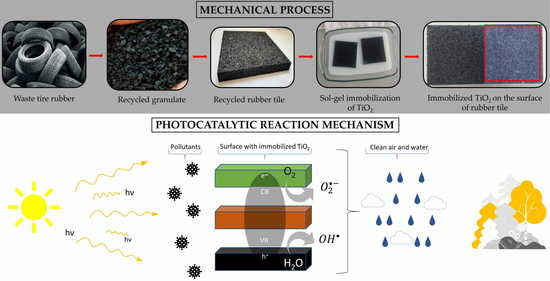
1. Introduction
An environmentally friendly and low-cost rubber recycling method is currently one of the biggest environmental challenges of the 21st century [
1]. The management of waste tires in Croatia has been progressively improved. The first company that recycled the rubber of used car tires was founded in 2005. The main goal was to reduce the harmful impact on the environment while reusing the valuable properties of rubber; in this case, the end goal was the production of recycled rubber tiles. The primary purpose of recycled rubber tiles is to provide protection from injuries on children’s playgrounds; they are also used for many other purposes [
2,
3]. Rehabilitation centers in closed and open spaces are much safer if the surface is covered with rubber tiles as opposed to, for example, ceramic tiles. Today, noise is defined as one of the factors that pollute the working and living environment, so it is important to note that rubber tiles reduce the noise level by up to 32 dB, which is important for places where sports fields and fields are located [
2,
4]. Rubber elements are used as sound insulators, anti-vibration mats, sealants of various types, roofing panels, buffers in harbors for the berthing of vessels, floating docks, and as various non-slip mats, etc. [
2,
5]. It is estimated that the construction industry will become the largest consumer of rubber granules and tiles due to the need to create sound barriers, structural stiffeners, and accessories for foundations, etc. [
2,
6]. The tiles are made of granules of different sizes (the technological process is explained in more detail in the methodology). This contributes to the roughness of the tiles and is responsible for anti-slip performance. Unfavorable weather conditions very often prevent the use of playgrounds or fields due to water retention, but rubber surfaces—due to their waterproofness—enable use regardless of the weather. “Tires are primarily vulcanized rubber, rubberized fabric, steel or fabric belts, and steel-wire-reinforced rubber beads. The most commonly used tire rubber is styrene-butadiene copolymer (SBR) and consists of about 75% butadiene and 25% styrene by weight [
7]”. Other elastomers akin to natural rubber are also used in differing amounts in tire compounds. The most-used fillers are carbon black, extender oil, and sulfur [
7].
Currently, the basic methods of waste-rubber recycling are shredding and grinding. To ensure the effective recycling of waste rubber, separating all unnecessary parts from the rubber (such as iron castings and textiles) is necessary [
1]. Old, exported tires give granules of various sizes with modern technology. In this paper, a special emphasis is placed on the recycled rubber tiles that line children’s playgrounds, sports and recreational facilities, and rehabilitation centers. Our main purpose is to extend the functionality of rubber tiles from an environmental point of view. Recently, people’s awareness of environmental pollution and its negative consequences has been increasingly awakened. Although industrial development affects air quality, it also encourages the advancement of technology and the interest of scientists, leading to its inevitability.
The application of heterogeneous photocatalysis in air purification has been developed over the last decades. The gas-phase photocatalysis was developed in work based on the photodecomposition of nitric oxide [
8]. Given that a wide range of air pollutants can be partially or completely degraded with TiO
2-assisted UV irradiation, over the years several studies have suggested using the solid–gas photocatalysis for environmental applications [
9,
10,
11,
12,
13]. Heterogeneous photocatalysis became popular after Fujishima and Honda discovered the UV-driven, photoelectrochemical water-splitting reaction with a TiO
2-based photoanode in 1972 [
14]. This is a process in which solar radiation, in synergy with a photocatalyst and in the presence of water molecules, creates a chain of redox reactions on the surface of the photocatalyst, resulting in inert products such as carbon dioxide, water, and other non-toxic species, depending on the structure of pollutant (as is shown in the graphical abstract). Under the source of UV radiation, the electrons in the photocatalyst’s valence band are promoted to its conduction band, thus leaving an empty space—or so-called hole (h+)—with a lack of negative charge (e−) in the valence band. These holes are crucial for the initiation of redox processes which lead to pollutant degradation because they produce hydroxyl radicals (-OH) in the presence of humidity. These radicals react non-selectively with pollutants, oxidizing them to harmless molecules [
15].
The advantage of photocatalysis is that it is an inexpensive, sustainable, and eco-friendly technology that results in a complete or partial decomposition of pollutants. The synergistic action of solar radiation and photocatalysts triggers redox reactions on photocatalysts surfaces which successfully remove sulphur dioxide, ammonia, unpleasant odors, volatile organic compounds, and nitrogen oxides, etc. [
16]. For example, one of the most-researched photocatalysts, titanium dioxide (TiO
2) irradiated with UV light, can decompose many organic compounds into water, carbon dioxide, and mineral acids or their salts [
17]. TiO
2 is widely used as an air purifier in various forms of coatings. For instance, Murugan et al. proved the performance of the self-cleaning coatings on building materials such as ceramic glazed tiles and glass windows. Nano TiO
2 films were generated in the form of a spray, dip, or as a flow coating method. The TiO
2 photocatalytic layer acts as a surface modification of such building materials, thus keeping the building exterior clean by utilizing solar energy [
18]. Wang et al. also used nano TiO
2 powders such as Degussa P25 for concrete coatings to enhance the photocatalytic activity. Furthermore, nano-TiO
2-based coatings have been studied to improve the air purification and self-cleaning properties of cement-based materials [
19], since those materials in buildings are directly and continuously exposed to various atmospheric pollutants and microorganisms under different weather conditions. Furthermore, new, industrially produced photocatalytic tiles by Bianchi et al. were made using a commercial micro-TiO
2. In addition to good photocatalytic performance, these tiles also meet standard requirements regarding porosity, durability, hardness, and vitrified surface. Photocatalytic degradation tests were performed using NO
x and methylene blue as modeled polls for confirming good performance in both phases, gas and liquid [
20]. Grčić et al. showed the application of solar photocatalysis for air purification by applying an alternative, best-available-technique TiO
2 photocatalysis. Toxic substances normally related to agriculture emissions, such as ammonia and methane, were included. Photocatalytic oxidation confirmed the continuous oxidation of NH
3 and CH
4 to CO and CO
2, and N
2, respectively [
15]. TiO
2 as a photocatalyst was also used in synthesized compounds to improve certain properties, such as TiO
2/ZnTiO
3 [
21], WO
3/TiO
2 [
22], N–V co-doped TiO
2 [
23], and TiO
2/zeolite composite [
24], etc. The idea to test the application of rubber tiles with a photocatalytic layer for passive air protection was developed with these above-mentioned applications in mind.
The use of secondary raw materials—in this case recycled tires, which are used to produce rubber tiles—already represents ecological progress for the purpose of environmental protection. Additionally, this work presents a solution for achieving the passive protection of air and human health by immobilizing TiO2 on the surface of recycled rubber tiles, based on which we will achieve a completely new purpose for substrates. The combination of tiles made from secondary raw materials and the achievement of photocatalytic decomposition of harmful compounds in the air gives additional value to this product in the context of a circular economy and environmental protection. Methods and results are provided in the following sections.
2. Materials and Methods
2.1. Rubber Tile Manufacturing
Rubber tiles made from recycled rubber were obtained from a recycling company, Gumiimpex-GRP, Ltd., Varaždin, Croatia. The process of manufacturing recycled rubber tiles begins with the collection of waste tires, followed by the acceptance, storage, sorting, mechanical processing (cutting and shredding), painting (depending on preference, occasion, and purpose), and finally production of the rubber tiles. The collected waste tires are divided and separated into passenger, cargo (dumper and truck), semi-truck, tractor, and forklift categories. Parts that are not of rubber origin, such as rims or tubes, must be separated. Mechanical processing cannot begin until the steel parts are separated. Cargo tires contain steel “bulks”, so the first step is to remove them. Separated rims and steel “bulks” represent secondary raw materials and are sold further on the market. After all the tires have been sorted and all the steel parts have been removed from them, the first part of the mechanical processing follows: cutting. Tires of larger dimensions, such as dumper tires, truck tires, etc., are cut, while those of smaller dimensions enter the process whole. The cutting of large tires is performed in a machine with large knives. The tires are cut so that they can enter the second part of the mechanical processing: shredding, which is performed using a “shredder”. The loader transfers cut tires into the shredder, which shreds the tires to a size of 100 × 250 mm. After the tires have been shredded for the first time, they pass through a vibrating screen and arrive on a conveyor belt which takes them to the raw material warehouse, where further shredding begins. After the tire is shredded to a size of 100 × 250 mm, it enters the first granulator via conveyor belt, where it is shredded to a maximum size of 30 mm. During this shredding, approximately 80% of the steel wire and textiles are released. The wire is transferred to the steel purification line, and the textile is separated pneumatically (i.e., by suction). Following this, the shredded rubber, which reaches a maximum size of 30 mm, enters the second granulator, where it is shredded to a maximum size of 12–14 mm. As with the previous granulator, steel wires and textiles are additionally separated. The third granulator shreds the rubber to a maximum size of 8–10 mm, and the fourth and fifth granulators shred the rubber to a maximum size of 3.0–3.5 mm. The last stage of purification from steel wires and textiles is carried out inside a rotating system for the separation of granules in which the iron and textiles are finally separated from the granules with additional suction and smaller magnets. After the sliced tires have undergone shredding through five granulators, the final raw material is obtained and is ready for further processing and the production of recycled rubber substrates. The final product is a granulate in size ranges of 0.0–0.5 mm, 0.5–2.0 mm, and 2.0–3.5 mm (
Figure 1).
After mechanical processing, the obtained rubber granulate is ready to produce tiles. The line to produce recycled rubber tiles consists of (1) an industrial mixer, (2) a press, (3) a material transport system, (4) a rubber granulate tank, (5) a polyurethane/binder tank, (6) a catalyst tank, and (7) a paint tank.
The tiles used in this work were made at a size of 1000 × 1000 × 10 mm. A total of 9 kg of rubber granulate, 380 g of binder (polyurethane STOBICOLL 352.00), and 5 g of catalyst (DABCO K 2097) were added to the bucket and mixed for 5 min using an industrial mixer (
Figure 2).
The listed ingredients were brought to the bucket using the material transport system. The catalyst was dosed with a pump and, depending on the weather conditions, it increased/decreased. After mixing, the mixture was placed into a pressing mold (
Figure 3), evenly distributed, and pressed under a high temperature (120 °C) for 4 min.
After 4 min, the tile was taken out of the mold and placed on its side to cool. Recycled rubber tiles are mostly composed of 20% of granules of a size 0.5–2.0 mm and 80% of granules of a size 2.0–3.5 mm. The final product is shown in
Figure 4.
In addition to the black rubber tiles, green and red rubber tiles were obtained for the experiments since colored rubber tiles are often used on children’s playgrounds. Depending on the customer’s wishes, needs, and the purpose of a certain granulate, the granules can be colored red, green, blue, or white, etc. Of course, coloring also increases the value of the product. The paints were stored in the paint tank and, depending on need, dosed using a pump.
2.1.1. Rubber Substrates Pre-Preparation
The recycled rubber substrate was first etched with a sodium hydroxide solution (NaOH, 1:10, w:V) according to the procedure described in [
25] to make the surface as rough as possible and to form -OH groups in order to achieve the binding of the TiO
2 photocatalyst on the rubber surface using the sol–gel method. The NaOH solution was prepared from granules (NaOH, Lachner, Neratovice, Czech Republic) with deionized water. Before soaking the rubber tiles into the prepared solution, the tiles were weighed and washed with ethanol (96%, Gram-mol), after which they were air-dried for 10 s. Afterwards, tiles were soaked in the NaOH solution for 40 min, after which they were washed with deionized water and dried for 24 h at 60 °C.
2.1.2. Photocatalyst Immobilization by the Sol–Gel Method
The photocatalyst TiO
2 (Evonik, Essen, Germany, Aeroxid
®, TiO
2 P25, 30 nm, 56 m
2/g, 75:25 anatase–rutile mass ratio) was immobilized on the rubber substrates using the sol–gel method described in [
26]. Three sets of experiments were conducted with black rubber tiles. Different proportions of TiO
2 were added to the sol–gel solution and were functionalized with appropriate amounts of tetraethyl orthosilicate (TEOS, ≥99.9% Sigma-Aldrich, Steinheim, Germany); 2 g TiO
2/5 mL TEOS, 4 g TiO
2/10 mL TEOS, and 10 g TiO
2/20 mL TEOS.
The sol–gel solution (the most perspective) was prepared by mixing 2 g of TiO2, 200 mL of deionized water, and 200 mL of ethanol (GramMol, 96%), followed by 10 min of stirring and a 3 min treatment in an ultrasonic bath. When the solution was well-stirred, 70 mL of acetic acid (99–100% p.a., LabExpert, Ljubljana, Slovenia) was added to adjust the pH to an acidic level of approximately 3–4. For better binding, 5 mL of TEOS was added to the solution. Afterwards, the solution was stirred at 50 °C for 1 h. The rubber substrates were soaked in a prepared solution for 10 min and then dried at 80 °C for 20 min. The soaking procedure was performed four times in a row, and the substrates were then left for one week at room temperature. The same procedure was used for samples with 4 g TiO2/10 mL TEOS and 10 g TiO2/20 mL TEOS.
Red and green rubber substrates were immobilized with the most perspective TiO2 mass proportion, based on the obtained results with black rubber substrates.
2.2. Characterization of Rubber Substrates
2.2.1. Scanning Electron Microscopy (SEM) and Energy Dispersion Spectroscopy (EDS)
The characterization of the immobilized layer in terms of crystal structure and morphology was investigated by SEM. SEM was performed with scanning electron microscopy using an FEG SEM Quanta 250 FEI microscope operating at 20 kV and a working distance set at 20 mm under low vacuum conditions (“as is”) without evaporation. EDS mapping was performed using an EDS detector, Oxford Pentafet. To select the best ratio of TiO2 immobilization (2, 4, or 10 g), each sample was cut into three layers to determine how deep the TiO2 had entered into the substrate. Three layers were horizontally cut at 2 mm, 4 mm, and 6 mm of height from the surface.
2.2.2. Fourier Transform Infrared Spectroscopy (FTIR)
FTIR was performed using a Bruker Vertex 70 in ATR (attenuated total reflectance) mode. The samples were pressed on a diamond. The absorbance data were collected between 400 and 4000 cm−1 with a spectral resolution of 1 cm−1 and 64 scans. The equipment was sourced from Bruker Corporation, Billerica, MA, USA.
2.3. Leaching Test
The possible leaching of tire constituents into the environment and their subsequent potential adverse impact is of great concern for recycling and reusing waste tires [
27]. To simulate natural leaching in a laboratory, a leaching test was used [
28] to quantify the leaching of hazardous substances from media into groundwater and other water systems. These tests have been standardized by international agencies such as the International Organization for Standardization (ISO) because their results can provide important information for understanding and addressing human health risks from contaminated soil [
29]. Various methods are available, but a selection that can precisely simulate the real-life scenario is challenging. Moreover, none of the laboratory leaching tests can replace the leaching behavior of materials in nature. Nevertheless, when used within the proper framework, the leaching test can ensure helpful information for environmental decision-making [
28]. According to evaluation, several countries and agencies have standardized leaching test methods [
29]. This paper reviews a tire–water chemical interaction based on the author’s research. Due to the metals and textiles present in tires, heavy metals are expected to be found. In this work, the samples were sliced and leached for 24 h with deionized water with a liquid/sample ratio (L/S) of 1/10. The leachates were then recovered by filtering the eluate through a 0.45 μm filter. The TDS (Total Dissolved Solids) were analyzed on Hach Lange Sension 156 multimeter and DOC (dissolved organic carbon) was analyzed on a Shimadzu TOC/TN analyzer. Chlorides, fluorides, and sulphates were analyzed on a Hach Lange DR 5000 spectrophotometer. Metals were analyzed on a PerkinElmer AAnalyst 800 spectrometer. The analysis was performed using three techniques: the flame technique (FAAS) for the elements zinc (Zn), chromium (Cr), and copper (Cu); the graphite technique (GFAAS) for arsenic (As), barium (Ba), cadmium (Cd), molybdenum (Mo), nickel (Ni), lead (Pb), selenium (Se), silicon (Si), and titanium (Ti); and the hydride technique (FIAS) for mercury (Hg) only. The results of the test were compared with the ordinance on the methods and conditions for the landfill of waste, categories and operational requirement for landfills [
30], and the Toxicity Characteristic Leaching Procedure (TCLP) [
31].
For the comparison with the limit values of eluate parameters in the ordinance on the methods and conditions of waste disposal categories and the operating conditions for landfills, due to the differences in the unit reporting, the conversion of measured leaching concentrations was performed in correspondence with the following conversion formula (Equation (1)) [
32]:
2.4. Photocatalytic Oxidation Testing Set Up
The photocatalytic activity of the most perspective rubber tiles with immobilized TiO
2 was tested in preliminary experiments. The degradation of ammonia (NH
3) in the air stream was tested in the semi-pilot photocatalytic wind tunnel (PWT) shown in
Figure 5. The flow-through reactor PWT resembles a solar simulator and can therefore be used for the testing of new photocatalytic materials such as photocatalytic rubber tiles. The reactor is made from a high-quality plexiglass material (thermoplastic, polymethyl methacrylate (PMMA)) consisting of two chambers separated by a screen with three slots for directional ventilation. The reaction chamber where photocatalytic tests are performed is followed by the measuring chamber. Rubber tiles were placed in the reaction chamber at a 10 cm distance from the irradiation source and positioned parallel to the air flow. In each experiment, there were four similar tiles (100 × 100 mm) in the reaction space, giving a total irradiated surface of 0.04 m
2. The PWT has one inlet connected to the source of NH
3, i.e., an aqueous solution with c(0)NH
3,aq = 200 ppm. The air flow was achieved using the air pump with a maximum velocity of 3.4 m/s placed before the NH
3 source. Arduino sensors for temperature and humidity were placed in the measuring chamber, and an outlet was attached to a rinse. The reaction chamber was covered with the irradiation emission panel. The panel contained three irradiation sources simulating solar spectra with a higher-UVB to average-spectra ratio: Sunlight Pro Compact UV-B 2.0 23 W (Trixie), Compact UV Sun 20 W (Lucky Reptile), and Compact Pro 8.0 UVB 23 W (Terra Exotica) [
15]. The incident irradiation intensities (I0, W m
−2) at the surface of the tiles were 13.3 and 24.6 for UVA and UVB irradiation, respectively, with >98% accuracy. Measurements were made with a UVP UVX radiometer following the previously established procedure [
15].
Several sets of experiments were performed to determine the extent of NH3 degradation in the PWT over rubber tiles with immobilized TiO2. Referent experiments were performed with irradiated rubber tiles (without immobilized TiO2) and in the dark to obtain the baseline of the NH3 concentration and respective residence time in the PWT. The adsorption of NH3 on the rubber tiles was studied in dark both with the referent rubber tiles and those with immobilized TiO2. The photocatalytic experiments were performed with the rubber tiles with immobilized TiO2 under irradiation. The duration of one experiment was 5 h. The continuous monitoring of gases CO, CO2, NOx, NO, NO2, HC, and H2S was conducted using a gas analyzer Testo 350 and a landfill gas analyzer GA5000 (Geotech, Leamington spa, Warwickshire, UK) for the monitoring of NH3.
4. Conclusions
The existing rubber tiles obtained from granules were successfully modified by a surface application of an anatase/rutile thin film from commercial TiO2 P25 by a modified sol–gel process using TEOS as an organic precursor (2 g TiO2/5 mL TEOS, 4 g TiO2/10 mL TEOS, and 10 g TiO2/20 mL TEOS). The SEM and EDS analyses of black rubber substrates showed that the optimal immobilization was achieved using the lowest TiO2 mass proportion (2 g TiO2/5 mL TEOS). On the samples with 4 g TiO2/10 mL TEOS and 10 g TiO2/20 mL TEOS, a crust was formed that cracked, and there was no penetration of TiO2 into the deeper layers. For this reason, these ratios were no longer used for further research.
The latter was confirmed by the SEM and EDS analyses of black rubber substrates by horizontal layer analysis, demonstrating the penetration and immobilization of the TiO2 nanoparticles. The same penetration and immobilization were observed in the red and green rubber substrates.
The FTIR analysis has shown the formation of the wideband at 3300 cm−1, which is attributed to -OH groups forming due to NaOH treatment. Furthermore, the bands at 950 and 800 cm−1, assigned to the Ti–O–Si and Si–O–Si groups after NaOH pretreatment and TiO2 immobilization, indicate that the immobilization of TiO2 on the newly formed -OH groups was successful. The formation of chemical bonds could also explain the low-Ti content in the leachate.
The stability and environmental impact of rubber substrates (black, red, and green) were investigated by a leaching test. Leached substances were the results of the rubber composition. As was previously mentioned, the results related to the leached Ti concentration indicate an efficient immobilization of TiO2. In contrast, Si concentrations assumed that the amount of TEOS used in the immobilization sol–gel method could be decreased, offering favorable start for the industrial development of modified rubber tiles.
Photocatalytic oxidation of NH3 was achieved by using black rubber tiles immobilized with 2 g of TiO2, confirming the photocatalytic oxidation of NH3 to N2. Furthermore, a decrease of NH3 in the presence of irradiation during the referent experiment without TiO2 layer indicates a possible wet catalytic oxidation which is a result of presence of metalloid particles in the rubber tiles. Therefore, the TiO2 layer on rubber tiles contributes to the protection of the rubber tiles against typical wear due to atmospheric exposure, confirming the use of these new materials for passive air protection.
Based on the given results, rubber substrates with the addition of 2 g of TiO2 have been shown to be the most perspective for further tests of air pollutant photocatalytic degradation.