corn stover with Phlebia brevispora NRRL-13108 for enhanced enzymatic hydrolysis and efficient ethanol production. Biotechnol. Prog. 2017, 33, 365–374. [Google Scholar] [CrossRef]
- Duan, X.-L.; Yuan, C.-G.; Jing, T.-T.; Yuan, X.-D. Removal of elemental mercury using large surface area micro-porous corn cob activated carbon by zinc chloride activation. Fuel 2019, 239, 830–840. [Google Scholar] [CrossRef]
- Abirami, S.; Priyalakshmi, M.; Soundariya, A.; Samrot, A.V.; Saigeetha, S.; Emilin, R.R.; Dhiva, S.; Inbathamizh, L. Antimicrobial activity, antiproliferative activity, amylase inhibitory activity and phytochemical analysis of ethanol extract of corn (Zea mays L.) silk. Curr. Res. Green Sustain. Chem. 2021, 4, 100089. [Google Scholar] [CrossRef]
- Žilić, S.; Janković, M.; Basić, Z.; Vančetović, J.; Maksimović, V. Antioxidant activity, phenolic profile, chlorophyll and mineral matter content of corn silk (Zea mays L): Comparison with medicinal herbs. J. Cereal Sci. 2016, 69, 363–370. [Google Scholar] [CrossRef]
- Lee, C.W.; Seo, J.Y.; Kim, S.-L.; Lee, J.; Choi, J.W.; Park, Y. Il Corn silk maysin ameliorates obesity in vitro and in vivo via suppression of lipogenesis, differentiation, and function of adipocytes. Biomed. Pharmacother. 2017, 93, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-López, L.; Vecino, X.; Barbosa-Pereira, L.; Moldes, A.B.; Cruz, J.M. A multifunctional extract from corn steep liquor: Antioxidant and surfactant activities. Food Funct. 2016, 7, 3724–3732. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.F.S.L.; Almeida, F.C.G.; Vinhas, G.M.; Sarubbo, L.A. Production of Bacterial Cellulose by Gluconacetobacter hansenii Using Corn Steep Liquor As Nutrient Sources. Front. Microbiol. 2017, 8, 2027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, M.-M.; Liu, E.-Q.; Bao, Y.; Duan, S.-L.; She, J.; Liu, H.; Wu, T.-T.; Cao, X.-Q.; Zhang, J.; Li, B.; et al. Low concentration of corn steep liquor promotes seed germination, plant growth, biomass production and flowering in soybean. Plant Growth Regul. 2019, 87, 29–37. [Google Scholar] [CrossRef]
- Loy, D.D.; Lundy, E.L. Chapter 23—Nutritional Properties and Feeding Value of Corn and Its Coproducts. In Corn, 3rd ed.; Serna-Saldivar, S.O., Ed.; AACC International Press: Oxford, UK, 2019; pp. 633–659. ISBN 978-0-12-811971-6. [Google Scholar]
- Kim, Y.; Mosier, N.S.; Hendrickson, R.; Ezeji, T.; Blaschek, H.; Dien, B.; Cotta, M.; Dale, B.; Ladisch, M.R. Composition of corn dry-grind ethanol by-products: DDGS, wet cake, and thin stillage. Bioresour. Technol. 2008, 99, 5165–5176. [Google Scholar] [CrossRef]
- Di Lena, G.; Ondrejíčková, P.; del Pulgar, J.S.; Cyprichová, V.; Ježovič, T.; Lucarini, M.; Lombardi Boccia, G.; Ferrari Nicoli, S.; Gabrielli, P.; Aguzzi, A.; et al. Towards a Valorization of Corn Bioethanol Side Streams: Chemical Characterization of Post Fermentation Corn Oil and Thin Stillage. Molecules 2020, 25, 3549. [Google Scholar] [CrossRef]
- Yang, X.; Nath, C.; Doering, A.; Goihl, J.; Baidoo, S.K. Effects of liquid feeding of corn condensed distiller’s solubles and whole stillage on growth performance, carcass characteristics, and sensory traits of pigs. J. Anim. Sci. Biotechnol. 2017, 8, 9. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.L.; Wang, P.; Zhou, C.H.; Li, P.; Tang, H.Y.; Zhang, J.B.; Cai, Y. Chemical composition and in vitro digestibility of corn stover during field exposure and their fermentation characteristics of silage prepared with microbial additives. Asian-Australas. J Anim. Sci. 2019, 32, 1854–1863. [Google Scholar] [CrossRef]
- Ma, Z.; Kasipandi, S.; Wen, Z.; Yu, L.; Cui, K.; Chen, H.; Li, Y. Highly efficient fractionation of corn stover into lignin monomers and cellulose-rich pulp over H2WO4. Appl. Catal. B Environ. 2021, 284, 119731. [Google Scholar] [CrossRef]
- Liu, Z.H.; Qin, L.; Zhu, J.Q.; Li, B.Z.; Yuan, Y.J. Simultaneous saccharification and fermentation of steam-exploded corn stover at high glucan loading and high temperature. Biotechnol. Biofuels 2014, 7, 167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Chen, W.; Zheng, B.; Yu, W.; Zheng, L.; Qu, Z.; Yan, X.; Wei, B.; Zhao, Z. Fermentation of NaHCO3-treated corn germ meal by Bacillus velezensis CL-4 promotes lignocellulose degradation and nutrient utilization. Appl. Microbiol. Biotechnol. 2022, 106, 6077–6094. [Google Scholar] [CrossRef] [PubMed]
- Azizi-Shotorkhoft, A.; Sharifi, A.; Mirmohammadi, D.; Baluch-Gharaei, H.; Rezaei, J. Effects of feeding different levels of corn steep liquor on the performance of fattening lambs. J. Anim. Physiol. Anim. Nutr. 2016, 100, 109–117. [Google Scholar] [CrossRef]
- Winkler-Moser, J.K.; Hwang, H.-S.; Byars, J.A.; Vaughn, S.F.; Aurandt-Pilgrim, J.; Kern, O. Variations in phytochemical content and composition in distillers corn oil from 30 U.S. ethanol plants. Ind. Crops Prod. 2023, 193, 116108. [Google Scholar] [CrossRef]
- Deepak, T.S.; Jayadeep, P.A. Nutraceutical potential of maize (Zea mays L.) (corn) lab-scale wet milling by-products in terms of β-sitosterol in fiber, gamma-tocopherol in germ, and lutein & zeaxanthin in gluten. Research Square Preprint. Available online: https://www.researchsquare.com/article/rs-1518914/v1 (accessed on 12 December 2022).
- Jiao, Y.; Li, D.; Chang, Y.; Xiao, Y. Effect of Freeze-Thaw Pretreatment on Extraction Yield and Antioxidant Bioactivity of Corn Carotenoids (Lutein and Zeaxanthin). J. Food Qual. 2018, 2018, 9843503. [Google Scholar] [CrossRef]
- Burlini, I.; Grandini, A.; Tacchini, M.; Maresca, I.; Guerrini, A.; Sacchetti, G. Different Strategies to Obtain Corn (Zea mays L.) Germ Extracts with Enhanced Antioxidant Properties. Nat. Prod. Commun. 2020, 15, 1–9. [Google Scholar] [CrossRef]
- Yadav, M.P.; Moreau, R.A.; Hicks, K.B. Phenolic Acids, Lipids, and Proteins Associated with Purified Corn Fiber Arabinoxylans. J. Agric. Food Chem. 2007, 55, 943–947. [Google Scholar] [CrossRef]
- Vazquez-Olivo, G.; López-Martínez, L.X.; Contreras-Angulo, L.; Heredia, J.B. Antioxidant Capacity of Lignin and Phenolic Compounds from Corn Stover. Waste Biomass Valor. 2019, 10, 95–102. [Google Scholar] [CrossRef]
- Johner, J.C.F.; de Meireles, M.A.A. Construction of a supercritical fluid extraction (SFE) equipment: Validation using annatto and fennel and extract analysis by thin layer chromatography coupled to image. Food Sci. Technol. 2016, 36, 210–247. [Google Scholar] [CrossRef]
- Santos, D.T.; Santana, Á.L.; Meireles, M.A.A.; Petenate, A.J.; Silva, E.K.; Albarelli, J.Q.; Johner, J.C.F.; Gomes, M.T.; Torres, R.A.D.C.; Hatami, T. (Eds.) A Detailed Design and Construction of a Supercritical Antisolvent Precipitation Equipment. In Supercritical Antisolvent Precipitation Process: Funtamentals, Applications and Perspectives; Springer Nature: Cham, Switzerland, 2019; Chapter 1. [Google Scholar]
- Santana, Á.L.; Zabot, G.L.; Osorio-Tobón, J.F.; Johner, J.C.F.; Coelho, A.S.; Schmiele, M.; Steel, C.J.; Meireles, M.A.A. Starch recovery from turmeric wastes using supercritical technology. J. Food Eng. 2017, 214, 266–276. [Google Scholar] [CrossRef]
- Carvalho, P.I.N.; Osorio-Tobón, J.F.; Rostagno, M.A.; Petenate, A.J.; Meireles, M.A.A. Techno-economic evaluation of the extraction of turmeric (Curcuma longa L.) oil and ar-turmerone using supercritical carbon dioxide. J. Supercrit. Fluids 2015, 105, 44–54. [Google Scholar] [CrossRef]
- Santana, Á.L.; Santos, D.T.; Meireles, M.A.A. Perspectives on small-scale integrated biorefineries using supercritical CO2 as a green solvent. Curr. Opin. Green Sustain. Chem. 2019, 18, 1–12. [Google Scholar] [CrossRef]
- Liu, J.; Lin, S.; Wang, Z.; Wang, C.; Wang, E.; Zhang, Y.; Liu, J. Supercritical fluid extraction of flavonoids from Maydis stigma and its nitrite-scavenging ability. Food Bioprod. Process. 2011, 89, 333–339. [Google Scholar] [CrossRef]
- Sun, J.; Ding, R.; Yin, J. Pretreatment corn ingredient biomass with high pressure CO2 for conversion to fermentable sugars via enzymatic hydrolysis of cellulose. Ind. Crops Prod. 2022, 177, 114518. [Google Scholar] [CrossRef]
- Yin, J.; Hao, L.; Yu, W.; Wang, E.; Zhao, M.; Xu, Q.; Liu, Y. Enzymatic hydrolysis enhancement of corn lignocellulose by supercritical CO2 combined with ultrasound pretreatment. Chin. J. Catal. 2014, 35, 763–769. [Google Scholar] [CrossRef]
- Cardenas-Toro, F.P.; Forster-Carneiro, T.; Rostagno, M.A.; Petenate, A.J.; Maugeri Filho, F.; Meireles, M.A.A. Integrated supercritical fluid extraction and subcritical water hydrolysis for the recovery of bioactive compounds from pressed palm fiber. J. Supercrit. Fluids 2014, 93, 42–48. [Google Scholar] [CrossRef]
- Zhang, J.; Wen, C.; Zhang, H.; Zandile, M.; Luo, X.; Duan, Y.; Ma, H. Structure of the zein protein as treated with subcritical water. Int. J. Food Prop. 2018, 21, 128–138. [Google Scholar] [CrossRef] [Green Version]
- Moraes, M.N.; Zabot, G.L.; Meireles, M.A.A. Extraction of tocotrienols from annatto seeds by a pseudo continuously operated SFE process integrated with low-pressure solvent extraction for bixin production. J. Supercrit. Fluids 2015, 96, 262–271. [Google Scholar] [CrossRef]
- Colombo, T.S.; Mazutti, M.A.; Di Luccio, M.; de Oliveira, D.; Oliveira, J.V. Enzymatic synthesis of soybean biodiesel using supercritical carbon dioxide as solvent in a continuous expanded-bed reactor. J. Supercrit. Fluids 2015, 97, 16–21. [Google Scholar] [CrossRef]
- Casademont, P.; García-Jarana, M.B.; Sánchez-Oneto, J.; Portela, J.R.; de la Ossa, E.J.M. Supercritical water gasification: A patents review. Rev. Chem. Eng. 2017, 33, 237–261. [Google Scholar] [CrossRef]
- Torres, A.R.C.; Santana, Á.L.; Santos, D.T.; Meireles, M.A.A. Perspectives on the application of supercritical antisolvent fractionation process for the purification of plant extracts: Effects of operating parameters and patent survey. Recent Pat. Eng. 2016, 10, 88–97. [Google Scholar] [CrossRef]
- Santana, Á.L.; Meireles, M.A.A. Coprecipitation of turmeric extracts and polyethylene glycol with compressed carbon dioxide. J. Supercrit. Fluids 2017, 125, 31–41. [Google Scholar] [CrossRef]
- Palazzo, I.; Campardelli, R.; Scognamiglio, M.; Reverchon, E. Zein/luteolin microparticles formation using a supercritical fluids assisted technique. Powder Technol. 2019, 356, 899–908. [Google Scholar] [CrossRef]
- Liu, X.; Jia, J.; Duan, S.; Zhou, X.; Xiang, A.; Lian, Z.; Ge, F. Zein/MCM-41 Nanocomposite Film Incorporated with Cinnamon Essential Oil Loaded by Modified Supercritical CO2 Impregnation for Long-Term Antibacterial Packaging. Pharmaceutics 2020, 12, 169. [Google Scholar] [CrossRef] [Green Version]
- Dias, A.L.B.; Hatami, T.; Viganó, J.; Santos de Araújo, E.J.; Mei, L.H.I.; Rezende, C.A.; Martínez, J. Role of supercritical CO2 impregnation variables on β-carotene loading into corn starch aerogel particles. J. CO2 Util. 2022, 63, 102125. [Google Scholar] [CrossRef]
- Chauvet, M.; Sauceau, M.; Fages, J. Extrusion assisted by supercritical CO2: A review on its application to biopolymers. J. Supercrit. Fluids 2017, 120, 408–420. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.-Y.; Ryu, G.-H. Physicochemical and antioxidant properties of extruded corn grits with corn fiber by CO2 injection extrusion process. J. Cereal Sci. 2013, 58, 110–116. [Google Scholar] [CrossRef]
- Turton, R.B.; Wallace, B.; Whiting, J.S.; Bhattacharyya, D. Analysis, Synthesis and Design of Chemical Processes, 3rd ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2009. [Google Scholar]
- Baldino, L.; De Luca, R.; Reverchon, E. Scale-up and economic analysis of a supercritical CO2 plant for antimalarial active compounds extraction. Chem. Eng. Trans. 2018, 64, 31–36. [Google Scholar]
- Chañi-Paucar, L.O.; Osorio-Tobón, J.F.; Johner, J.C.F.; Meireles, M.A.A. A comparative and economic study of the extraction of oil from Baru (Dipteryx alata) seeds by supercritical CO2 with and without mechanical pressing. Heliyon 2021, 7, e05971. [Google Scholar] [CrossRef] [PubMed]
- Koxholt, M.; Altieri, P.A.; Marentis, R.T.; Trzasko, P.T. Process for Purifying Starches. U.S. Patent 8216628B, 10 July 2012. [Google Scholar]
- DeLine, K.E.; Claycamp, D.L.; Fetherston, D.; Marentis, R.T. Carbon Dioxide Corn Germ Oil Extraction System. U.S. Patent US8603328B2, 3 November 2009. [Google Scholar]
- Zhao, F.; Liu, D.; Cai, S.; Wang, H.; Fu, H. Method for Extracting Corn Embryo Oil by Supercritical Carbon Dioxide. CN Patent 101077990A, 4 July 2007. [Google Scholar]
- Kilambi, S.; Kadam, K.L. Solvo-Thermal Fractionation of Biomass. U.S. Patent 8282738B2, 16 July 2009. [Google Scholar]
- Xu, J.; Gu, S.; Wu, H.; Zuo, Z.; Wei, W.; Hu, S.; Zhang, K.; Xu, X. Method for Improving Enzymolysis and Xylose Conversion Efficiency of Corn Straws by Supercritical Carbon Dioxide Coupled NaOH Pretreatment. CN Patent 112708647A, 24 December 2020. [Google Scholar]
- Yu, D.; Xu, J.; Zhang, Q.; Zhang, S.; Wang, J.; Jiang, L.; Hu, L.; Wang, L.; Qi, Y.; Zhang, J.; et al. Method for Synthesizing Corn Oil Rich in Caprylin Enzymatically under Supercritical Carbon Dioxide (CO2) State. CN Patent 102210356B, 1 April 2011. [Google Scholar]




| Moisture | Protein | Fat | Ash | Carbohydrates | Reference | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total * | Fiber | Starch | Glucan | Cellulose | Lignin | Xylan | ||||||
| DDGS | 10 | 27.5 | 10.5 | 4.1 | 47.9 | 32.8 | 5.2 | 21.2 | 16 | - | 8.2 | [59,60] |
| TS | 91.78 | 0.12 | 1.75 | 0.74 | 5.61 | 1.27 | - | - | - | - | - | [61] |
| CDS | 68.51 | 8.11 | 1.86 | 4.17 | 17.35 | 0.32 | - | - | - | - | - | [62] |
| Stover | 11.8 | 9.01 | 0 | 0 | 79.19 | 17.9 | - | 31.7 | 32.9–43.1 | 5.4–12.6–14.8 | 17.1 | [17,63,64,65] |
| Gluten feed | 10 | 20.3 | 3 | 7.4 | 59.3 | 32 | 15.5 | - | - | - | - | [59] |
| Gluten meal | 10 | 28.37 | 2.2 | 5.83 | 53.6 | 5.88–7.3 | 13.9 | 13.64 | - | [59,66] | ||
| Germ meal | 10 | 25.12 | 2.73 | 3.8 | 58.35 | 35.5 | 17.8 | - | - | - | - | [13,59] |
| Steep liquor | 50 | 16.4 | 2.3 | 1.5 | 29.8 | 1.8 | 5.7–14.8 | - | - | - | - | [7,59,67] |
| Substance | DDGS | Germ Meal | Gluten Feed Fiber | Gluten Meal | Thin Stillage | Stover | Reference |
|---|---|---|---|---|---|---|---|
| Carotenoids | Lutein: 101 µg/g | Lutein: 0.072 µg/g | Lutein: 5.24 µg/g | Lutein: 2.5–15 µg/g | Lutein: 31 µg/g | - | [42,61,68,69,70] |
| Zeaxanthin: 69 µg/g | Zeaxanthin: 0.989 µg/g | Zeaxanthin: 6.61 µg/g | Zeaxanthin: 5–35 µg/g | Zeaxanthin: 32.5 µg/g Beta cryptoxanthin: 0.70 µg/g | - | ||
| Beta cryptoxanthin: 47 µg/g | Beta carotene: 0.16 µg/g | Beta-carotene:0.299 µg/g | Beta-carotene:1.22 µg/g | - | - | ||
| - | - | - | - | - | - | ||
| Tocopherols | Alpha-tocopherol: 0.108 µg/g | Alpha-tocopherol: 31.74 µg/g | Alpha-tocopherol: 0.28 µg/g | Alpha-tocopherol: 0.18 µg/g | Alpha-tocopherol: 37.3 µg/g | - | [14,69] |
| Gamma tocopherol: 0.069 µg/g | Gamma tocopherol: 535.59 µg/g | Gamma tocopherol: 9.78 µg/g | Gamma tocopherol: 13.96 µg/g | Gamma tocopherol: 143 µg/g | - | ||
| Delta tocopherol: 0.0182 µg/g | Delta tocopherol:17.38 µg/g | Delta tocopherol: 1.08 µg/g | Delta tocopherol: 0.97 µg/g | Delta tocopherol: 4.46 µg/g | - | ||
| Tocotrienols | Alpha Tocotrienol: 0.0093 µg/g | Alpha Tocotrienol: 0.32 µg/g | Alpha Tocotrienol: 1.32 µg/g | Alpha Tocotrienol: Non identified | Alpha Tocotrienol: 29.6 µg/g | - | [14,69] |
| Gamma tocotrienol: 0.014 µg/g | Gamma tocotrienol: 3.67 µg/g | Gamma tocotrienol: 6.61 µg/g | Gamma tocotrienol: 4.52 µg/g | Gamma tocotrienol: 38.5 µg/g | - | ||
| Delta tocotrienol: 0.24 µg/g | Delta tocotrienol: 0.54 µg/g | Delta tocotrienol: 0.50 µg/g | Delta tocotrienol 0.09 µg/g | - | |||
| Phenolic compounds | Ferulic acid: 7010 µg/g | Ferulic acid: 9870—636,540 µg/g | Ferulic acid: 1020–4200 µg/g | - | - | Ferulic acid: 990.20–3515.50 µg/g- | [39,71,72,73] |
| p-Coumaric acid: 530 µg/g | - | p-Coumaric acid: 60–340 µg/g | - | - | p-Coumaric acid: 2027.45–7307.75 µg/g | ||
| Caffeic acid: 770 µg/g | - | - | - | - | - | ||
| Vanillic acid: 500 µg/g | - | - | - | - | - | ||
| Sinapic acid: 8780 µg/g | - | - | - | - | - | ||
| Phytosterols | Campesterol: 2916 µg/g | Campesterol: 87 µg/g | Campesterol: 201.1 µg/g | Campesterol: 87.2 µg/g | Beta sitosterol: 1467 µg/g | Campesterol: 226.4 µg/g | [17,61,68,69] |
| Stigmasterol: 861 µg/g | Stigmasterol: 343.8 µg/g | Stigmasterol: 476.1 µg/g | Beta sitosterol: 1342.5 µg/g | Squalene: 168 µg/g | Stigmasterol: 319.6 µg/g | ||
| Sitosterol: 9066 µg/g | Beta sitosterol: 311.1 µg/g | Beta sitosterol: 2943.6 µg/g | - | Δ-5 avenasterol: 455 µg/g | Beta-sitosterol: 735.6 µg/g | ||
| Sitostanol: 4186 µg/g | - | - | - | - | - |
| Process | Byproduct | Conditions | Reference |
|---|---|---|---|
| SFE | Germ | Solvent: CO2 | [21] |
| Cosolvent: acetone, ethanol, and hexane | |||
| Temperature: 45–85 °C | |||
| Pressure: 15–25 MPa | |||
| CO2 flow rate: 3 L/h | |||
| Cosolvent flow rate: 0.1 mL/min | |||
| SFE | Germ | Solvent: CO2 | [71] |
| Temperature: 80 °C | |||
| Pressure: 30 MPa | |||
| CO2 flow rate: 2.5 L/min | |||
| Time: 10 min | |||
| SFE | Germ | Solvent: CO2 | [43] |
| Temperature: 45 °C | |||
| Pressure: 40 MPa | |||
| CO2 flow rate: 5.6 g/min | |||
| SFE | Silk | Solvent: CO2 | [79] |
| Temperature: 40–60 °C | |||
| Pressure: 25–45 MPa | |||
| CO2 flow rate: 20 L/h | |||
| Cosolvent flow rate: 1.3 mL/g | |||
| Time: 120 min | |||
| Hydrolysis assisted with SC-CO2 | Stover: cob and stalk | Solvent: CO2 and water present in raw material | [80] |
| Temperature: 40–70 °C | |||
| Pressure: 35–45 MPa | |||
| Time: Non identified | |||
| Hydrolysis assisted with SC-CO2 | Stover: cob and stalk treated with ultrasound | Solvent: CO2 and water present in raw material | [81] |
| Temperature: 170 °C | |||
| Pressure 20 MPa | |||
| Time: 30 min | |||
| Hydrolysis assisted with SW | Zein | Temperature: 110–170 °C | [83] |
| Pressure: 0.1–0.8 MPa | |||
| Time: 20–120 min | |||
| Transesterification | Oil | Solvent: SC-CO2 | [22] |
| Temperature: 95 °C | |||
| Pressure: 9.65 MPa | |||
| Catalyst: Nafion NR50 | |||
| Time: 240 min | |||
| SWG | Straw | Temperature: 450–550 °C | [23] |
| Pressure: 41 MPa | |||
| Catalyst: K2CO3 | |||
| Time: 10–50 min | |||
| Encapsulation | Zein | Antisolvent: SC-CO2 | [18] |
| Solvent: Ethanol:water (94:6, v/v) | |||
| Temperature:40 °C | |||
| Pressure: 7–16 MPa | |||
| Solution flow rate: 0.02 g/mL | |||
| CO2 flow rate: 20–60 g/min | |||
| Encapsulation | Zein | Antisolvent: CO2 | [89] |
| Solvent: Aqueous mixtures with ethanol and acetone | |||
| Temperature: 60 °C | |||
| Pressure: 8.2–10 MPa | |||
| Solution flow rate: non-identified | |||
| CO2 flow rate: non-identified | |||
| Impregnation | Zein | Temperature: 40 °C | [90] |
| Pressure: 15 MPa | |||
| Time: 60 min | |||
| Impregnation | Starch | Temperature: 40–60 °C | [91] |
| Pressure: 15–30 MPa | |||
| Number of cycles: 1–4 | |||
| Depressurization rate: 0.25–2.61 MPa/min | |||
| Extrusion | Fiber | Temperature: 90–120 °C | [93] |
| Pressure: non-identified | |||
| Moisture: 30% | |||
| CO2 flow rate: 200 mL/min | |||
| Screw speed: 100 g/min |
| Material | Process | Conditions Used | Product | Patent Number | Location | Reference |
|---|---|---|---|---|---|---|
| Starch (solid and slurry at 20–50% solids) | Supercritical fluid extraction | Solvent: CO2 and ethanol Temperature: 50–120 °C Pressure: >30 MPa Solvent to the raw material ratio: 1–10 | Deodorized starch | US8216628B | United States | [97] |
| Germ meal | Supercritical fluid extraction | Solvent: CO2 Temperature: 20–110 °C Pressure: 11–64 MPa Solvent to the raw material ratio: 2–30 | Oil | US8603328B2 | United States | [98] |
| Germ meal | Supercritical fluid extraction coupled with fractionation | Solvent: CO2 Extractor: 40 °C and 8 MPa–20 MPa Separator 1: 35 °C and 8 MPa–6 MPa Separator 2: 35 °C and 6 MPa Flow rate: 20 L/h | Fractionated oils | CN101077990A | China | [99] |
| Stover | Supercritical hydrolysis | Solvent: water Temperature: 264 °C Pressure: 7.58 MPa Time: 20 min | Monosaccharides | US8282738B2 | United States | [100] |
| Straw | Supercritical hydrolysis | Solvent: CO2 and water/C1–C5 alcohol Temperature: 35–70 °C Pressure: Non identified Time: 60 min | Monosaccharides | CN112708647A | China | [101] |
| Germ oil | Enzymatic reaction | Enzymatic reaction Temperature: 45–65 ℃ Catalyst: Novozym 435 at 3–5% Pressure: 8–13 MPa Mixing speed: 120 r/min, Reaction time: 18–28 h | Structured lipid rich in caprylin | CN102210356B | China | [102] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santana, Á.L.; Meireles, M.A.A. Valorization of Cereal Byproducts with Supercritical Technology: The Case of Corn. Processes 2023, 11, 289. https://doi.org/10.3390/pr11010289
Santana ÁL, Meireles MAA. Valorization of Cereal Byproducts with Supercritical Technology: The Case of Corn. Processes. 2023; 11(1):289. https://doi.org/10.3390/pr11010289
Chicago/Turabian StyleSantana, Ádina L., and Maria Angela A. Meireles. 2023. "Valorization of Cereal Byproducts with Supercritical Technology: The Case of Corn" Processes 11, no. 1: 289. https://doi.org/10.3390/pr11010289