Pinch Analysis for Heat Integration of Pulverized Coke Chemical Looping Gasification Coupled with Coke-Oven Gas to Methanol and Ammonia
Abstract
:1. Introduction
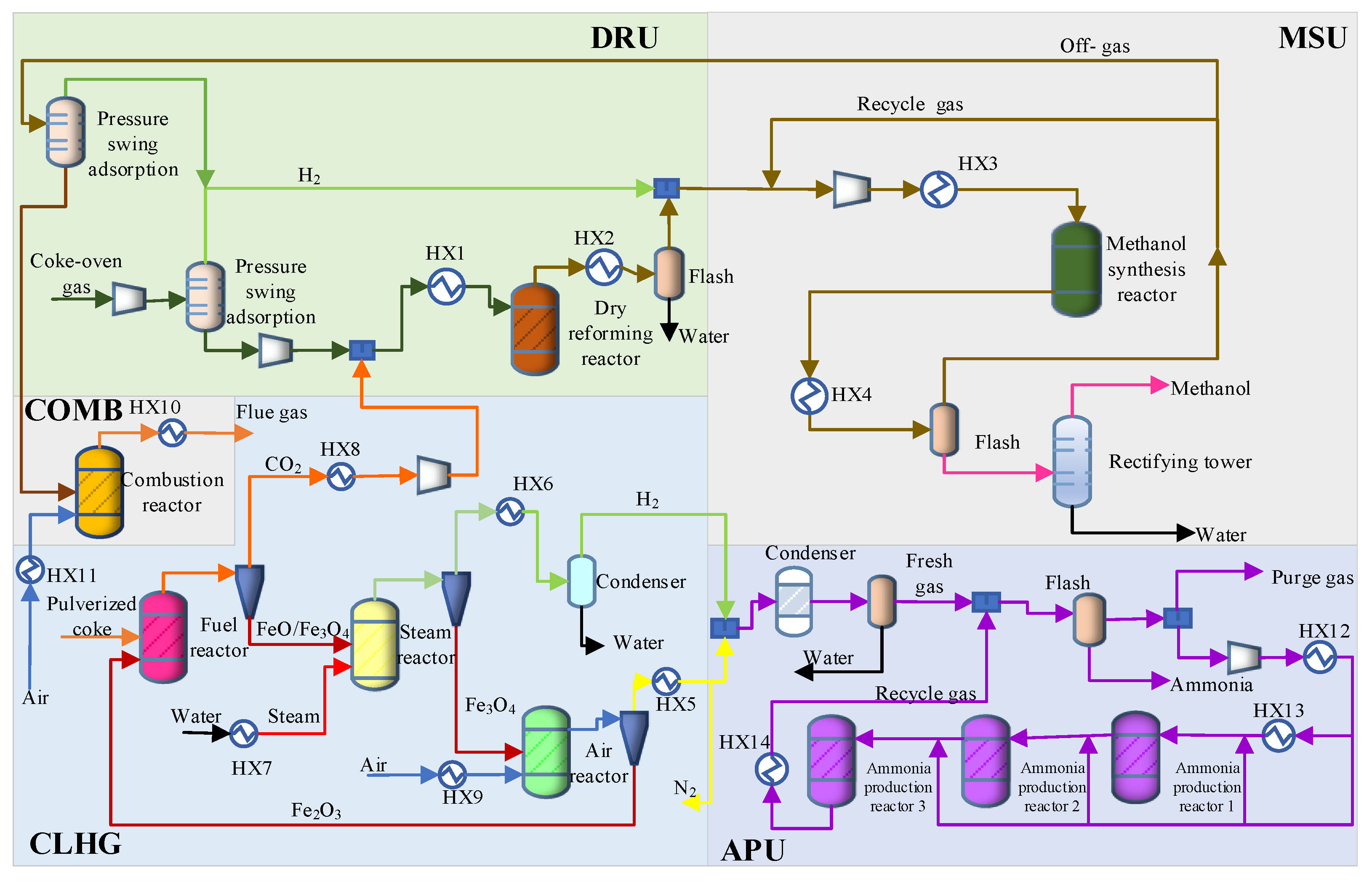
2. Process Description
2.1. DRU
2.2. MSU
2.3. CLHG
2.4. COMB
2.5. APU
2.6. Model Assumptions and Validation of the System
- (1)
- There was no heat loss between the units of the system;
- (2)
- All parameters of the reactor were constant during the reaction;
- (3)
- The equilibrium was reached very fast—reaction rates of all processes were very high;
- (4)
- There was no temperature gradient, during the heating of the reactants.
3. Pinch Technology
- Data gathering is used to extract stream data from different processing units, considering the total heat and mass balances of the system to calculate heat exchanged for all hot and cold streams. The data gathering considers two aspects. (1) The entire network system is divided into three heat exchange units, namely, CLHG + COMB, DRU + MSU, and APU. (2) The system is treated as a whole, and the dates of all cold and hot streams are collected simultaneously;
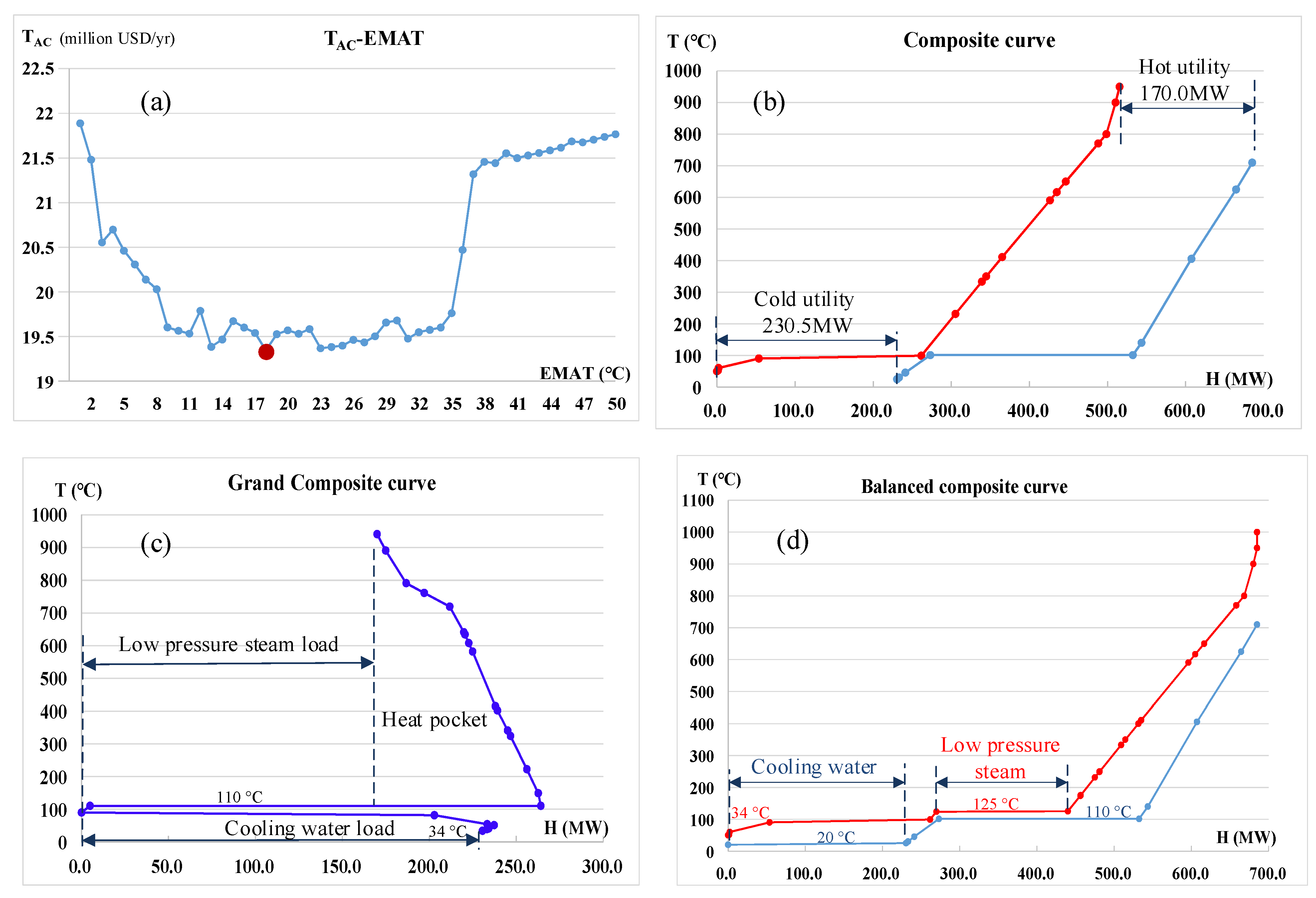
- The estimate of ΔTmin in each heat exchange unit based on the minimum TAC according to TAC vs. ΔTmin diagrams;
- The drawing composite curve, grand composite curve, and balanced composite curve are used to determine the amount of energy consumption and utility required;
- Pinch analysis is applying to design the HENs from two aspects: (1) the HEN in each heat exchange unit is designed individually, and then the total site is analyzed when solving interplant integration; and (2) the HENs of the three heat exchange units are treated as a whole and are designed simultaneously;
- Through heat integration from the above two aspects, the theoretical maximum heat recovery or the minimum energy requirement is obtained. Then, the economic feasibility of the two abovementioned schemes is compared basis the TAC;
- The results of the previous steps provide insights for the heat recovery and utilization of the HEN system.
4. Results and Discussion
4.1. Heat Integration of the Three Heat Exchange Units
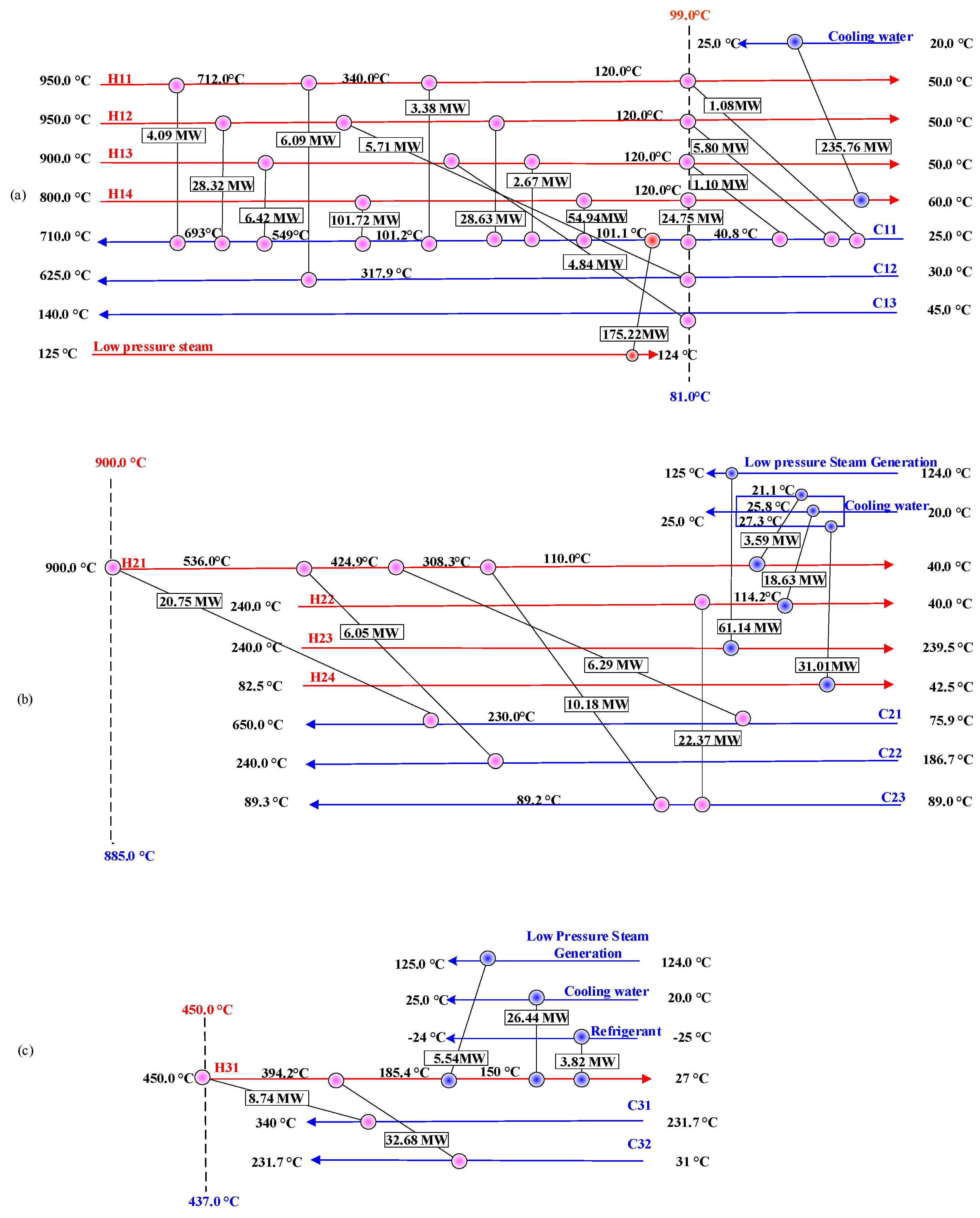
4.1.1. CLHG + COMB
4.1.2. DRU + MSU
4.1.3. APU
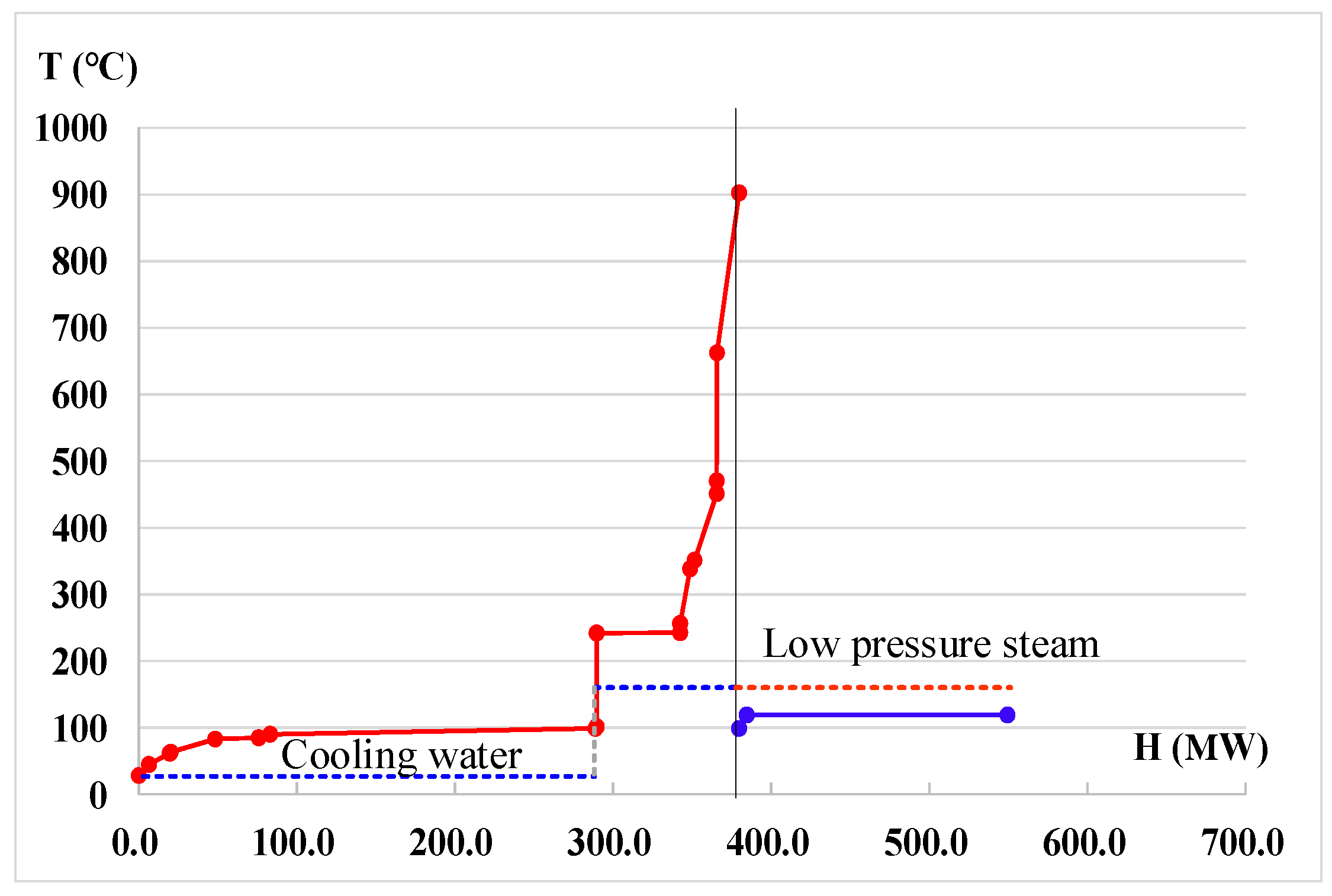
4.2. Heat Integration of the System as a Whole
4.3. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| Abbreviations | |
| R | molar ratio of (H2-CO2)/(CO+CO2) |
| PC | pulverized coke |
| APU | ammonia production unit |
| CLC | chemical looping combustion |
| COG | coke-oven gas |
| DRU | dry reforming unit |
| HEN | heat exchanger network |
| MSU | methanol synthesis unit |
| TAC | total annual cost |
| CLHG | chemical looping hydrogen generation |
| COMB | combustion unit |
| PCCLHG-CGTMA | PC CLHG assisted COG to methanol and ammonia |
| Notations in formulation | |
| A | heat transfer surface area |
| C | cost |
| N | number |
| Q | heat duty |
| U | heat transfer coefficient |
| m | mass flow |
| CC | capital cost |
| CP | heat capacity flow rate |
| Cp | specific heat capacity |
| CU | cold utility |
| HU | hot utility |
| OC | operation cost |
| PL | the plant life cycle |
| ROR | the rate of return |
| temperature differences at cold end of the heat exchange interval | |
| temperature differences at hot end of the heat exchange interval | |
| the log means temperature difference | |
| ΔTmin | minimum heat exchange temperature difference at pinch point |
| Subscripts | |
| C | relating to cold stream |
| H | relating to hot stream |
| i | relating to the individual heat transfer device in each zone |
| CU | cold utility |
| HU | hot utility |
| tot | relating to the total quantity in each zone |
References
- Keshavarz, A.; Mirvakili, A.; Chahibakhsh, S.; Shariati, A.; Rahimpour, M.R. Simultaneous methanol production and separation in the methanol synthesis reactor to increase methanol production. Chem. Eng. Process.—Process Intensif. 2020, 158, 108176. [Google Scholar] [CrossRef]
- Ishaq, H.; Dincer, I. Dynamic modelling of a solar hydrogen system for power and ammonia production. Int. J. Hydrog. Energy 2021, 46, 13985–14004. [Google Scholar] [CrossRef]
- NBS (National Bureau of Statistics). China Energy Statistics Yearbook; NBS: Beijing, China, 2021.
- Yi, Q.; Gong, M.-H.; Huang, Y.; Feng, J.; Hao, Y.-H.; Zhang, J.-L.; Li, W.-Y. Process development of coke oven gas to methanol integrated with CO2 recycle for satisfactory techno-economic performance. Energy 2016, 112, 618–628. [Google Scholar] [CrossRef]
- Yang, Z.; Ding, W.; Zhang, Y.; Lu, X.; Zhang, Y.; Shen, P. Catalytic partial oxidation of coke oven gas to syngas in an oxygen permeation membrane reactor combined with NiO/MgO catalyst. Int. J. Hydrog. Energy 2010, 35, 6239–6247. [Google Scholar] [CrossRef]
- Razzaq, R.; Li, C.; Zhang, S. Coke oven gas: Availability, properties, purification, and utilization in China. Fuel 2013, 113, 287–299. [Google Scholar] [CrossRef]
- Yi, Q.; Li, W.; Feng, J.; Xie, K. Carbon cycle in advanced coal chemical engineering. Chem. Soc. Rev. 2015, 44, 5409–5445. [Google Scholar] [CrossRef]
- Gong, M.-H.; Yi, Q.; Huang, Y.; Wu, G.-S.; Hao, Y.-H.; Feng, J.; Li, W.-Y. Coke oven gas to methanol process integrated with CO2 recycle for high energy efficiency, economic benefits and low emissions. Energy Convers. Manag. 2017, 133, 318–331. [Google Scholar] [CrossRef]
- Xiang, D.; Xiang, J.; Liu, S.; Sun, Z.; Jiang, Y.; Dong, Z.; Tao, Q.; Cao, Y. A chemical looping scheme of co-feeding of coke-oven gas and pulverized coke toward polygeneration of olefins and ammonia. Chem. Eng. J. 2018, 334, 1754–1765. [Google Scholar] [CrossRef]
- Zhu, X.; Shen, T.; Bollas, G.; Shen, L. Design and operation of a multi-stage reactor system for chemical looping combustion process. Fuel Process. Technol. 2021, 215, 106748. [Google Scholar] [CrossRef]
- Abuelgasim, S.; Wang, W.; Abdalazeez, A. A brief review for chemical looping combustion as a promising CO2 capture technology: Fundamentals and progress. Sci. Total Environ. 2021, 764, 142892. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Z.; Zhang, Y.; Zhang, Y.; Zhao, B. Thermodynamic analysis of using chemical-looping combustion in Allam-Z cycle instead of common combustion. Energy Convers. Manag. 2022, 254, 115229. [Google Scholar] [CrossRef]
- Luo, M.; Yi, Y.; Wang, S.; Wang, Z.; Du, M.; Pan, J.; Wang, Q. Review of hydrogen production using chemical-looping technology. Renew. Sustain. Energy Rev. 2018, 81, 3186–3214. [Google Scholar] [CrossRef]
- Hou, F.; Kou, X.; Wang, Y.; Li, Y. Diffusion transport characteristic of carbon monoxide within calcium sulfate slit in chemical looping hydrogen production: Molecular dynamics simulation. Int. J. Hydrog. Energy 2022, in press. [Google Scholar] [CrossRef]
- Adanez, J.; Abad, A.; Garcia-Labiano, F.; Gayan, P.; de Diego, L.F. Progress in Chemical-Looping Combustion and Reforming technologies. Prog. Energy Combust. Sci. 2012, 38, 215–282. [Google Scholar] [CrossRef]
- Nourbakhsh, H.; Khani, Y.; Zamaniyan, A.; Bahadoran, F. Hydrogen and syngas production through dynamic chemical looping reforming-decomposition of methane. Int. J. Hydrog. Energy 2022, 47, 9835–9852. [Google Scholar] [CrossRef]
- Qing, M.; Jin, B.; Ma, J.; Zou, X.; Wang, X.; Zheng, C.; Zhao, H. Thermodynamic and economic performance of oxy-combustion power plants integrating chemical looping air separation. Energy 2020, 206, 118136. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, R.; Shang, J.; Zhang, H.; Yang, B.; Wu, Z. Thermal behavior and kinetics analysis from liquid chemical looping gasification of cellulose with bismuth-based and antimony-based oxygen carriers. Fuel 2022, 321, 124047. [Google Scholar] [CrossRef]
- Salkuyeh, Y.K.; Adams, T.A. A new power, methanol, and DME polygeneration process using integrated chemical looping systems. Energy Convers. Manag. 2014, 88, 411–425. [Google Scholar] [CrossRef]
- Zhang, D.; Duan, R.; Li, H.; Yang, Q.; Zhou, H. Optimal design, thermodynamic, cost and CO2 emission analyses of coal-to-methanol process integrated with chemical looping air separation and hydrogen technology. Energy 2020, 203, 117876. [Google Scholar] [CrossRef]
- Chen, S.; Lior, N.; Xiang, W. Coal gasification integration with solid oxide fuel cell and chemical looping combustion for high-efficiency power generation with inherent CO2 capture. Appl. Energy 2015, 146, 298–312. [Google Scholar] [CrossRef]
- Xiang, D.; Huang, W.; Huang, P. A novel coke-oven gas-to-natural gas and hydrogen process by integrating chemical looping hydrogen with methanation. Energy 2018, 165, 1024–1033. [Google Scholar] [CrossRef]
- Dong, X.; Zhou, Y. Concept design and techno-economic performance of hydrogen and ammonia co-generation by coke-oven gas-pressure swing adsorption integrated with chemical looping hydrogen process. Appl. Energy 2018, 229, 1024–1034. [Google Scholar]
- Xiang, D.; Li, P.; Liu, L. Concept design, technical performance, and GHG emissions analysis of petroleum coke direct chemical looping hydrogen highly integrated with gasification for methanol production process. Sci. Total Environ. 2022, 838, 156652. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, Y.; Yi, Q.; Li, T.; Wang, J.; Bao, W.; Chang, L. Highly flexible and energy-efficient process for converting coke-oven gas and pulverized coke into methanol and ammonia using chemical looping technology. Energy Convers. Manag. 2021, 248, 114796. [Google Scholar] [CrossRef]
- Bandyopadhyay, R.; Alkilde, O.F.; Upadhyayula, S. Applying pinch and exergy analysis for energy efficient design of diesel hydrotreating unit. J. Clean. Prod. 2019, 232, 337–349. [Google Scholar] [CrossRef]
- Brunet, R.; Boer, D.; Guillén-Gosálbez, G.; Jiménez, L. Reducing the cost, environmental impact and energy consumption of biofuel processes through heat integration. Chem. Eng. Res. Des. 2015, 93, 203–212. [Google Scholar] [CrossRef]
- Shemfe, M.B.; Fidalgo, B.; Gu, S. Heat integration for bio-oil hydroprocessing coupled with aqueous phase steam reforming. Chem. Eng. Res. Des. 2016, 107, 73–80. [Google Scholar] [CrossRef]
- Lei, G.; Yu, J.; Kang, H.; Li, Q.; Zheng, H.; Wang, T.; Yang, L.; Xuan, Z. A novel intercooled series expansion refrigeration/liquefaction cycle using pinch technology. Appl. Therm. Eng. 2019, 163, 114336. [Google Scholar] [CrossRef]
- Ebrahim, M.; Kawari, A. Pinch technology: An efficient tool for chemical-plant energy and capital-cost saving. Appl. Energy 2000, 65, 45–49. [Google Scholar] [CrossRef]
- Chen, Q.; Li, S.; Li, J. Energy efficiency optimization of methanol synthesis and distillation units. Chem. Eng. 2012, 40, 1–5. [Google Scholar] [CrossRef]
- Song, C.; Liu, Q.; Ji, N.; Kansha, Y.; Tsutsumi, A. Optimization of steam methane reforming coupled with pressure swing adsorption hydrogen production process by heat integration. Appl. Energy 2015, 154, 392–401. [Google Scholar] [CrossRef]
- Liu, X.; Liang, J.; Xiang, D.; Yang, S.; Qian, Y. A proposed coal-to-methanol process with CO2 capture combined Organic Rankine Cycle (ORC) for waste heat recovery. J. Clean. Prod. 2016, 129, 53–64. [Google Scholar] [CrossRef]
- Bokun, C.; Yu, Q.; Siyu, Y. Integration of thermo-vapor compressors with phenol and ammonia recovery process for coal gasification wastewater treatment system. Energy 2019, 166, 108–117. [Google Scholar] [CrossRef]
- Liu, S.; Yang, L.; Chen, B.; Yang, S.; Qian, Y. Comprehensive energy analysis and integration of coal-based MTO process. Energy 2021, 214, 119060. [Google Scholar] [CrossRef]
- Kim, Y.; Lim, J.; Shim, J.Y.; Lee, H.; Cho, H.; Kim, J. Optimizing wastewater heat recovery systems in textile dyeing processes using pinch analysis. Appl. Therm. Eng. 2022, 214, 118880. [Google Scholar] [CrossRef]
- El-Halwagi, M.M.; Gabriel, F.; Harell, D. Rigorous graphical targeting for resource conservation via material recycle/reuse networks. Ind. Eng. Chem. Res. 2003, 42, 4319–4328. [Google Scholar] [CrossRef]
- Manan, Z.A.; Alwi, S.; Ujang, Z. Water pinch analysis for an urban system: A case study on the Sultan Ismail Mosque at the Universiti Teknologi Malaysia (UTM). Desalination 2006, 194, 52–68. [Google Scholar] [CrossRef]
- Tan, R.R.; Foo, D.C.Y. Pinch analysis approach to carbon-constrained energy sector planning. Energy 2007, 32, 1422–1429. [Google Scholar] [CrossRef]
- Marques, J.P.; Matos, H.A.; Oliveira, N.M.C.; Nunes, C.P. State-of-the-art review of targeting and design methodologies for hydrogen network synthesis. Int. J. Hydrog. Energy 2017, 42, 376–404. [Google Scholar] [CrossRef]
- Alwi, S.; Rozali, N.; Abdul-Manan, Z.; Klemes, J.J. A process integration targeting method for hybrid power systems. Energy 2012, 44, 6–10. [Google Scholar] [CrossRef]
- Singhvi, A.; Shenoy, U.V. Aggregate Planning in Supply Chains by Pinch Analysis. Chem. Eng. Res. Des. 2002, 80, 597–605. [Google Scholar] [CrossRef]
- Tan, R.R.; Aviso, K.B.; Foo, D. Carbon emissions pinch analysis of economic systems. J. Clean. Prod. 2018, 182, 863–871. [Google Scholar] [CrossRef]
- Gai, L.; Varbanov, P.S.; Walmsley, T.G. Critical analysis of process integration options for joule-cycle and conventional heat pumps. Energies 2020, 13, 635. [Google Scholar] [CrossRef]
- Yang, A.; Jin, S.; Shen, W.; Cui, P.; Chien, I.L.; Ren, J. Investigation of energy-saving azeotropic dividing wall column to achieve cleaner production via heat exchanger network and heat pump technique. J. Clean. Prod. 2019, 234, 410–422. [Google Scholar] [CrossRef]
- Kamel, D.; Gadalla, M.; Ashour, F. New set of Graphical Axes for Grassroots Design of Heat Exchanger Networks for Chemical Engineering Applications. In Computer Aided Chemical Engineering; Pierucci, S., Manenti, F., Bozzano, G.L., Manca, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 48, pp. 637–642. [Google Scholar]
- Fu, D.; Nguyen, T.; Lai, Y.; Lin, L.; Dong, Z.; Lyu, M. Improved pinch-based method to calculate the capital cost target of heat exchanger network via evolving the spaghetti structure towards low-cost matching. J. Clean. Prod. 2022, 343, 131022. [Google Scholar] [CrossRef]
- Araújo, A.; Skogestad, S. Control structure design for the ammonia synthesis process. Comput. Chem. Eng. 2008, 32, 2920–2932. [Google Scholar] [CrossRef]
- Yang, Q.; Yan, M.; Zhang, L.; Xia, X.; Wang, X. Thermodynamic analysis of chemical looping coupling process for coproducing syngas and hydrogen with in situ CO2 utilization. Energy Convers. Manag. 2021, 231, 113845. [Google Scholar] [CrossRef]
- Puig-Gamero, M.; Argudo-Santamaria, J.; Valverde, J.; Sánchez, P.; Sanchez-Silva, L. Three integrated process simulation using aspen plus®: Pine gasification, syngas cleaning and methanol synthesis. Energy Convers. Manag. 2018, 177, 416–427. [Google Scholar] [CrossRef]
- Linnhoff, B.; Mason, D.R.; Wardle, I. Understanding heat exchanger networks. Comput. Chem. Eng. 1979, 3, 295–302. [Google Scholar] [CrossRef]
- Linnhoff, B.; Hindmarsh, E. The pinch design method for heat exchanger networks. Chem. Eng. Sci. 1983, 38, 745–763. [Google Scholar] [CrossRef]
- Panjeshahi, M.H.; Ghasemian Langeroudi, E.; Tahouni, N. Retrofit of ammonia plant for improving energy efficiency. Energy 2008, 33, 46–64. [Google Scholar] [CrossRef]
- Quijera, J.A.; Garcia, A.; Alriols, M.G.; Labidi, J. Heat integration options based on pinch and exergy analyses of a thermosolar and heat pump in a fish tinning industrial process. Energy 2013, 55, 23–37. [Google Scholar] [CrossRef]
- Linnhoff, B.; Akinradewo, C.G. Linking process simulation and process integration. Comput. Chem. Eng. 1999, 23, S945–S953. [Google Scholar] [CrossRef]
- Aspelund, A.; Berstad, D.O.; Gundersen, T. An Extended Pinch Analysis and Design procedure utilizing pressure based exergy for subambient cooling. Appl. Therm. Eng. 2007, 27, 2633–2649. [Google Scholar] [CrossRef]
- Anantharaman, R.; Abbas, O.S.; Gundersen, T. Energy Level Composite Curves—A new graphical methodology for the integration of energy intensive processes. Appl. Therm. Eng. 2006, 26, 1378–1384. [Google Scholar] [CrossRef]
- Mehdizadeh-Fard, M.; Pourfayaz, F.; Mehrpooya, M.; Kasaeian, A. Improving energy efficiency in a complex natural gas refinery using combined pinch and advanced exergy analyses. Appl. Therm. Eng. 2018, 137, 341–355. [Google Scholar] [CrossRef]
- Sun, L. Energy System Optimization in Process Industries—Heat Exchanger Network and Steam Power System; Chemical Industry Press: Beijing, China, 2021. [Google Scholar]
- Mano, T.B.; Guillén-Gosálbez, G.; Jiménez, L.; Ravagnani, M.A.S.S. Synthesis of heat exchanger networks with economic and environmental assessment using fuzzy-Analytic Hierarchy Process. Chem. Eng. Sci. 2019, 195, 185–200. [Google Scholar] [CrossRef]





| Units | T/°C | P/MPa | Molar Ratio | Key Component Conversion Rate/% | Relative Error | ||
|---|---|---|---|---|---|---|---|
| Calculation Formula | Literature | Aspen | |||||
| DRU | 800 | 0.1 | CH4/CO2 = 1 | 89 [8] | 86 | 3% | |
| 97 [8] | 94 | ||||||
| 103 [8] | 106 | ||||||
| MSU | 240 | 5 | H2/CO = 2 | 35 [50] | 33 | 2% | |
| APU | 450 | 20.3 | H2/N2 = 3 | 24 [48] | 27 | 0~3% | |
| 27 [48] | 27 | ||||||
| 100 [48] | 100 | ||||||
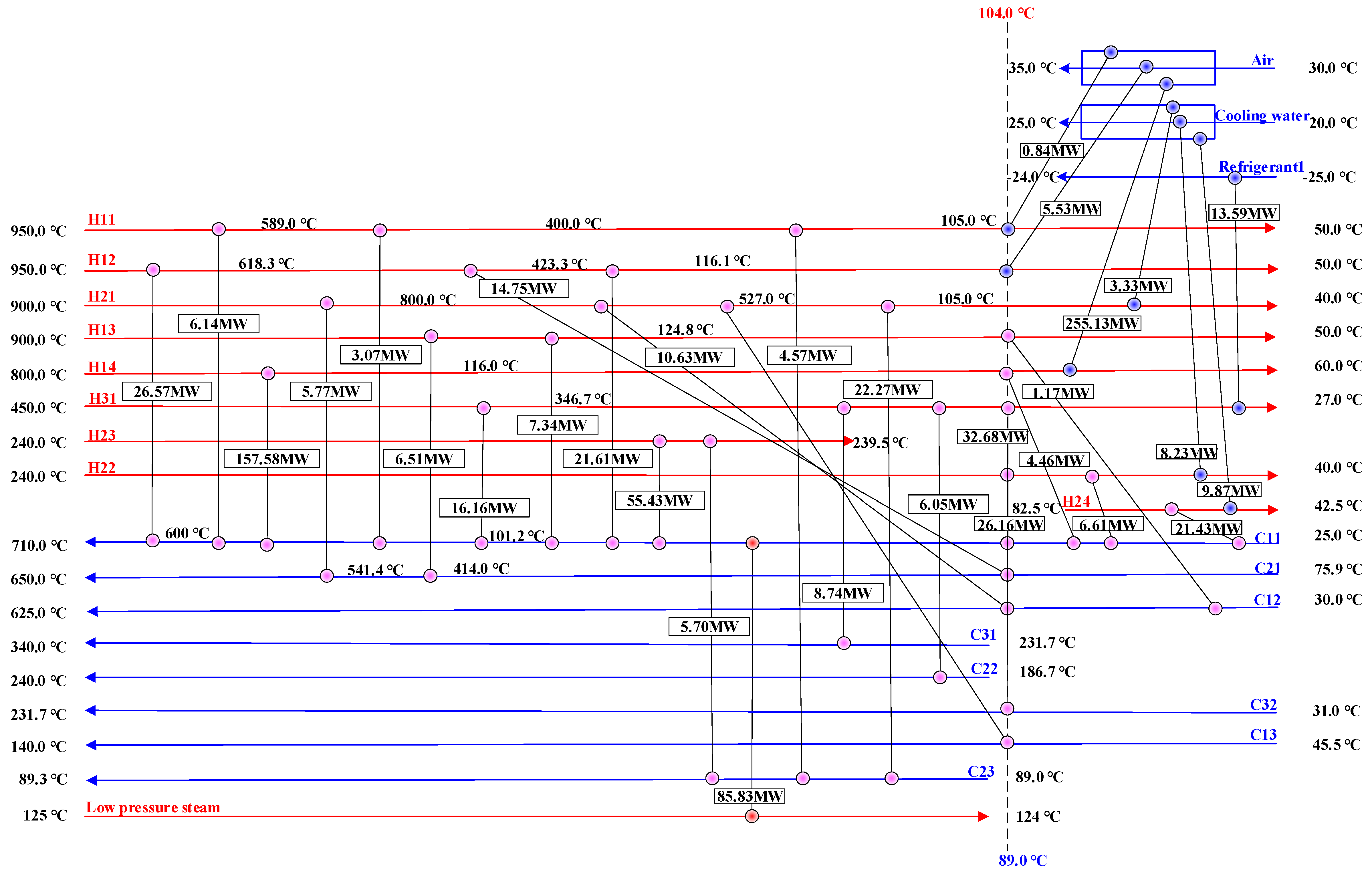
| Heat Exchange Units | Heat Exchanger | Stream No. | Supply Temperature | Target Temperature | Heat Load | Mass Flow |
|---|---|---|---|---|---|---|
| °C | °C | kW | kg/s | |||
| CLHG + COMB | HX5 | H11 | 950 | 50 | 14,633 | 14.56 |
| HX10 | H12 | 950 | 50 | 68,470 | 62.77 | |
| HX8 | H13 | 900 | 50 | 15,026 | 15.61 | |
| HX6 | H14 | 800 | 60 | 417,174 | 104.00 | |
| HX7 | C11 | 25 | 710 | 438,123 | 111.10 | |
| HX9 | C12 | 30 | 625 | 11,798 | 18.65 | |
| HX11 | C13 | 45 | 140 | 4844 | 50.40 | |
| DRU + MSU | HX2 | H21 | 900 | 40 | 46,853.93 | 29.28 |
| HX4 | H22 | 240 | 40 | 41,003.93 | 45.41 | |
| MSU- | H23 | 240 | 239.5 | 61,133.81 | - | |
| Distillation column-Condenser | H24 | 82.5 | 42.5 | 31,018.03 | 24.32 | |
| HX1 | C21 | 75.9 | 650 | 27,039.55 | 29.28 | |
| HX3 | C22 | 186.7 | 240 | 6046.52 | 45.41 | |
| Distillation column-Reboiler | C23 | 89 | 89.3 | 32,548.64 | 258.34 | |
| APU | HX14 | H31 | 450 | 27 | 77,222.50 | 47.13 |
| HX13 | C31 | 231.7 | 340 | 8739.17 | 23.56 | |
| HX12 | C32 | 31 | 231.7 | 32,678.36 | 47.13 |
| Item | CLHG + COMB | DRU + MSU | APU | The Whole System |
|---|---|---|---|---|
| Total Area (m2) | 269,212.1 | 13,740 | 2013 | 330,200 |
| Heating (million USD/y) | 11.06 | 0 | 0 | 5.417 |
| Cooling (million USD/y) | 1.58 | −3.29 | 0.1763 | 1.327 |
| Operating (million USD/y) | 12.64 | −3.29 | 0.1763 | 6.744 |
| Capital (million USD) | 47.16 | 2.523 | 0.4343 | 57.99 |
| Total Cost (million USD/y) | 18.85 | −2.958 | 0.2334 | 14.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Zhao, Y.; Huang, Y.; Wang, J.; Bao, W.; Chang, L.; Shi, L.; Yi, Q. Pinch Analysis for Heat Integration of Pulverized Coke Chemical Looping Gasification Coupled with Coke-Oven Gas to Methanol and Ammonia. Processes 2022, 10, 1879. https://doi.org/10.3390/pr10091879
Zhao Y, Zhao Y, Huang Y, Wang J, Bao W, Chang L, Shi L, Yi Q. Pinch Analysis for Heat Integration of Pulverized Coke Chemical Looping Gasification Coupled with Coke-Oven Gas to Methanol and Ammonia. Processes. 2022; 10(9):1879. https://doi.org/10.3390/pr10091879
Chicago/Turabian StyleZhao, Yaxian, Yingjie Zhao, Yi Huang, Jiancheng Wang, Weiren Bao, Liping Chang, Lijuan Shi, and Qun Yi. 2022. "Pinch Analysis for Heat Integration of Pulverized Coke Chemical Looping Gasification Coupled with Coke-Oven Gas to Methanol and Ammonia" Processes 10, no. 9: 1879. https://doi.org/10.3390/pr10091879






