Bamboo Chopstick Biochar Electrodes and Enhanced Nitrate Removal from Groundwater
Abstract
:1. Introduction
2. Materials and Methods
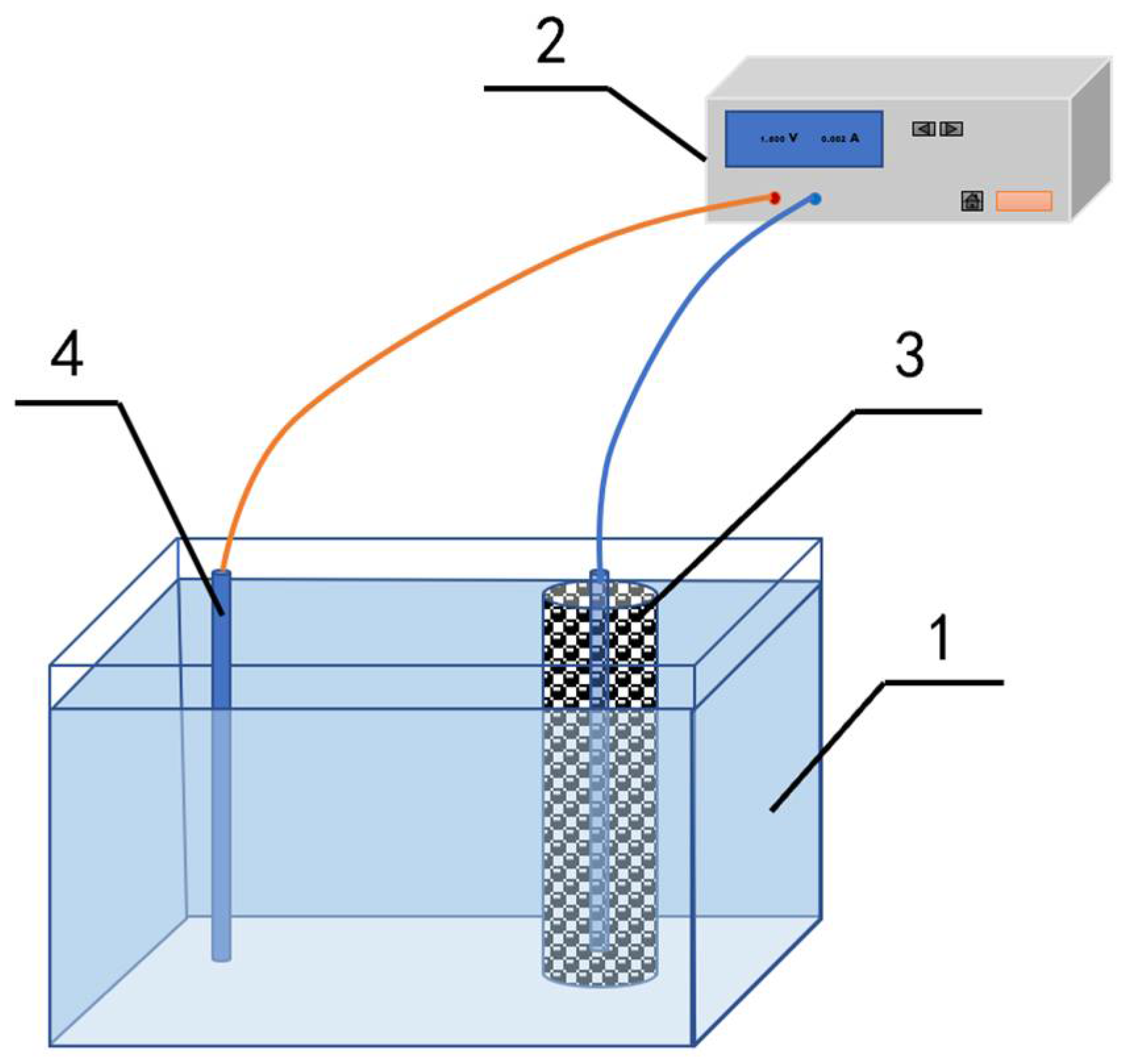
2.1. Preparation of Biochar Electrode
2.2. Adsorption of Nitrate
2.3. Synergetic Adsorption and Electroreduction of Nitrate
2.4. Analytical Methods
3. Results and Discussions
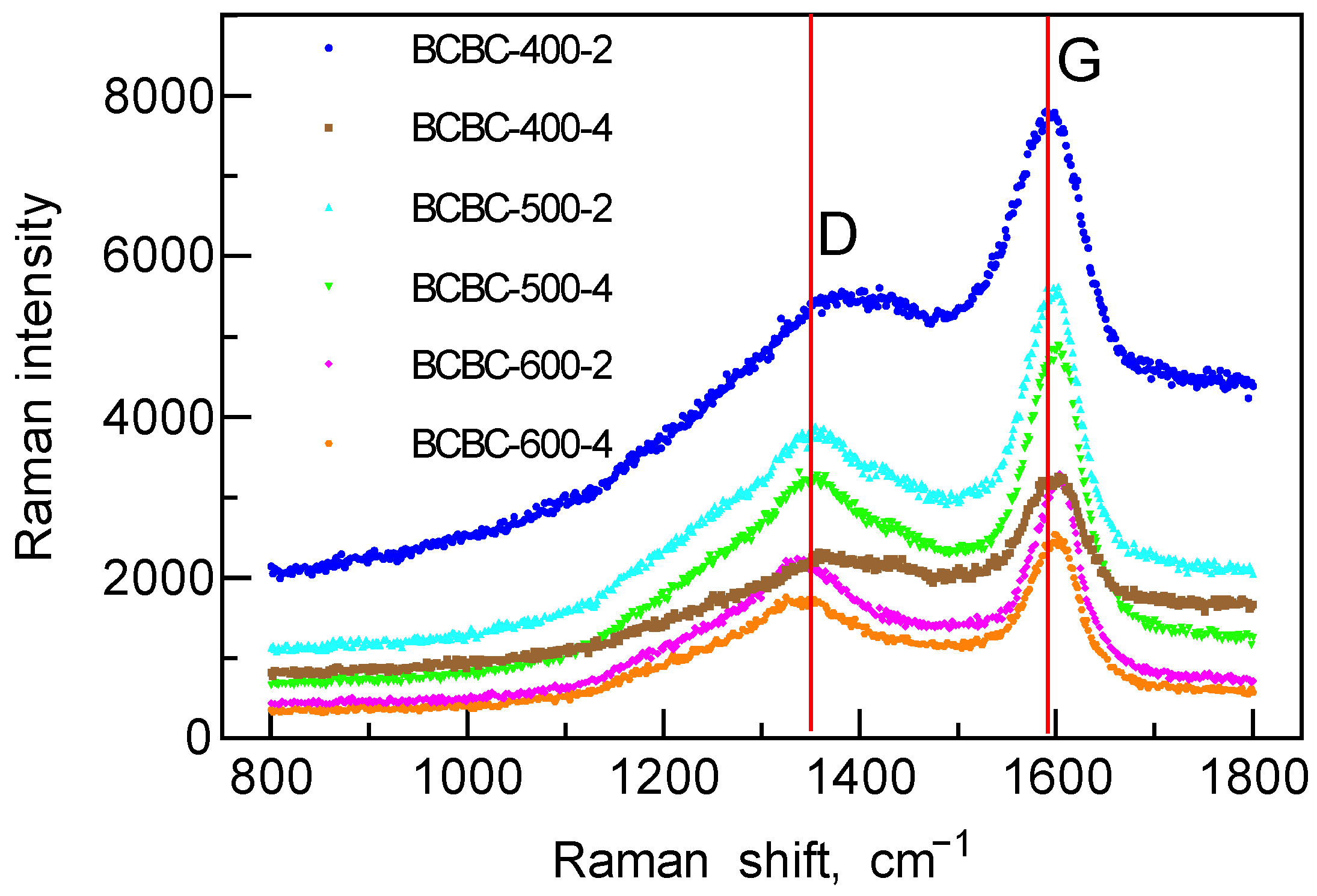
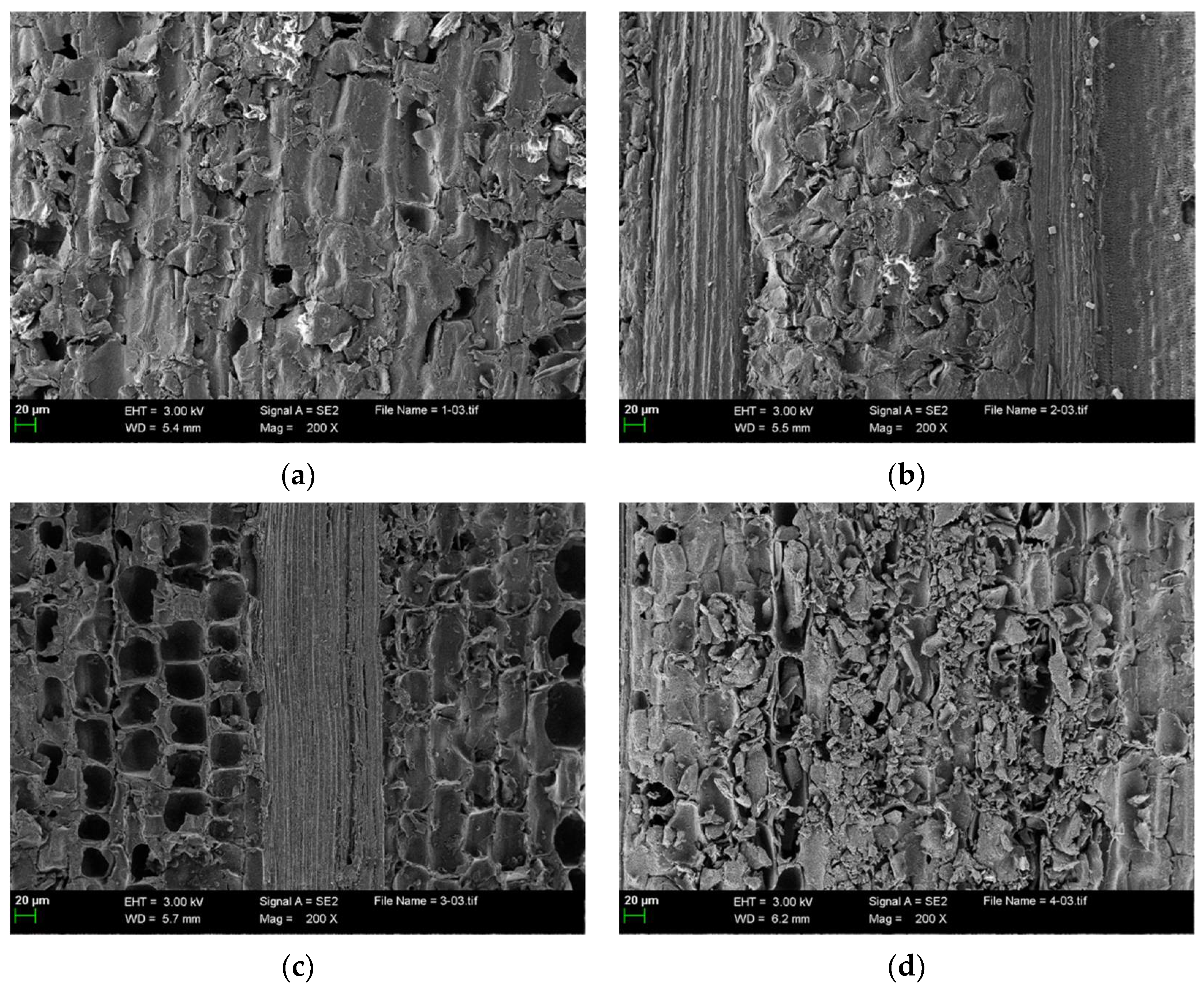
3.1. Physicochemical Properties of the Biochars
3.2. Selection of the Biochar Electrode Material
3.3. Adsorption of Nitrate by Biochar Electrodes
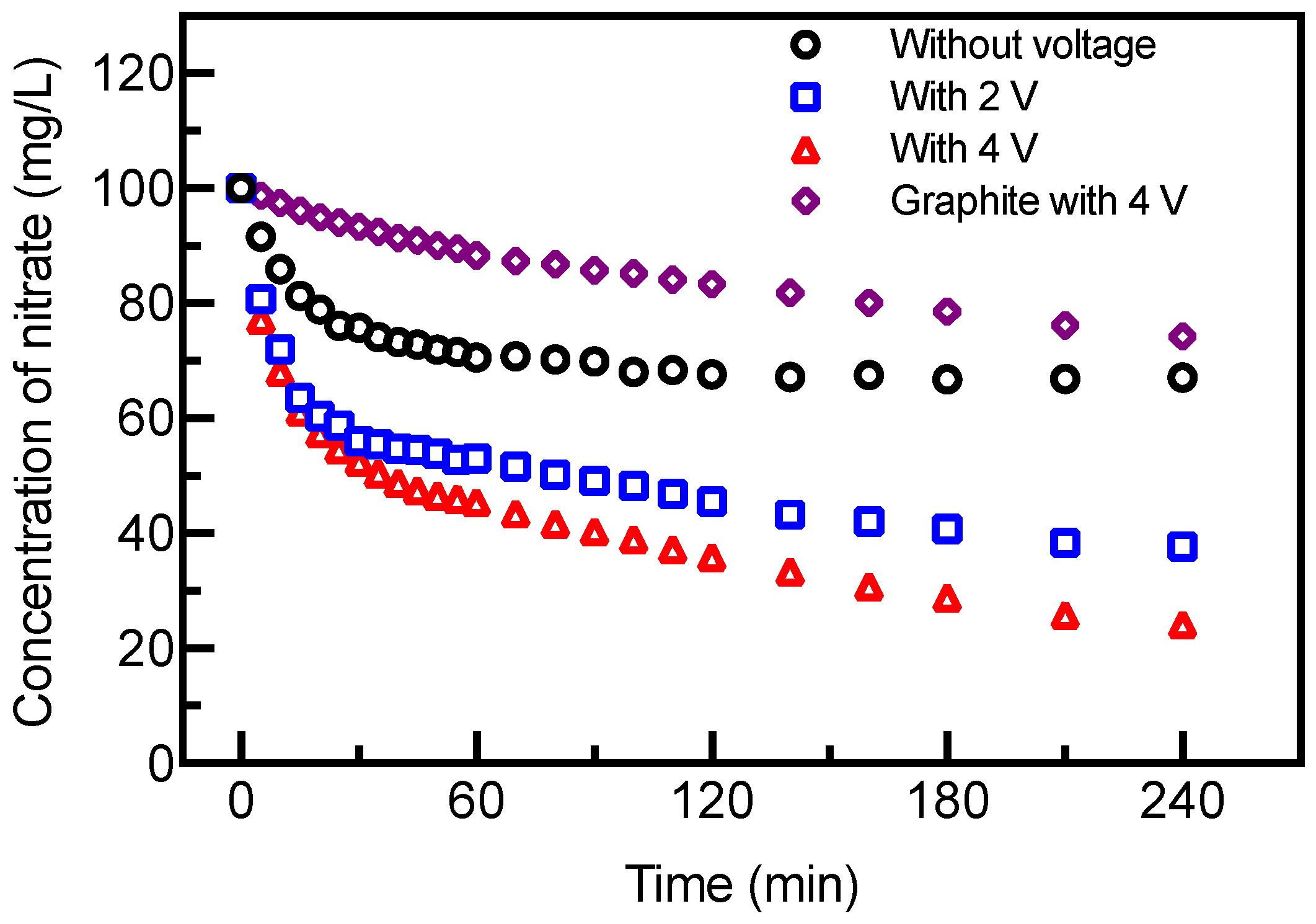
3.4. Nitrate Removal under Electrochemical Enhancement
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khanoranga; Khalid, S. An Assessment of Groundwater Quality for Irrigation and Drinking Purposes around Brick Kilns in Three Districts of Balochistan Province, Pakistan, through Water Quality Index and Multivariate Statistical Approaches. J. Geochem. Explor. 2019, 197, 14–26. [Google Scholar] [CrossRef]
- Su, H.; Kang, W.; Li, Y.; Li, Z. Fluoride and Nitrate Contamination of Groundwater in the Loess Plateau, China: Sources and Related Human Health Risks. Environ. Pollut. 2021, 286, 117287. [Google Scholar] [CrossRef] [PubMed]
- Kaur, L.; Rishi, M.S.; Siddiqui, A.U. Deterministic and Probabilistic Health Risk Assessment Techniques to Evaluate Non-Carcinogenic Human Health Risk (NHHR) Due to Fluoride and Nitrate in Groundwater of Panipat, Haryana, India. Environ. Pollut. 2020, 259, 113711. [Google Scholar] [CrossRef] [PubMed]
- Meghdadi, A.; Javar, N. Quantification of Spatial and Seasonal Variations in the Proportional Contribution of Nitrate Sources Using a Multi-Isotope Approach and Bayesian Isotope Mixing Model. Environ. Pollut. 2018, 235, 207–222. [Google Scholar] [CrossRef]
- Biddau, R.; Cidu, R.; Da Pelo, S.; Carletti, A.; Ghiglieri, G.; Pittalis, D. Source and Fate of Nitrate in Contaminated Groundwater Systems: Assessing Spatial and Temporal Variations by Hydrogeochemistry and Multiple Stable Isotope Tools. Sci. Total Environ. 2019, 647, 1121–1136. [Google Scholar] [CrossRef]
- Urresti-Estala, B.; Vadillo-Pérez, I.; Jiménez-Gavilán, P.; Soler, A.; Sánchez-García, D.; Carrasco-Cantos, F. Application of Stable Isotopes (Δ34S-SO4, Δ18O-SO4, Δ15N-NO3, Δ18O-NO3) to Determine Natural Background and Contamination Sources in the Guadalhorce River Basin (Southern Spain). Sci. Total Environ. 2015, 506–507, 46–57. [Google Scholar] [CrossRef]
- Espejo-Herrera, N.; Cantor, K.P.; Malats, N.; Silverman, D.T.; Tardón, A.; García-Closas, R.; Serra, C.; Kogevinas, M.; Villanueva, C.M. Nitrate in Drinking Water and Bladder Cancer Risk in Spain. Environ. Res. 2015, 137, 299–307. [Google Scholar] [CrossRef]
- Shrock, D.L.; Krasowski, M.D. Chapter 23.5-Methemoglobinemia Due to Dietary Nitrate. In Toxicology Cases for the Clinical and Forensic Laboratory; Ketha, H., Garg, U., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 469–472. ISBN 978-0-12-815846-3. [Google Scholar]
- Temkin, A.; Evans, S.; Manidis, T.; Campbell, C.; Naidenko, O.V. Exposure-Based Assessment and Economic Valuation of Adverse Birth Outcomes and Cancer Risk Due to Nitrate in United States Drinking Water. Environ. Res. 2019, 176, 108442. [Google Scholar] [CrossRef]
- Keerio, H.A.; Bae, W. Experimental Investigation of Substrate Shock and Environmental Ammonium Concentration on the Stability of Ammonia-Oxidizing Bacteria (AOB). Water 2020, 12, 223. [Google Scholar] [CrossRef]
- Keerio, H.A.; Bae, W.; Park, J.; Kim, M. Substrate Uptake, Loss, and Reserve in Ammonia-Oxidizing Bacteria (AOB) under Different Substrate Availabilities. Process Biochem. 2020, 91, 303–310. [Google Scholar] [CrossRef]
- Li, W.-W.; Yu, H.-Q. Electro-Assisted Groundwater Bioremediation: Fundamentals, Challenges and Future Perspectives. Bioresour. Technol. 2015, 196, 677–684. [Google Scholar] [CrossRef]
- Garcia-Segura, S.; Lanzarini-Lopes, M.; Hristovski, K.; Westerhoff, P. Electrocatalytic Reduction of Nitrate: Fundamentals to Full-Scale Water Treatment Applications. Appl. Catal. B-Environ. 2018, 236, 546–568. [Google Scholar] [CrossRef]
- Kato, M.; Okui, M.; Taguchi, S.; Yagi, I. Electrocatalytic Nitrate Reduction on Well-Defined Surfaces of Tin-Modified Platinum, Palladium and Platinum-Palladium Single Crystalline Electrodes in Acidic and Neutral Media. J. Electroanal. Chem. 2017, 800, 46–53. [Google Scholar] [CrossRef]
- Sanjuán, I.; García-Cruz, L.; Solla-Gullón, J.; Expósito, E.; Montiel, V. Bi–Sn Nanoparticles for Electrochemical Denitrification: Activity and Selectivity towards N2 Formation. Electrochim. Acta 2020, 340, 135914. [Google Scholar] [CrossRef]
- Martínez, J.; Ortiz, A.; Ortiz, I. State-of-the-Art and Perspectives of the Catalytic and Electrocatalytic Reduction of Aqueous Nitrates. Appl. Catal. B-Environ. 2017, 207, 42–59. [Google Scholar] [CrossRef]
- Epsztein, R.; Nir, O.; Lahav, O.; Green, M. Selective Nitrate Removal from Groundwater Using a Hybrid Nanofiltration–Reverse Osmosis Filtration Scheme. Chem. Eng. J. 2015, 279, 372–378. [Google Scholar] [CrossRef]
- Aliaskari, M.; Schäfer, A.I. Nitrate, Arsenic and Fluoride Removal by Electrodialysis from Brackish Groundwater. Water Res. 2021, 190, 116683. [Google Scholar] [CrossRef]
- Xu, X.; Gao, B.; Yue, Q.; Li, Q.; Wang, Y. Nitrate Adsorption by Multiple Biomaterial Based Resins: Application of Pilot-Scale and Lab-Scale Products. Chem. Eng. J. 2013, 234, 397–405. [Google Scholar] [CrossRef]
- Song, H.; Zhou, Y.; Li, A.; Mueller, S. Selective Removal of Nitrate from Water by a Macroporous Strong Basic Anion Exchange Resin. Desalination 2012, 296, 53–60. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, H.; Shen, F.; Yang, G.; Zhang, Y.; Zeng, Y.; Wang, L.; Xiao, H.; Deng, S. Biochar Produced from Oak Sawdust by Lanthanum (La)-Involved Pyrolysis for Adsorption of Ammonium (NH4+), Nitrate (NO3−), and Phosphate (PO43−). Chemosphere 2015, 119, 646–653. [Google Scholar] [CrossRef]
- Yin, Q.; Wang, R.; Zhao, Z. Application of Mg–Al-Modified Biochar for Simultaneous Removal of Ammonium, Nitrate, and Phosphate from Eutrophic Water. J. Clean. Prod. 2018, 176, 230–240. [Google Scholar] [CrossRef]
- Xue, L.; Gao, B.; Wan, Y.; Fang, J.; Wang, S.; Li, Y.; Muñoz-Carpena, R.; Yang, L. High efficiency and selectivity of MgFe-LDH modified wheat-straw biochar in the removal of nitrate from aqueous solutions. J. Taiwan Inst. Chem. Eng. 2016, 63, 312–317. [Google Scholar] [CrossRef]
- Zhang, M.; Song, G.; Gelardi, D.L.; Huang, L.; Khan, E.; Mašek, O.; Parikh, S.J.; Ok, Y.S. Evaluating Biochar and Its Modifications for the Removal of Ammonium, Nitrate, and Phosphate in Water. Water Res. 2020, 186, 116303. [Google Scholar] [CrossRef] [PubMed]
- Long, L.; Xue, Y.; Hu, X.; Zhu, Y. Study on the Influence of Surface Potential on the Nitrate Adsorption Capacity of Metal Modified Biochar. Environ. Sci. Pollut. Res. Int. 2019, 26, 3065–3074. [Google Scholar] [CrossRef]
- Yao, F.; Yang, Q.; Yan, M.; Li, X.; Chen, F.; Zhong, Y.; Yin, H.; Chen, S.; Fu, J.; Wang, D.; et al. Synergistic Adsorption and Electrocatalytic Reduction of Bromate by Pd/N-Doped Loofah Sponge-Derived Biochar Electrode. J. Hazard. Mater. 2020, 386, 121651. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, W.; Hu, J.; Xin, G.; Chen, Z.; Wan, C.; Wang, S.; Zhang, Q. A Novel Biochar Electrode for Efficient Electroreduction of Nitrate: Selective and Regulation of Halogen. Chemosphere 2022, 288, 132400. [Google Scholar] [CrossRef]
- Chen, Y.H.; Chang, C.C.; Chang, C.Y.; Yuan, M.H.; Ji, D.R.; Shie, J.L.; Lee, C.H.; Chen, Y.H.; Chang, W.R.; Yang, T.Y.; et al. Production of a Solid Bio-Fuel from Waste Bamboo Chopsticks by Torrefaction for Cofiring with Coal. J. Anal. Appl. Pyrol. 2017, 126, 315–322. [Google Scholar] [CrossRef]
- Xia, Y.; Lu, D.; Qi, Y.; Chen, H.; Zhao, Y.; Bai, Y.; Zhu, L.; Geng, N.; Xu, C.; Hua, E. Removal of Nitrate from Agricultural Runoff in Biochar Electrode Based Biofilm Reactor: Performance and Enhancement Mechanisms. Chemosphere 2022, 301, 134744. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, Y.-X.; Wu, W.-X.; Shi, D.-Z.; Yang, M.; Zhong, Z.-K. Evaluation of Biochar Effects on Nitrogen Retention and Leaching in Multi-Layered Soil Columns. Water Air Soil Pollut. 2010, 213, 47–55. [Google Scholar] [CrossRef]
- Sun, B.; Skyllas-Kazacos, M. Chemical Modification of Graphite Electrode Materials for Vanadium Redox Flow Battery Application—Part II. Acid Treatments. Electrochim. Acta 1992, 37, 2459–2465. [Google Scholar] [CrossRef]
- Jazini, R.; Soleimani, M.; Mirghaffari, N. Characterization of Barley Straw Biochar Produced in Various Temperatures and Its Effect on Lead and Cadmium Removal from Aqueous Solutions. Water Environ. J. 2018, 32, 125–133. [Google Scholar] [CrossRef]
- Novak, J.; Lima, I.; Xing, B.; Gaskin, J.; Steiner, C.; Das, K.C.; Ahmedna, M.; Rehrah, D.; Watts, D.; Busscher, W. Characterization of Designer Biochar Produced at Different Temperatures and Their Effects on a Loamy Sand. Ann. Environ. Sci. 2009, 3, 195–206. [Google Scholar]
- Gai, X.; Wang, H.; Liu, J.; Zhai, L.; Liu, S.; Ren, T.; Liu, H. Effects of Feedstock and Pyrolysis Temperature on Biochar Adsorption of Ammonium and Nitrate. PLoS ONE 2014, 9, e113888. [Google Scholar] [CrossRef]
- Wu, W.; Yang, M.; Feng, Q.; McGrouther, K.; Wang, H.; Lu, H.; Chen, Y. Chemical Characterization of Rice Straw-Derived Biochar for Soil Amendment. Biomass Bioenerg. 2012, 47, 268–276. [Google Scholar] [CrossRef]
- Gabhi, R.S.; Kirk, D.W.; Jia, C.Q. Preliminary Investigation of Electrical Conductivity of Monolithic Biochar. Carbon 2017, 116, 435–442. [Google Scholar] [CrossRef]
- Yu, J.; Sun, L.; Berrueco, C.; Fidalgo, B.; Paterson, N.; Millan, M. Influence of Temperature and Particle Size on Structural Characteristics of Chars from Beechwood Pyrolysis. J. Anal. Appl. Pyrol. 2018, 130, 127–134. [Google Scholar] [CrossRef]
- Tan, X.-F.; Zhu, S.-S.; Wang, R.-P.; Chen, Y.-D.; Show, P.-L.; Zhang, F.-F.; Ho, S.-H. Role of Biochar Surface Characteristics in the Adsorption of Aromatic Compounds: Pore Structure and Functional Groups. Chin. Chem. Lett. 2021, 32, 2939–2946. [Google Scholar] [CrossRef]
- Hollister, C.C.; Bisogni, J.J.; Lehmann, J. Ammonium, Nitrate, and Phosphate Sorption to and Solute Leaching from Biochars Prepared from Corn Stover (Zea mays L.) and Oak Wood (Quercus spp.). J. Environ. Qual. 2013, 42, 137–144. [Google Scholar] [CrossRef]
- Ohe, K.; Nagae, Y.; Nakamura, S.; Baba, Y. Removal of Nitrate Anion by Carbonaceous Materials Prepared from Bamboo and Coconut Shell. J. Chem. Eng. 2003, 36, 511–515. [Google Scholar] [CrossRef]
- Kameyama, K.; Miyamoto, T.; Iwata, Y.; Shiono, T. Influences of Feedstock and Pyrolysis Temperature on the Nitrate Adsorption of Biochar. Soil Sci. Plant Nutr. 2016, 62, 180–184. [Google Scholar] [CrossRef]


| Biochars 1 | Temperature (°C) | Yield (%) | Component (%) | Atomic Ratio | |||||
|---|---|---|---|---|---|---|---|---|---|
| C | H | O | N | C:N | O:C | H:C | |||
| BCBC-400-2 | 400 | 47.4 | 69.82 | 1.16 | 27.85 | 0.88 | 92.6 | 0.30 | 0.20 |
| BCBC-400-4 | 46.9 | 68.65 | 1.10 | 25.73 | 0.92 | 87.1 | 0.28 | 0.19 | |
| BCBC-500-2 | 500 | 42.3 | 65.77 | 0.93 | 26.01 | 1.55 | 49.5 | 0.30 | 0.17 |
| BCBC-500-4 | 38.6 | 64.13 | 0.68 | 28.56 | 1.09 | 68.6 | 0.33 | 0.13 | |
| BCBC-600-2 | 600 | 35.7 | 71.61 | 0.77 | 21.32 | 1.07 | 78.1 | 0.22 | 0.13 |
| BCBC-600-4 | 32.1 | 78.76 | 0.76 | 18.52 | 0.96 | 95.7 | 0.18 | 0.12 | |
| Biochars | pH | EC (μS/cm) | Ash Content (%) | Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Size (nm) |
|---|---|---|---|---|---|---|
| BCBC-400-2 | 9.5 | 16.9 | 3.72 | 2.42 | 0.0035 | 57.35 |
| BCBC-400-4 | 9.6 | 47.9 | 3.45 | 1.56 | 0.0022 | 56.73 |
| BCBC-500-2 | 10.2 | 25.1 | 5.28 | 2.18 | 0.0031 | 57.76 |
| BCBC-500-4 | 10.2 | 63.7 | 6.37 | 9.17 | 0.0031 | 13.36 |
| BCBC-600-2 | 10.1 | 8869.2 | 9.22 | 179.2 | 0.085 | 19.03 |
| BCBC-600-4 | 10.6 | 1503.8 | 8.75 | 307.5 | 0.138 | 18.03 |
| Biochars | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| KL (L/mg) | Qm (mg/g) | R2 | KF (mg/g) | 1/n | R2 | |
| BCBC-400-2 | 17.59 | 0.688 | 0.902 | 0.0385 | 0.900 | 0.971 |
| BCBC-500-2 | 7.36 | 2.139 | 0.947 | 0.0173 | 0.988 | 0.997 |
| BCBC-600-2 | 0.0132 | 16.39 | 0.944 | 0.234 | 0.877 | 0.979 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geng, N.; Ren, B.; Xu, B.; Li, D.; Xia, Y.; Xu, C.; Hua, E. Bamboo Chopstick Biochar Electrodes and Enhanced Nitrate Removal from Groundwater. Processes 2022, 10, 1740. https://doi.org/10.3390/pr10091740
Geng N, Ren B, Xu B, Li D, Xia Y, Xu C, Hua E. Bamboo Chopstick Biochar Electrodes and Enhanced Nitrate Removal from Groundwater. Processes. 2022; 10(9):1740. https://doi.org/10.3390/pr10091740
Chicago/Turabian StyleGeng, Nan, Beifei Ren, Bailong Xu, Dongfeng Li, Yinfeng Xia, Cundong Xu, and Ertian Hua. 2022. "Bamboo Chopstick Biochar Electrodes and Enhanced Nitrate Removal from Groundwater" Processes 10, no. 9: 1740. https://doi.org/10.3390/pr10091740





