Cadmium in Rice Is Affected by Fertilizer-Borne Chloride and Sulfate Anions: Long-Term Field Versus Pot Experiments
Abstract
:1. Introduction
2. Methodology
2.1. Experimental Site and Design
2.2. Soil Pot Experiment
2.3. Soil and Plant Analyses
2.3.1. Processing of the Total Cd Concentration in Plant Samples
2.3.2. Processing of the Total Cd Concentration in Soil Samples
2.3.3. Processing of the Soil Samples for the EDTA-Cd Concentration
2.3.4. Quality Assurance and Control
2.3.5. Determination of Total Cl− in Soil
2.3.6. Determination of Total SO42− in Soil
2.3.7. Soil Characterization
2.3.8. Statistical Analysis
3. Results
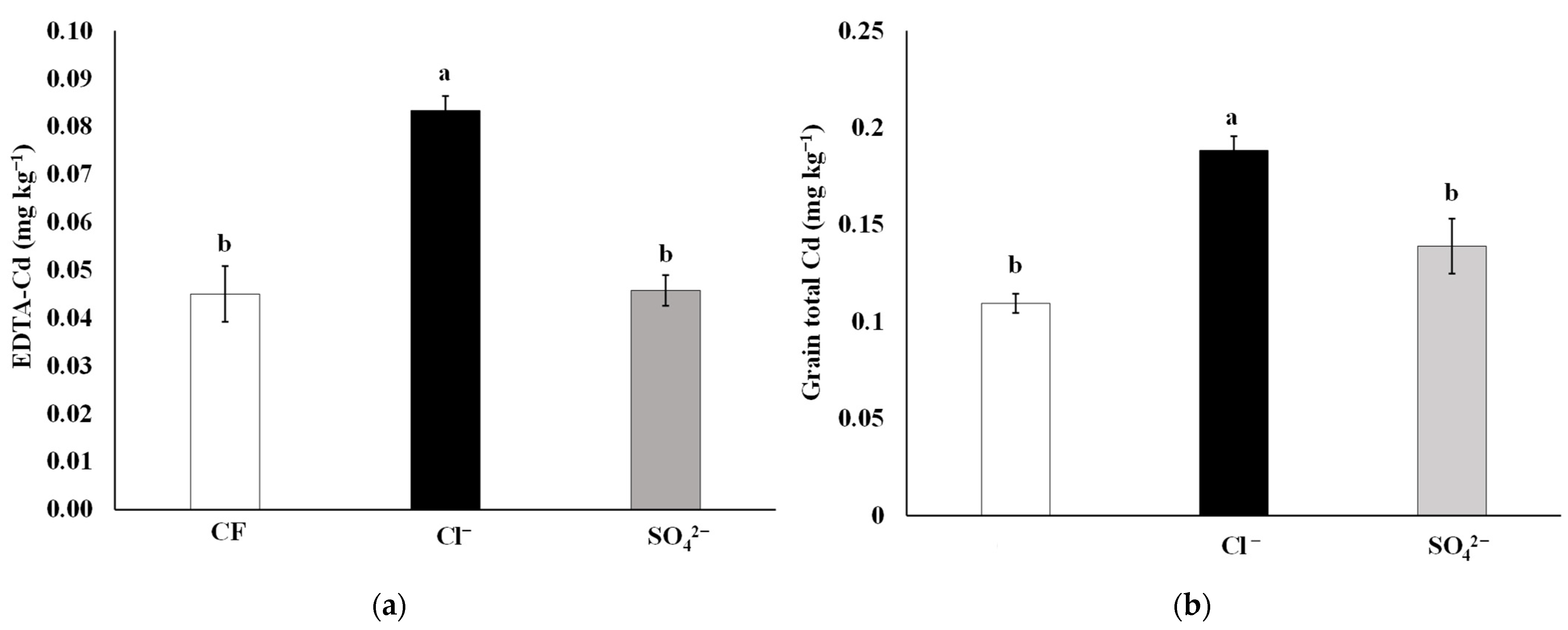
3.1. Available and Total Cd in the Soil and Rice Grains
3.2. Effects of Cl−- and SO42−-Based Fertilizers on Cd Concentrations in the Pot Experiment Rice Roots and Shoots
3.3. Prediction of Cd Accumulation in Rice Grain in Long Term Experiment
3.4. Prediction of Cd Accumulation in Rice Shoot in Short Term Experiment
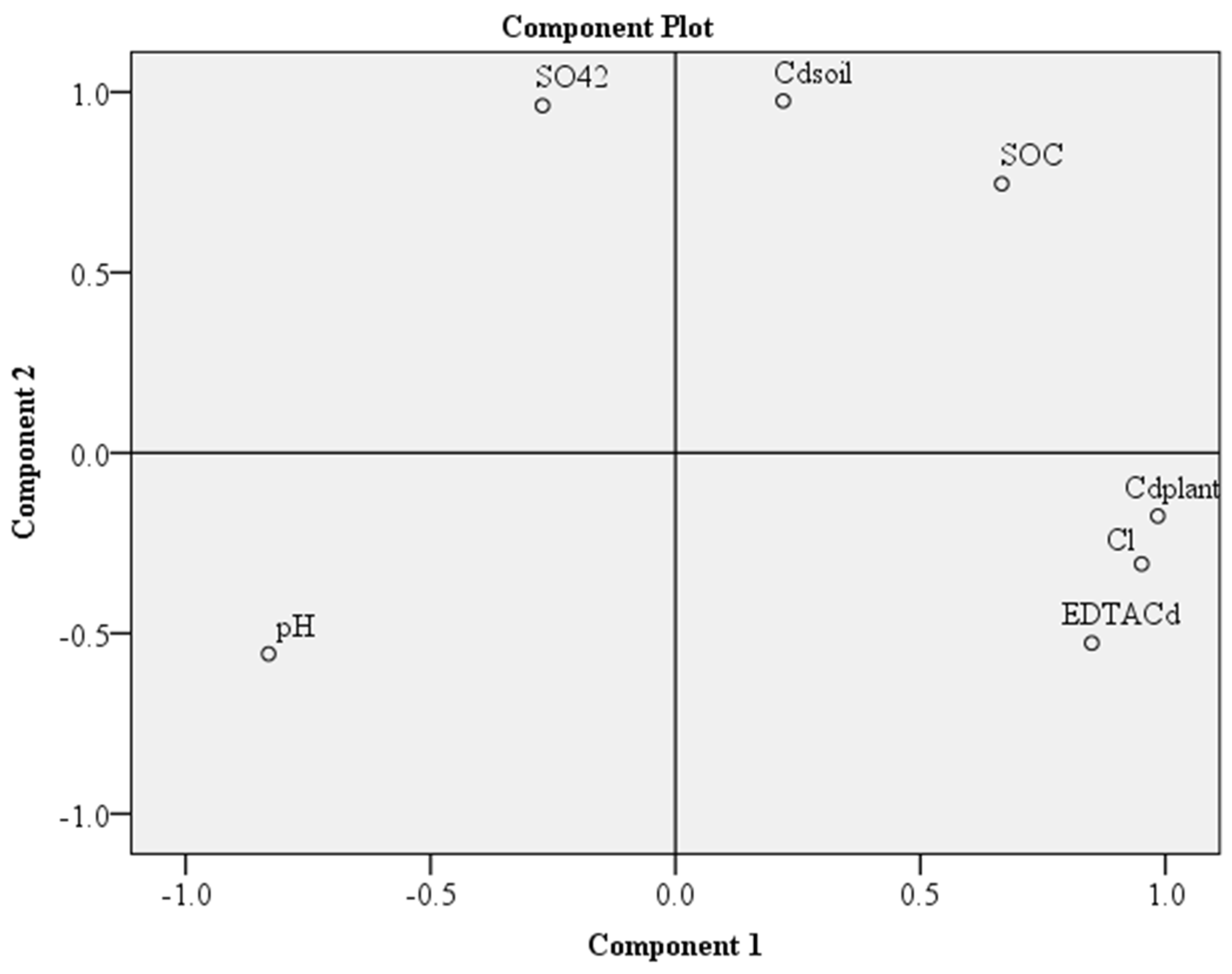
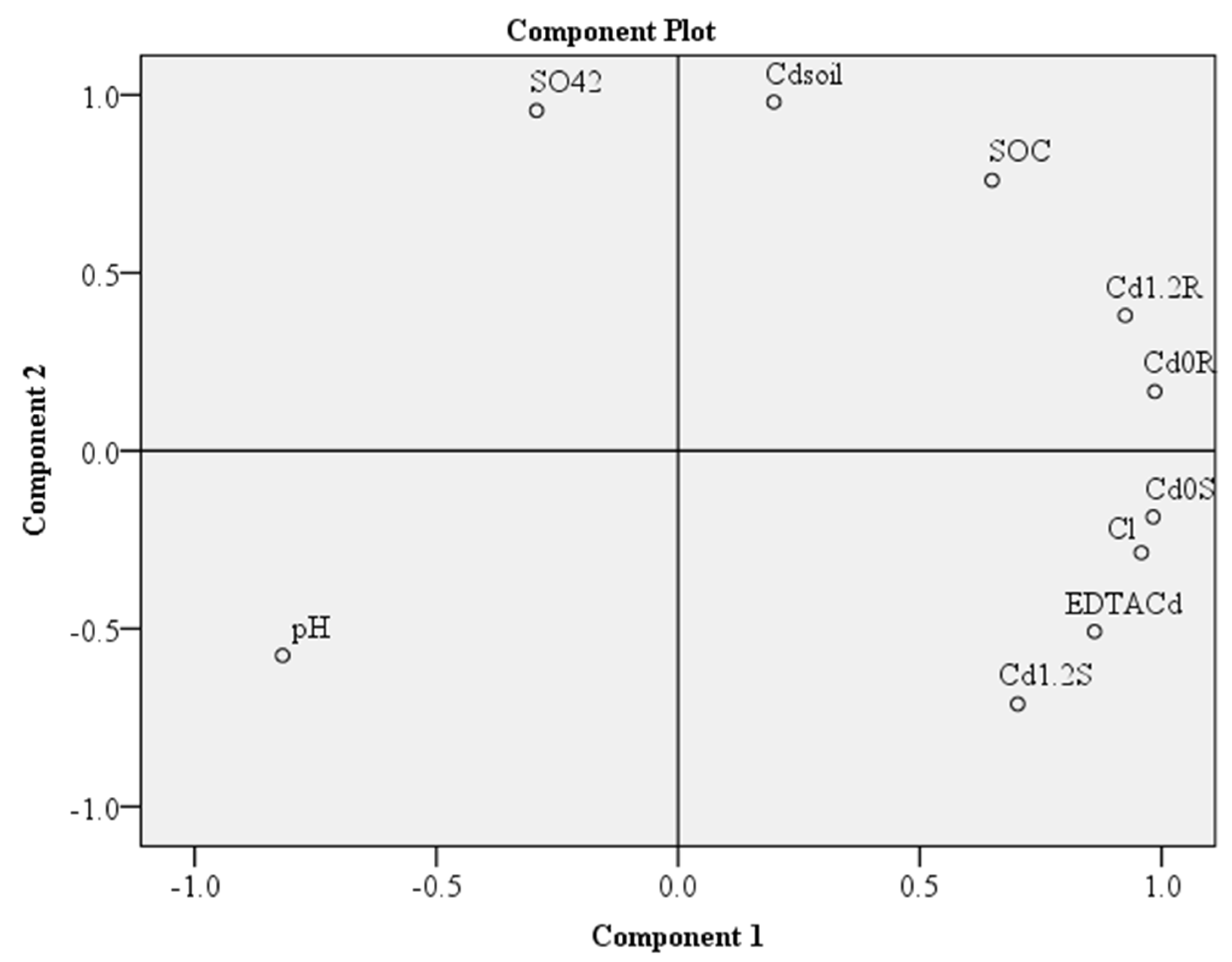
3.5. PCA Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Livera, J.; McLaughlin, M.J.; Hettiarachchi, G.M.; Kirby, J.K.; Beak, D.G. Cadmium solubility in paddy soils: Effects of soil oxidation, metal sulfides and competitive ions. Sci. Total Environ. 2011, 409, 1489–1497. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-H.; Wang, S.-L.; Lin, J.-H.; Chen, Y.-M.; Wang, M.-K. Dynamics of cadmium concentration in contaminated rice paddy soils with submerging time. Paddy Water Environ. 2012, 11, 483–491. [Google Scholar] [CrossRef]
- Wan, Y.; Camara, A.Y.; Yu, Y.; Wang, Q.; Guo, T.; Zhu, L.; Li, H. Cadmium dynamics in soil pore water and uptake by rice: Influences of soil-applied selenite with different water managements. Environ. Pollut. 2018, 240, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.-Y.; Liu, C.; Zhu, J.; Li, F.; Deng, D.-M.; Wang, Q.; Liu, C. Cadmium availability in rice paddy fields from a mining area: The effects of soil properties highlighting iron fractions and pH value. Environ. Pollut. 2016, 209, 38–45. [Google Scholar] [CrossRef]
- Yuan, C.; Li, F.; Cao, W.; Yang, Z.; Hu, M.; Sun, W. Cadmium solubility in paddy soil amended with organic matter, sulfate, and iron oxide in alternative watering conditions. J. Hazard. Mater. 2019, 378, 120672. [Google Scholar] [CrossRef]
- Huang, L.; Yang, X.; Xie, Z.; Li, S.; Liang, X.; Hu, Z. Residual effects of sulfur application prior to oilseed rape cultivation on cadmium accumulation in brown rice under an oilseed rape–rice rotation pot experiment. Ecotoxicol. Environ. Saf. 2021, 225, 112765. [Google Scholar] [CrossRef]
- Li, H.; Li, Z.; Khaliq, M.A.; Xie, T.; Chen, Y.; Wang, G. Chlorine weaken the immobilization of Cd in soil-rice systems by biochar. Chemosphere 2019, 235, 1172–1179. [Google Scholar] [CrossRef]
- Ohtani, T.; Kawabata, M.; Sase, A.; Fukami, M. Cadmium and Nutrient Heavy Metals Uptake by Rice, Barley, and Spinach as Affected by Four Ammonium Salts. J. Plant Nutr. 2007, 30, 599–610. [Google Scholar] [CrossRef]
- Zhang, W.; Lin, K.; Zhou, J.; Zhang, W.; Liu, L.; Zhang, Q. Cadmium accumulation, sub-cellular distribution and chemical forms in rice seedling in the presence of sulfur. Environ. Toxicol. Pharmacol. 2014, 37, 348–353. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Yamaguchi, N. Chemical speciation of cadmium and sulfur K-edge XANES spectroscopy in flooded paddy soils amended with zerovalent iron. Soil Sci. Soc. Am. J. 2013, 77, 1189–1198. [Google Scholar] [CrossRef] [Green Version]
- Weggler, K.; McLaughlin, M.J.; Graham, R.D. Effect of Chloride in Soil Solution on the Plant Availability of Biosolid-Borne Cadmium. J. Environ. Qual. 2004, 33, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Hussain, B.; Ashraf, M.N.; Abbas, A.; Li, J.; Farooq, M. Cadmium stress in paddy fields: Effects of soil conditions and remediation strategies. Sci. Total Environ. 2021, 754, 142188. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Shao, S.; Ni, H.; Fu, Z.; Hu, L.; Zhou, Y.; Min, X.; She, S.; Chen, S.; Huang, M.; et al. Current status, spatial features, health risks, and potential driving factors of soil heavy metal pollution in China at province level. Environ. Pollut. 2020, 266, 114961. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L. Molecular mechanism of rice responses to cadmium stress. Chin. J. Rice Sci. 2013, 27, 539–544. [Google Scholar]
- Zhai, L.; Liao, X.; Chen, T.; Yan, X.; Xie, H.; Wu, B.; Wang, L. Regional assessment of cadmium pollution in agricultural lands and the potential health risk related to intensive mining activities: A case study in Chenzhou City, China. J. Environ. Sci. 2008, 20, 696–703. [Google Scholar] [CrossRef]
- Hu, Y.; Cheng, H.; Tao, S. The challenges and solutions for cadmium-contaminated rice in China: A critical review. Environ. Int. 2016, 92, 515–532. [Google Scholar] [CrossRef]
- Liu, X.; Tian, G.; Jiang, D.; Zhang, C.; Kong, L. Cadmium (Cd) distribution and contamination in Chinese paddy soils on national scale. Environ. Sci. Pollut. Res. Int. 2016, 23, 17941–17952. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, L.; Tang, Z.; Huang, X.Y.; Ma, J.F.; Zhao, F.J. Producing cadmium-free Indica rice by overexpressing OsHMA3. Environ. Int. 2019, 126, 619–626. [Google Scholar] [CrossRef]
- Gao, P.; Huang, J.; Wang, Y.; Li, L.; Sun, Y.; Zhang, T.; Peng, F. Effects of nearly four decades of long-term fertilization on the availability, fraction and environmental risk of cadmium and arsenic in red soils. J. Environ. Manag. 2021, 295, 113097. [Google Scholar] [CrossRef]
- Xu, D.; Zhu, Q.; Ros, G.; Cai, Z.; Wen, S.; Xu, M.; Zhang, F.; de Vries, W. Calculation of spatially explicit amounts and intervals of agricultural lime applications at county-level in China. Sci. Total Environ. 2022, 806, 150955. [Google Scholar] [CrossRef]
- Zhou, S.; Liu, J.; Xu, M.; Lv, J.; Sun, N. Accumulation, availability, and uptake of heavy metals in a red soil after 22-year fertilization and cropping. Environ. Sci. Pollut. Res. Int. 2015, 22, 15154–15163. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Tan, C.; Liu, L.; Zhu, P.; Peng, C.; Luo, Y.; Christie, P. Cadmium bioavailability in surface soils receiving long-term applications of inorganic fertilizers and pig manure. Geoderma 2012, 173, 224–230. [Google Scholar] [CrossRef]
- Huang, Q.; Yu, Y.; Wan, Y.; Wang, Q.; Luo, Z.; Qiao, Y.; Su, D.; Li, H. Effects of continuous fertilization on bioavailability and fractionation of cadmium in soil and its uptake by rice (Oryza sativa L.). J. Environ. Manag. 2018, 215, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Hussain, B.; Li, J.; Ma, Y.; Chen, Y.; Wu, C.; Ullah, A.; Tahir, N. A Field Evidence of Cd, Zn and Cu Accumulation in Soil and Rice Grains after Long-Term (27 Years) Application of Swine and Green Manures in a Paddy Soil. Sustainability 2021, 13, 2404. [Google Scholar] [CrossRef]
- Smolders, E.; Lambregts, R.M.; McLaughlin, M.J.; Tiller, K.G. Effect of soil solution chloride on cadmium availability to Swiss chard. J. Environ. Qual. 1998, 27, 426–431. [Google Scholar] [CrossRef]
- Grant, C.A.; Bailey, L.D.; Therrien, M.C. The effect of N, P and KCI fertilizers on grain yield and Cd concentration of malting barley. Fertil. Res. 1996, 45, 153–161. [Google Scholar] [CrossRef]
- Smolders, E.; McLaughlin, M.J. Chloride Increases Cadmium Uptake in Swiss Chard in a Resin-buffered Nutrient Solution. Soil Sci. Soc. Am. J. 1996, 60, 1443–1447. [Google Scholar] [CrossRef]
- McLaughlin, M.J.; Lambrechts, R.M.; Smolders, E.; Smart, M.K. Effects of sulfate on cadmium uptake by Swiss chard: II. Effects due to sulfate addition to soil. Plant Soil 1998, 202, 217–222. [Google Scholar] [CrossRef]
- Zhao, Z.-Q.; Zhu, Y.-G.; Li, H.-Y.; Smith, S.E.; Smith, F.A. Effects of forms and rates of potassium fertilizers on cadmium uptake by two cultivars of spring wheat (Triticum aestivum L.). Environ. Int. 2003, 29, 973–978. [Google Scholar] [CrossRef]
- Norvell, W.A.; Wu, J.; Hopkins, D.G.; Welch, R.M. Association of Cadmium in Durum Wheat Grain with Soil Chloride and Chelate-Extractable Soil Cadmium. Soil Sci. Soc. Am. J. 2000, 64, 2162–2168. [Google Scholar] [CrossRef]
- McLaughlin, M.J.; Tiller, K.; Smart, M. Speciation of cadmium in soil solutions of saline/sodic soils and relationship with cadmium concentrations in potato tubers (Solanum tuberosum L.). Soil Res. 1997, 35, 183–198. [Google Scholar] [CrossRef]
- Wang, Y.P.; Li, Q.B.; Hui, W.; Shi, J.Y.; Lin, Q.; Chen, X.C.; Chen, Y.X. Effect of sulphur on soil Cu/Zn availability and microbial community composition. J. Hazardous. Mater. 2018, 159, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Hoefer, C.; Santner, J.; Puschenreiter, M.; Wenzel, W.W. Localized metal solubilization in the rhizosphere of salix smithiana upon sulfur application. Environ. Sci. Technol. 2015, 49, 4522–4529. [Google Scholar] [CrossRef] [PubMed]
- Capaldi, F.R.; Gratao, P.L.; Reis, A.R.; Lima, L.W.; Azevedo, R.A. Sulfur metabolism and stress defense responses in plants. Trop. Plant Biol. 2015, 8, 60–73. [Google Scholar] [CrossRef] [Green Version]
- Clemens, S.; Ma, J.F. Toxic heavy metal and metalloid accumulation in crop plants and foods. Annu. Rev. Plant Biol. 2016, 67, 489–512. [Google Scholar] [CrossRef] [Green Version]
- Li, L.Z.; Liu, X.L.; Peijnenburg, W.J.G.M.; Zhao, J.M.; Chen, X.B.; Yu, J.B.; Wu, H.F. Pathways of cadmium fluxes in the root of the halophyte Suaeda salsa. Ecotoxicol. Environ. Saf. 2012, 75, 1–7. [Google Scholar] [CrossRef]
- Takahashi, H.; Kopriva, S.; Giordano, M.; Saito, K.; Hell, R. Sulfur assimilation in photosynthetic organisms: Molecular functions and regulations of transporters and assimilatory enzymes. Annu. Rev. Plant Biol. 2011, 62, 157–184. [Google Scholar] [CrossRef]
- Wang, M.; Chen, S.; Zheng, H.; Li, S.; Chen, L.; Wang, D. The responses of cadmium phytotoxicity in rice and the microbial community in contaminated paddy soils for the application of different long-term N fertilizers. Chemosphere 2020, 238, 124700. [Google Scholar] [CrossRef]
- Mugford, S.G.; Lee, B.-R.; Koprivova, A.; Matthewman, C.; Kopriva, S. Control of sulfur partitioning between primary and secondary metabolism. Plant J. 2011, 65, 96–105. [Google Scholar] [CrossRef]
- Zakari, S.; Jiang, X.; Zhu, X.; Liu, W.; Allakonon, M.G.B.; Singh, A.K.; Chen, C.; Zou, X.; Akponikpè, P.B.I.; Dossa, G.G.O.; et al. Influence of sulfur amendments on heavy metals phytoextraction from agricultural contaminated soils: A meta-analysis. Environ. Pollut. 2021, 288, 117820. [Google Scholar] [CrossRef]
- Shen, C.; Fu, H.-L.; Liao, Q.; Huang, B.; Fan, X.; Liu, X.-Y.; Xin, J.-L.; Huang, Y.-Y. Transcriptome analysis and physiological indicators reveal the role of sulfur in cadmium accumulation and transportation in water spinach (Ipomoea aquatica Forsk.). Ecotoxicol. Environ. Saf. 2021, 225, 112787. [Google Scholar] [CrossRef] [PubMed]
- IARRP. National Farmland Ecosystem Observation and Research Station in Qiyang. Available online: https://iarrp.caas.cn/en/research/supportdepartmentsorganizations/289714.htm (accessed on 24 April 2022).
- da Silva, Y.J.; do Nascimento, C.W.; Biondi, C.M. Comparison of USEPA digestion methods to heavy metals in soil samples. Environ. Monit. Assess. 2014, 186, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Burridge, J.C.; Hewitt, I.J. A comparison of two soil-extraction procedures for the determination of EDTA-extractable copper and manganese. Commun. Soil Sci. Plant Anal. 1987, 18, 301–310. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Wang, Y.; Tsang, D.C.W.; Yang, X.; Beiyuan, J.; Yin, M.; Xiao, T.; Jiang, Y.; Lin, W.; et al. Emerging risks of toxic metal(loid)s in soil-vegetables influenced by steel-making activities and isotopic source apportionment. Environ. Int. 2021, 146, 106207. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lu, W.; Zhang, N.; Su, D.; Zeer, L.; Du, H.; Hu, K. Collaborative Assessment and Health Risk of Heavy Metals in Soils and Tea Leaves in the Southwest Region of China. Int. J. Environ. Res. Public Health 2021, 18, 10151. [Google Scholar] [CrossRef]
- Yang, C.-C.; Liang, C.-H. A modified colorimetric method to determine the chloride profile from the ponding test. J. Chin. Inst. Eng. 2014, 37, 419–427. [Google Scholar] [CrossRef]
- Yang, C.C.; Chiang, C.T. Relation between the chloride migration coefficients of concrete from the colourimetric method and the chloride profile method. J. Chin. Inst. Eng. 2009, 32, 801–809. [Google Scholar] [CrossRef]
- Wall, L.L.; Gehrke, C.W.; Suzuki, J. An automated turbidimetric method for total sulfur in plant tissue and sulfate sulfur in soils. Commun. Soil Sci. Plant Anal. 1980, 11, 1087–1103. [Google Scholar] [CrossRef]
- XLSTAT, A. Data Analysis and Statistics Software for Microsoft Excel; Addinsoft: New York, NY, USA, 2013. [Google Scholar]
- Ishikawa, N.; Ishioka, G.; Yanaka, M.; Takata, K.; Murakami, M. Effects of Ammonium Chloride Fertilizer and its Application Stage on Cadmium Concentrations in Wheat (Triticum aestivum L.) Grain. Plant Prod. Sci. 2015, 18, 137–145. [Google Scholar] [CrossRef] [Green Version]
- Havlin, J.; Beaton, J.D.; Tisdale, S.L.; Nelson, W.L. Soil Fertility and Fertilizers. An Introduction to Nutrient Management; Pearson Education: Bengaluru, India, 1985. [Google Scholar]
- Liu, X.; Hu, C.; Zhu, Z.; Riaz, M.; Liu, X.; Dong, Z.; Liu, Y.; Wu, S.; Tan, Z.; Tan, Q. Migration of Chlorine in Plant–Soil–Leaching System and Its Effects on the Yield and Fruit Quality of Sweet Orange. Front. Plant Sci. 2021, 12, 744843. [Google Scholar] [CrossRef]
- de Souza, T.R.; Quaggio, J.A.; Silva, G.O. Dinâmica de íons e acidificação do solo nos sistemas de fertirrigação e adubação sólida na citricultura. Rev. Bras. Frutic. 2006, 28, 501–505. [Google Scholar] [CrossRef]
- Quaggio, J.A. Acidez e Calagem em Solos Tropicais; Instituto Agronômico: Londrina, Brazil, 2000. [Google Scholar]
- Li, J.-y.; Liu, Z.-d.; Zhao, W.-z.; Masud, M.; Xu, R.-k. Alkaline slag is more effective than phosphogypsum in the amelioration of subsoil acidity in an Ultisol profile. Soil Tillage Res. 2015, 149, 21–32. [Google Scholar] [CrossRef]
- Bolan, N.S.; Adriano, D.C.; Curtin, D. Soil acidification and liming interactions with nutrient and heavy metal transformation and bioavailability. Adv. Agron. 2003, 78, 5–272. [Google Scholar]
- Kabata-Pendias, A.; Pendias, H. Trace Elements in Soils; Boca Ratón: New York, NY, USA, 2001. [Google Scholar]
- Shi, Y.; Qiu, L.; Guo, L.; Man, J.; Shang, B.; Pu, R.; Ou, X.; Dai, C.; Liu, P.; Yang, Y.; et al. K Fertilizers Reduce the Accumulation of Cd in Panax notoginseng (Burk.) F.H. by Improving the Quality of the Microbial Community. Front. Plant Sci. 2020, 11, 888. [Google Scholar] [CrossRef] [PubMed]
- Rasa, K.; Peltovuori, T.; Hartikainen, H. Effects of de-icing chemicals sodium chloride and potassium formate on cadmium solubility in a coarse mineral soil. Sci. Total Environ. 2006, 366, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Makino, T.; Takano, H.; Kamiya, T.; Itou, T.; Sekiya, N.; Inahara, M.; Sakurai, Y. Restoration of cadmium-contaminated paddy soils by washing with ferric chloride: Cd extraction mechanism and bench-scale verification. Chemosphere 2008, 70, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Zhou, Y.Q.; Liang, C.H. Evaluation of Phosphate Fertilizers for the Immobilization of Cd in Contaminated Soils. PLoS ONE 2015, 10, e0124022. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Yu, H.; Sun, X.; Yang, J.; Wang, D.; Shen, L.; Pan, Y.; Wu, Y.; Wang, Q.; Zhao, Y. Effects of sulfur application on cadmium bioaccumulation in tobacco and its possible mechanisms of rhizospheric microorganisms. J. Hazard. Mater. 2019, 368, 308–315. [Google Scholar] [CrossRef]
- Weggler-Beaton, K.; McLaughlin, M.J.; Graham, R.D. Salinity increases cadmium uptake by wheat and Swiss chard from soil amended with biosolids. Aust. J. Soil Res. 2000, 38, 37–45. [Google Scholar] [CrossRef]
- Zhang, D.; Du, G.; Chen, D.; Shi, G.; Rao, W.; Li, X.; Jiang, Y.; Liu, S.; Wang, D. Effect of elemental sulfur and gypsum application on the bioavailability and redistribution of cadmium during rice growth. Sci. Total Environ. 2019, 657, 1460–1467. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, M.; Chen, S.; Li, S.; Lei, X. Sulfur application modifies cadmium availability and transfer in the soil-rice system under unstable pe + pH conditions. Ecotoxicol. Environ. Saf. 2019, 184, 109641. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.Z.; Qin, M.L.; Lin, X.Y.; Zhu, Z.W.; Chen, M.X. Sulfur supply reduces cadmium uptake and translocation in rice grains (Oryza sativa L.) by enhancing iron plaque formation, cadmium chelation and vacuolar sequestration. Environ. Pollut. 2018, 238, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Song, J.; Wu, L.; Chen, Z. Worldwide cadmium accumulation in soybean grains and feasibility of food production on contaminated calcareous soils. Environ. Pollut. 2021, 269, 116153. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Li, X.; Wang, D.-c.; Rao, W.; Du, G.-H.; Yang, J.; Hua, D.-l. Influence of Sulfur on the Formation of Fe-Mn Plaque on Root and Uptake of Cd by Rice (Oryza sativa L.). Environ. Sci. 2015, 36, 1877–1887. [Google Scholar]
- Ye, X.; Li, H.; Ma, Y.; Wu, L.; Sun, B. The bioaccumulation of Cd in rice grains in paddy soils as affected and predicted by soil properties. J. Soils Sediments 2014, 14, 1407–1416. [Google Scholar] [CrossRef]
- Wu, J.; Song, Q.; Zhou, J.; Wu, Y.; Liu, X.; Liu, J.; Zhou, L.; Wu, Z.; Wu, W. Cadmium threshold for acidic and multi-metal contaminated soil according to Oryza sativa L. Cadmium accumulation: Influential factors and prediction model. Ecotoxicol. Environ. Saf. 2021, 208, 111420. [Google Scholar] [CrossRef]
- Ning, C.-C.; Gao, P.-d.; Wang, B.-Q.; Lin, W.-P.; Jiang, N.-H.; Cai, K.-Z. Impacts of chemical fertilizer reduction and organic amendments supplementation on soil nutrient, enzyme activity and heavy metal content. J. Integr. Agric. 2017, 16, 1819–1831. [Google Scholar] [CrossRef] [Green Version]
- McCauley, A.; Jones, C.; Jacobsen, J. Soil pH and organic matter. Nutr. Manag. Modul. 2009, 8, 1–12. [Google Scholar]


| Treatments | CF | Cl− | SO42− | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Fertilizer type | Urea | KH2PO4 | KCl | NH4Cl | KH2PO4 | KCl | (NH4)2SO4 | KH2PO4 | K2SO4 |
| Amount in long-term experiments (kg plot−1) | 0.82 | 1.13 | 0.88 | 1.2 | 0.75 | 0.73 | 1.88 | 1.13 | 1.13 |
| Amount in pot experiment (g kg−1) | 0.155 | 0.215 | 0.17 | 0.23 | 0.145 | 0.14 | 0.365 | 0.215 | 0.215 |
| Treatments | Soil pH | SOC (g kg−1) | TN (g kg−1) | TP (g kg−1) | TK (g kg−1) | Total Cl− (g kg−1) | Total SO42− (g kg−1) | Total Cd (mg kg−1) |
|---|---|---|---|---|---|---|---|---|
| CF | 6.19 ± 0.04 a | 16.4 ± 1.3 a | 1.75 ± 0.18 a | 0.34 ± 0.07 a | 54.4 ± 4.1 ab | 0.04 ± 0.00 b | 0.63 ± 0.01 b | 0.39 ± 0.003 a |
| Cl− | 5.86 ± 0.08 b | 17.9 ± 1.3 a | 1.78 ± 0.15 a | 0.25 ± 0.05 a | 55.6 ± 2.8 a | 0.08 ± 0.01 a | 0.42 ± 0.01 c | 0.40 ± 0.002 a |
| SO42− | 5.82 ± 0.09 b | 15.7 ± 2.0 a | 1.63 ± 0.09 a | 0.26 ± 0.06 a | 51.9 ± 2.5 b | 0.05 ± 0.00 b | 1.10 ± 0.02 a | 0.44 ± 0.031 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, B.; Ma, Y.; Li, J.; Gao, J.; Ullah, A.; Tahir, N. Cadmium in Rice Is Affected by Fertilizer-Borne Chloride and Sulfate Anions: Long-Term Field Versus Pot Experiments. Processes 2022, 10, 1253. https://doi.org/10.3390/pr10071253
Hussain B, Ma Y, Li J, Gao J, Ullah A, Tahir N. Cadmium in Rice Is Affected by Fertilizer-Borne Chloride and Sulfate Anions: Long-Term Field Versus Pot Experiments. Processes. 2022; 10(7):1253. https://doi.org/10.3390/pr10071253
Chicago/Turabian StyleHussain, Babar, Yibing Ma, Jumei Li, Jusheng Gao, Aman Ullah, and Nazia Tahir. 2022. "Cadmium in Rice Is Affected by Fertilizer-Borne Chloride and Sulfate Anions: Long-Term Field Versus Pot Experiments" Processes 10, no. 7: 1253. https://doi.org/10.3390/pr10071253






