Biodegradation of Naphthalene and Anthracene by Aspergillus glaucus Strain Isolated from Antarctic Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Microorganism and Culture Conditions
2.3. Biomass Measurement
2.4. Enzyme Analyses
2.5. Extraction of PAHs and GC-MS Analysis
2.6. DNA Isolation Procedure
2.7. PCR Conditions and DNA Sequencing
| Primers | Sequence (5′→3′) | Source |
|---|---|---|
| for 18S rDNA Pffor Pfrev | AGGGATGTATTTATTAGATAAAAAATCAA CGCAGTAGTTAGTCTTCAGTAAATC PCR conditions: initial step, 95 °C, 5 min; 35 cycles amplification, 95 °C, 30 s; 58 °C, 30 s; 72 °C, 45 s; extension step, 72 °C, 7 min | [64] |
| for catechol dioxygenase AFKF1 AFKR1 AFKF2 AFKR2 AFKF3 AFKR3 AFKF4 AFKR4 | GCTACTCATCGATTTGACC GCGGCCTCATCAGCAAGCTT TTGTCTGTGACGTGATCGGG TGCCACACCTCCACCGTCGC TCATGCACGGCCGGGTGATC GGGTGTCGGTCCATGAGCTC GTCCGACGCCGTATCCTGTT CTACGCCTGGTCCGCCACCA PCR conditions: initial step, 95 °C, 5 min; 35 cycles amplification, 95 °C, 30 s; 60 °C, 30 s; 72 °C, 60 s; extension step, 72 °C, 5 min | Primers for detection of catechol dioxygenase coding genes were designed in this study on the basis of Aspergillus fumigatus Af293 sequence NCBI Acc. No. XM_744333 [43] |
3. Results and Discussion
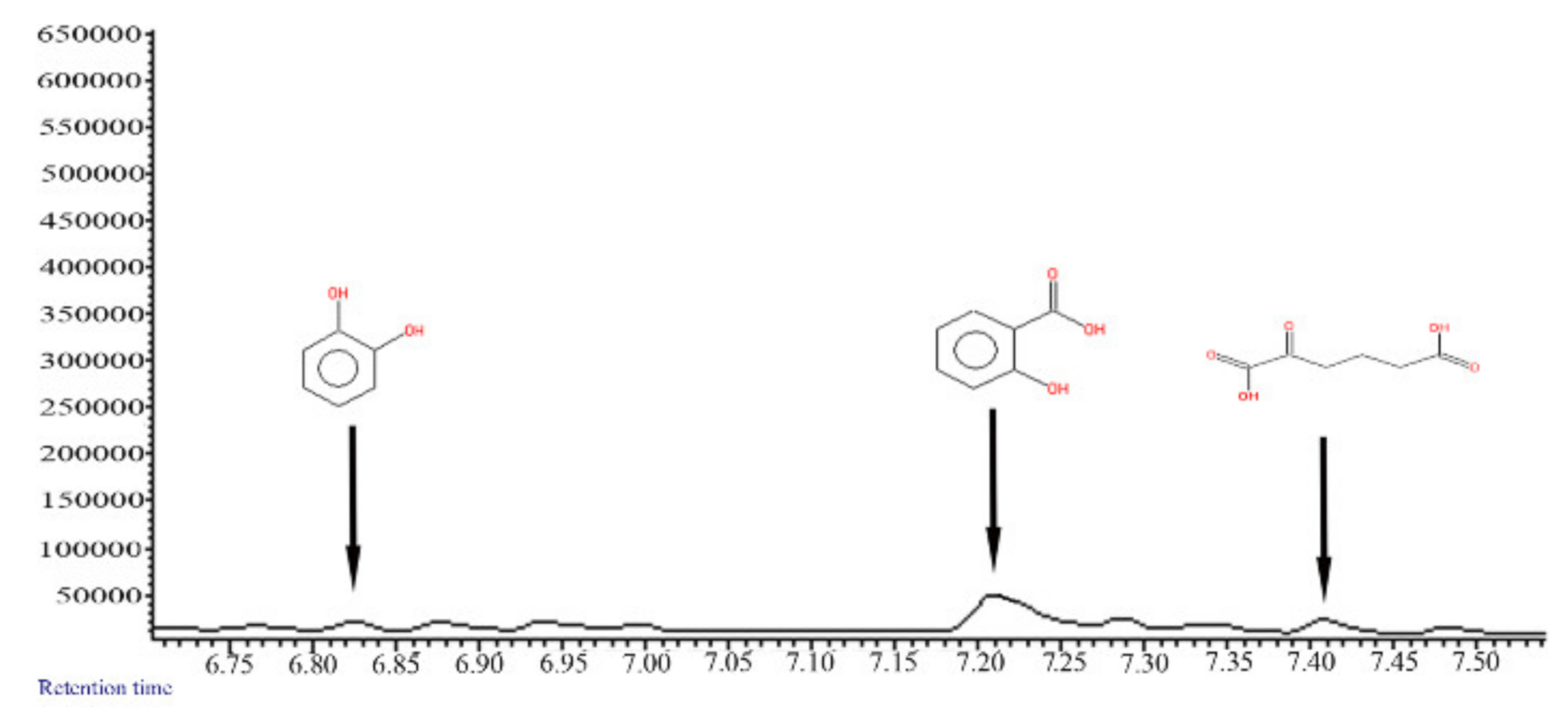
3.1. Degradation of PAHs
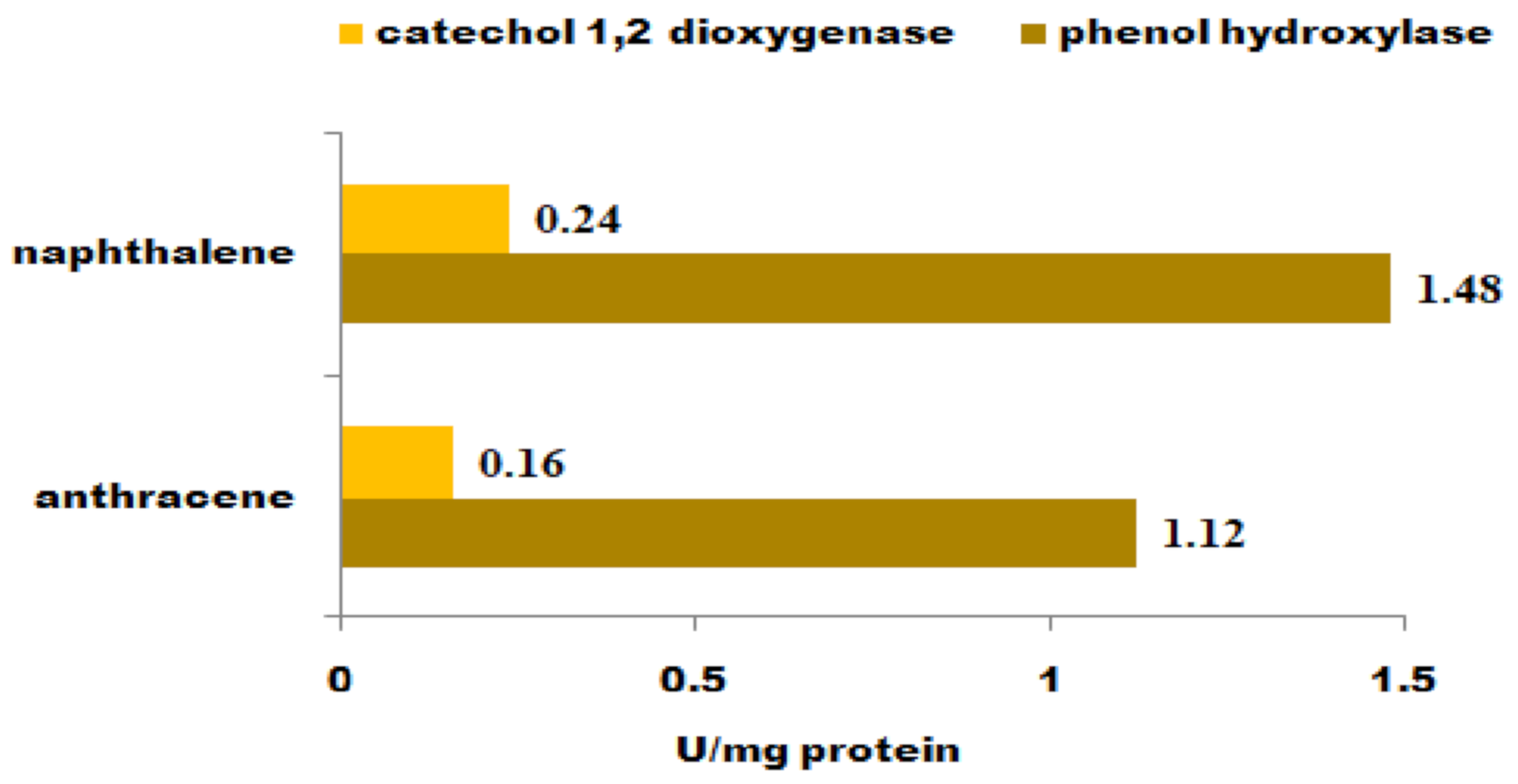
3.2. Enzyme Analyses
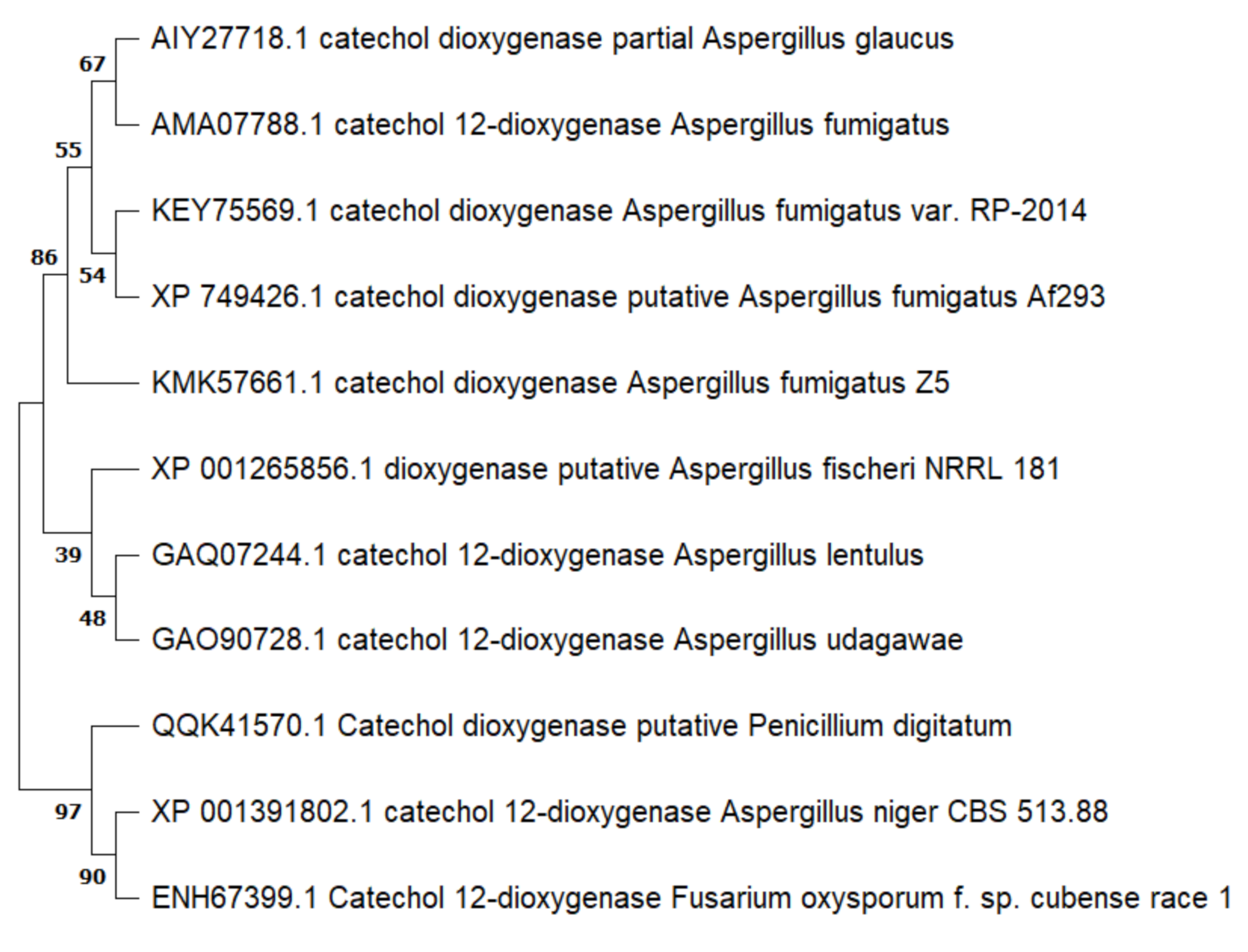
3.3. Sequence Analyses of Putative Catechol 1,2-dioxygenase Gene
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Voulvoulis, N. Water Reuse from a Circular Economy Perspective and Potential Risks from an Unregulated Approach. Cur. Opin. Environ. Sci. Health 2018, 2, 32–45. [Google Scholar] [CrossRef]
- Morris, J.; Georgiou, I.; Guenther, E.; Caucci, S. Barriers in Implementation of Wastewater Reuse: Identifying the Way Forward in Closing the Loop. Circ. Econ. Sust. 2021, 1, 413–433. [Google Scholar] [CrossRef]
- Bratkova, S.; Alexieva, Z.; Angelov Nikolova, A.K.; Genova, P.; Ivanov, R.; Gerginova, M.; Peneva, N.; Beschkov, V. Efficiency of microbial fuel cells based on the sulfate reduction by lactate and glucose. Int. J. Environ. Sci. Technol. 2019, 16, 6145–6156. [Google Scholar] [CrossRef]
- Apollon, W.; Rusyn, I.; González-Gamboa, N.; Kuleshova, T.; Luna-Maldonado, A.I.; Vidales-Contreras, J.A.; Kamaraj, S.-K. Improvement of zero waste sustainable recovery using microbial energy generation systems: A comprehensive review. Sci. Total Environ. 2022, 817, 153055. [Google Scholar] [CrossRef]
- Zhang, X.; Cheng, S.; Zhu, C.; Sun, S. Microbial PAH-degradation in soil: Degradation pathways and contributing factors. Pedosphere 2006, 16, 555–565. [Google Scholar] [CrossRef]
- Alexieva, Z.; Yemendzhiev, H.; Zlateva, P. Cresols utilization by Trametes versicolor and substrate interactions in the mixture with phenol. Biodegradation 2010, 21, 625–635. [Google Scholar] [CrossRef]
- Hamdi, H.; Benzarti, S.; Aoyama, I.; Jedidi, N. Rehabilitation of degraded soils containing aged PAHs based on phytoremediation with alfalfa (Medicago sativa L.). Int. Biodeterior. Biodegrad. 2012, 67, 40–47. [Google Scholar] [CrossRef]
- Sun, G.-D.; Jin, J.-H.; Xu, Y.; Zhong, Z.-P.; Liu, Y.; Liu, Z.-P. Isolation of a high molecular weight polycyclic aromatic hydrocarbon degrading strain and its enhancing the removal of HMW-PAHs from heavily contaminated soil. Int. Biodeterior. Biodegrad. 2014, 90, 23–28. [Google Scholar] [CrossRef]
- Liu, Y.-Q.; Maulidiany, N.; Zeng, P.; Heo, S. Decolourization of azo, anthraquinone and triphenylmethane dyes using aerobic granules: Acclimatization and long-term stability. Chemosphere 2021, 263, 128312. [Google Scholar] [CrossRef]
- Malhotra, M.; Gupta, D.; Sahani, J.; Singh, S. Microbial Degradation of Phenol and Phenolic Compounds. In Recent Advances in Microbial Degradation; Inamuddin, Ahamed, M.I., Prasad, R., Eds.; Environmental and Microbial Biotechnology; Springer: Singapore, 2021. [Google Scholar] [CrossRef]
- Potin, O.; Rafin, C.; Veignie, E. Bioremediation of an aged polycyclic aromatic hydrocarbon (PAHs)-contaminated soil by filamentous fungi isolated from the soil. Int. Biodeterior. Biodegrad. 2004, 54, 45–52. [Google Scholar] [CrossRef]
- Haritash, A.; Kaushik, C. Biodegradation aspects of polycyclic aromatic hydrocarbons (PAHs): A review. J. Hazard. Mater. 2009, 169, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Cerniglia, C.; Sutherland, J. Degradation of polycyclic aromatic hydrocarbons by fungi. In Handbook of Hydrocarbon and Lipid Microbiology; Timmis, K.N., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 2079–2110. [Google Scholar]
- Ye, J.-S.; Yin, H.; Qiang, J.; Peng, H.; Qin, H.-M. Biodegradation of anthracene by Aspergillus fumigatus. J. Hazard. Mater. 2011, 185, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Jang, Y.; Choi, Y.; Kim, M.; Lee, J.; Lee, H.; Hong, J.; Lee, Y.M.; Kim, G.; Kim, J. Biotechnological procedures to select white rot fungi for the degradation of PAHs. J. Microbiol. Methods 2014, 97, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Marchand, C.; St-Arnaud, M.; Hogland, W.; Bell, T. Petroleum hydrocarbon capacity of bacteria and fungi isolated from petroleum-contaminated soil. Int. Biodeterior. Biodegrad. 2017, 116, 48–57. [Google Scholar] [CrossRef]
- Leonardi, V.; Šašek, V.; Petruccioli, M.; D’Annibale, A.; Erbanova, P.; Cajthaml, T. Bioavailability modification and fungal biodegradation of PAHs in aged industrial soils. Int. Biodeterior. Biodegrad. 2007, 60, 165–170. [Google Scholar] [CrossRef]
- Szczepaniak, Z.; Cyplik, P.; Juzwa, W.; Czarny, J.; Staninska, J.; Piotrowska-Cyplik, A. Antibacterial effect of the Trichoderma viride fungi on soil microbiome during PAH’s biodegradation. Int. Biodeterior. Biodegrad. 2015, 104, 170–177. [Google Scholar] [CrossRef]
- Lawal, A.T. Polycyclic aromatic hydrocarbons. A review. Cogent Environ. Sci. 2017, 3, 1339841. [Google Scholar] [CrossRef]
- García-Díaz, C.; Ponce-Noyola, M.; Esparza-García, F.; Rivera-Orduña, F.; Barrera-Cortés, J. PAH removal of high molecular weight by characterized bacterial strains from different organic sources. Int. Biodeterior. Biodegrad. 2013, 85, 311–322. [Google Scholar] [CrossRef]
- Thion, C.; Cébron, A.; Beguiristain, T.; Leyval, C. Inoculation of PAH-degrading strains of Fusarium solani and Arthrobacter oxydans in rhizospheric sand and soil microcosms: Microbial interactions and PAH dissipation. Biodegradation 2013, 24, 569–581. [Google Scholar] [CrossRef]
- Ferradji, F.; Mnif, S.; Badis, A.; Rebbani, S.; Fodil, D.; Eddouaouda, K.; Sayadi, S. Naphthalene and crude oil degradation by biosurfactant producing Streptomyces spp. isolated from Mitidja plain soil (North of Algeria). Int. Biodeterior. Biodegrad. 2014, 86, 300–308. [Google Scholar] [CrossRef]
- Pandey, P.; Pathak, H.; Saurabh, D. Microbial ecology of hydrocarbon degradation in the soil: A Review. Res. J. Environ. Toxicol. 2016, 10, 1–15. [Google Scholar] [CrossRef]
- Aranda, E. Promising approaches towards biotransformation of polycyclic aromatic hydrocarbons with Ascomycota fungi. Curr. Opin. Biotechnol. 2016, 38, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Isaac, P.; Lozada, M.; Dionisi, H.; Estevez, M.; Ferrero, M. Differential expression of the catabolic nahAc gene and its effect on PAH degradation in Pseudomonas strains isolated from contaminated Patagonian coasts. Int. Biodeterior. Biodegrad. 2015, 105, 1–6. [Google Scholar] [CrossRef]
- Kuppusamy, S.; Thavamani, P.; Venkatesvarlu, K.; Lee, Y.; Naidu, R.; Megharaj, M. Remediation approaches for polycyclic aromatic hydrocarbons (PAHs) contaminated soils: Technological constraints, emerging trends and future directions. Chemosphere 2017, 168, 944–968. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Nandy, A.; Taylor, N.; Venkatesan, S.; Ozhukil, K.; Vinayaraj, O.; Karan, K.; Thangadurai, V.; Tsesmetzis, N.; Gieg, L. Bioelectrochemical remediation of phenanthrene in a microbial fuel cell using an anaerobic consortium enriched from a hydrocarbon-contaminated site. J. Hazard. Mater. 2019, 389, 121845. [Google Scholar] [CrossRef]
- Mauger, S.; Monard, C.; Thion, C.; Vandenkoornhuyse, P. Contribution of single-cell omics to microbial ecology. Trends Ecol. Evol. 2022, 37, 67–78. [Google Scholar] [CrossRef]
- Peng, R.-H.; Xiong, A.-S.; Xue, Y.; Fu, X.-Y.; Gao, F.; Zhao, W.; Tian, Y.-S.; Yao, Q.-H. Microbial biodegradation of polyaromatic hydrocarbons. FEMS Microbiol. Rev. 2008, 32, 927–955. [Google Scholar] [CrossRef]
- Seo, J.; Keum, Y.; Hu, Y.; Lee, S.; Li, Q. Degradation of phenanthrene by Burkholderia sp. C3: Initial 1,2- and 3,4-dioxygenation and meta- and ortho-cleavage of naphthalene-1,2-diol. Biodegradation 2007, 18, 123–131. [Google Scholar] [CrossRef]
- Brzeszcz, J.; Kaszycki, P. Aerobic bacteria degrading both n-alkanes and aromatic hydrocarbons: An undervalued strategy for metabolic diversity and flexibility. Biodegradation 2018, 29, 359. [Google Scholar] [CrossRef]
- Hammel, K.; Green, B.; Gai, W. Ring fission of anthracene by eucaryote. Proc. Natl. Acad. Sci. USA 1991, 88, 10605–10608. [Google Scholar] [CrossRef]
- Wang, C.; Sun, H.; Li, J.; Li, Y.; Zhang, Q. Enzyme activities during degradation of polycyclic aromatic hydrocarbons by white rot fungus Phanerochaete chrysosporium in soils. Chemosphere 2009, 77, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Harms, H.; Schlosser, D.; Wich, L. Untapped potential: Exploiting fungi in bioremediation of hazardous chemicals. Nat. Rev. Microbiol. 2011, 9, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Ichinose, H.; Wariishi, H. Flavin-containing monooxygenases from Phanerochaete chrysosporium responsible for fungal metabolism of phenolic compounds. Biodegradation 2012, 23, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Adenipekun, C.; Ipeaiyeda, A.; Olayonwa, A.; Egbewale, S. Biodegradation of polycyclic aromatic hydrocarbons (PAHs), in spent and fresh cutting fluids contaminated soils by Pleurotus pulmonarius (Fries). Quelet and Pleurotusos treatus (Jacq.) Fr. P. Kumm. Afr. J. Biotechnol. 2015, 48, 661–667. [Google Scholar] [CrossRef]
- Kadri, T.; Rouissi, T.; Brar, S.; Cledon, M.; Sarma, S.; Verma, M. Biodegradation of polycyclic aromatic hydrocarbons (PAHs) by fungal enzymes: A review. J. Environ. Sci. 2017, 51, 52–74. [Google Scholar] [CrossRef]
- Cerniglia, C. Fungal metabolism of polycyclic aromatic hydrocarbons: Past, present and future applications in bioremediation. J. Ind. Microbiol. Biotechnol. 1997, 19, 324–333. [Google Scholar] [CrossRef]
- Schmidt, S.; Christensen, J.; Johnsen, A. Fungal PAH-metabolites resist mineralization by soil microorganisms. Environ. Sci. Technol. 2010, 44, 1677–1682. [Google Scholar] [CrossRef]
- Zang, S.; Lian, B.; Wang, J.; Yang, Y. Biodegradation of 2-naphtol and its metabolites by coupling Aspergillus niger with Bacillus subtilis. J. Environ. Sci. 2010, 22, 669–674. [Google Scholar] [CrossRef]
- Tortella, G.; Diez, M. Fungal diversity and use in decomposition of environmental pollutants. Crit. Rev. Microbiol. 2005, 31, 197–212. [Google Scholar] [CrossRef]
- Ghosal, D.; Ghosh Sh Dutta, T.; Ahn, Y. Current State of Knowledge in Microbial Degradation of Polycyclic Aromatic Hydrocarbons (PAHs): A Review. Front. Microbiol. 2016, 7, 1837. [Google Scholar] [CrossRef]
- Gerginova, M.; Manasiev, J.; Yemendzhiev, H.; Terziyska, A.; Peneva, N.; Alexieva, Z. Biodegradation of phenol by Antarctic strains of Aspergillus fumigatus. Z. Nat. 2013, 68C, 384–393. [Google Scholar] [CrossRef]
- Mineki, S.; Suzuki, K.; Iwata, K.; Nakajima, D.; Goto, S. Degradation of polyaromatic hydrocarbons by fungi isolated from soil in Japan. Polycycl. Aromat. Compd. 2015, 35, 120–128. [Google Scholar] [CrossRef]
- Mohammed, D.; Khudeir, S.; Al-Jubouri, M. The optimum conditions for naphthalene biodegradation by filamentous fungi. Iraqi J. Sci. 2014, 55, 1780–1791. [Google Scholar]
- Reyes-Cesar, A.; Absalon, A.; Fernandez, F.; Gonzalez, J.; Cortes-Espinosa, D. Biodegradation of a mixture of PAHs by non-ligninolytic fungal strains isolated from crude oil-contaminated soil. World J. Microb. Biot. 2014, 30, 999–1009. [Google Scholar] [CrossRef] [PubMed]
- Marco-Urrea, E.; García-Romera, I.; Aranda, E. Potential of non-ligninolytic fungi in bioremediation of chlorinated and polycyclic aromatic hydrocarbons. N. Biotechnol. 2015, 32, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Rosales, C.; Fullana, N.; Musto, H.; Castro-Sowinsk, S. Antarctic DNA moving forward: Genomic plasticity and biotechnological potential. FEMS Microbiol. Lett. 2012, 331, 1–9. [Google Scholar] [CrossRef]
- Amrani, A.; Dumas, A.-S.; Wick, L.; Yergeau, E.; Berthomé, R. “Omics” Insights into PAH degradation toward improved green remediation biotechnologies. Environ. Sci. Technol. 2015, 49, 11281–11291. [Google Scholar] [CrossRef]
- Giudice, A.; Bruni, V.; De Domenico, M.; Michaud, L. Psychrophiles-cold-adapted hydrocarbon degrading microorganisms. In Handbook of Hydrocarbon and Lipid Microbiology; Timmis, K.N., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 1897–1921. [Google Scholar] [CrossRef]
- Okere, U.; Cabrerizo, A.; Dachs, J.; Jones, K.; Kirk, T. Semple Biodegradation of phenanthrene by indigenous microorganisms in soils from Livingstone Island, Antarctika. FEMS Microbiol. Lett. 2012, 329, 69–77. [Google Scholar] [CrossRef]
- Cury, J.; Jurelevicius, D.; Villela, H.D.M.; Jesus, H.; Peixoto, R.; Schaefer, C.E.G.R.; Bicedo, M.; Seldin, L.; Rosado, A. Microbial diversity and hydrocarbon depletion in low and high diesel-polluted soil samples from Keller Peninsula, South Shetland Islands. Antarct. Sci. 2015, 27, 263–273. [Google Scholar] [CrossRef][Green Version]
- Litova, K.; Manasiev, J.; Gerginova, M.; Peneva, N.; Alexieva, Z. Sequence analysis of gene coding key enzymes responsible for degradation of phenolic compounds by Aspergillus fumigatus strain AL3. J. BioSci. Biotechnol. 2015, SE, 195–203. [Google Scholar]
- Gerginova, M.; Litova, K.; Manasiev, J.; Peneva, N.; Bibova, R.; Krastanov, A.; Alexieva, Z. Studies on biodegradation of phenolic compounds by Aspergillus glaucus strain. In Microbes in the Spotlight: Recent Progress in the Understanding of Beneficial and Harmful Microorganisms; Méndez-Vilas, A., Ed.; BrownWalker Press: Boca Raton, FL, USA, 2016; pp. 135–139. [Google Scholar]
- Elster, J.; Margesin, R.; Wagner, D.; Häggblom, M. Polar and Alpine Microbiology—Earth’s cryobiosphere. FEMS Microbiol. Ecol. 2017, 93, fiw221. [Google Scholar] [CrossRef] [PubMed]
- Ogaki, M.B. Antarctic Marine Fungi and Their Potential Application in Bioremediation. In Marine Microbial Bioremediation; Vala, A., Dudhagara, D., Dave, B., Eds.; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar] [CrossRef]
- Tossi, S.; Kostadinova, N.; Krumova, E.; Pashova, S.; Dishliiska, V.; Spassova, B.; Vassilev, S.; Angelova, M. Antioxidant enzyme activity of filamentous fungi isolated from Livingston Island, Maritime Antarctica. Polar Biol. 2010, 33, 1227–1237. [Google Scholar] [CrossRef]
- Neujahr, H.; Gaal, A. Phenol Hydroxylase from Yeast. Purification and properties of the enzyme from yeast Trichosporon cutaneum. Eur. J. Biochem. 1973, 35, 386–400. [Google Scholar] [CrossRef] [PubMed]
- Varga, J.; Neujah, H. Purification and Properties of Catechol 1,2-Oxygenase from Trichosporon cutaneum. Eur. J. Biochem. 1970, 12, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M. A rapid and sensitive for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Kiiskinen, L.-L.; Ratto, M.; Kruus, K. Screening for novel laccase-producing microbes. J. Appl. Microbiol. 2004, 97, 640–646. [Google Scholar] [CrossRef]
- Dincheva, I.; Badjakov, I.; Kondakova, V.; Batchvarova, R. Metabolic profiling of red raspberry (Robus idaeus) during fruit development and ripening. Int. J. Agric. Sci. Res. 2013, 3, 81–88. [Google Scholar]
- Maniatis, T.; Fritsch, E.; Sambrook, J. Molecular Cloning: A Laboratory Manual; Sambrook, J., Ed.; Cold Spring Harbor Laboratory: Cold Spring Harbor, NY, USA, 1982. [Google Scholar]
- Jaeger, E.; Carroll, N.; Choudhury, S.; Dunlop, A.; Towler, H.; Matheson, M.; Adamson, P.; Okhravi, N. Rapid Detection and Identification of Candida, Aspergillus, and Fusarium Species in Ocular Samples Using Nested PCR. J. Clin. Microbiol. 2000, 38, 2902–2908. [Google Scholar] [CrossRef]
- Alexieva, Z.; Yemendzhiev, H.; Tossi, S.; Krumova, E.; Angelova, M.; Terziyska, A.; Peneva, N.; Gerginova, M. Growth of fungal strains isolated from Livingston Island on phenolic compounds—Biodegradation potential. In Microbes in Applied Research: Current Advances and Challenges; Mendez-Vilas, A., Ed.; World Scientific Publishing Co.: Singapore, 2012; pp. 131–134. [Google Scholar] [CrossRef]
- Kathiresan, K. Polythene and plastic-degrading microbes in an Indian mangrove soil. Rev. Biol. Trop. 2003, 51, 629–633. [Google Scholar]
- Prabhat, S.; Bhattacharyya, S.; Vishal, V.; Kalyan, R.; Vijai, K.; Pandey, K.; Singh, M. Studies on isolation and identification of active microorganisms during degradation of polyethylene/starch film. Int. Res. J. Environ. Sci. 2013, 2, 83–85. [Google Scholar]
- Habe, H.; Omori, T. Genetics of polycyclic aromatic hydrocarbon metabolism in diverse aerobic bacteria. Biosci. Biotechnol. Biochem. 2003, 67, 225–243. [Google Scholar] [CrossRef] [PubMed]
- Mallick, S.; Chatterjee, S.; Dutta, T. A novel degradation pathway in the assimilation of phenanthrene by Staphylococcus sp. strain PN/Y via meta-cleavage of 2-hydroxy-1-naphthoic acid: Formation of trans-2,3-dioxo-5-(29-hydroxyphenyl)-pent-4-enoic acid. Microbiology 2007, 153, 2104–2115. [Google Scholar] [CrossRef] [PubMed]
- Mallick, S.; Chakraborty, J.; Dutta, T. Role of oxygenases in guiding diverse metabolic pathways in the bacterial degradation of low-molecular-weight polycyclic aromatic hydrocarbons: A review. Crit. Rev. Microbiol. 2011, 37, 64–97. [Google Scholar] [CrossRef] [PubMed]
- Kunihiro, M.; Ozeki, Y.; Nogy, Y.; Hamamura, N.; Kanaly, R. Benz[a]anthracene biotransformation and production of ring fission products by Sphingobium sp. strain KK22. Appl. Environ. Microbiol. 2013, 79, 4410–4420. [Google Scholar] [CrossRef]
- Coon, M.; Persson, A.; French, J. On the possible relationship of cytochrome P-450 to alcohol metabolism: Fundamental aspects of the microsomal hydroxylation system, including properties and interactions of the components. Adv. Exp. Med. Biol. 1980, 132, 11–22. [Google Scholar] [CrossRef]
- Koop, D. Hydroxylation of p-nitrophenol by rabbit ethanol-inducible cytochrome P-450 isozyme 3a. Mol. Pharmacol. 1986, 29, 399–404. [Google Scholar]
- Koop, D.; Laethem, C.; Schnie, G. Identification of ethanol-inducible P450 isozyme 3a (P450IIE1) as a benzene and phenol hydroxylase. Toxicol. Appl. Pharmacol. 1989, 98, 278–288. [Google Scholar] [CrossRef]
- Evans, W. The microbiological degradation of aromatic compounds. J. Gen. Microbiol. 1963, 32, 177–184. [Google Scholar] [CrossRef]
- Guo, C.; Dang, Z.; Wong, Y.; Tam, N. Biodegradation ability and dioxgenase genes of PAH-degrading Sphingomonas and Mycobacterium strains isolated from mangrove sediments. Int. Biodeterior. Biodegrad. 2010, 64, 419–426. [Google Scholar] [CrossRef]
- Fuchs, G.; Boll, M.; Heider, J. Microbial degradation of aromatic compounds—From one strategy to four. Nat. Rev. Microbiol. 2011, 9, 803–816. [Google Scholar]
- Pugazhendi, A.; Qari, H.; Al-Badry Basahi, J.; Godon, J.; Dhavamani, J. Role of a halothermophilic bacterial consortium for the biodegradation of PAHs and the treatment of petroleum wastewater at extreme conditions. Int. Biodeterior. Biodegrad. 2017, 121, 44–54. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
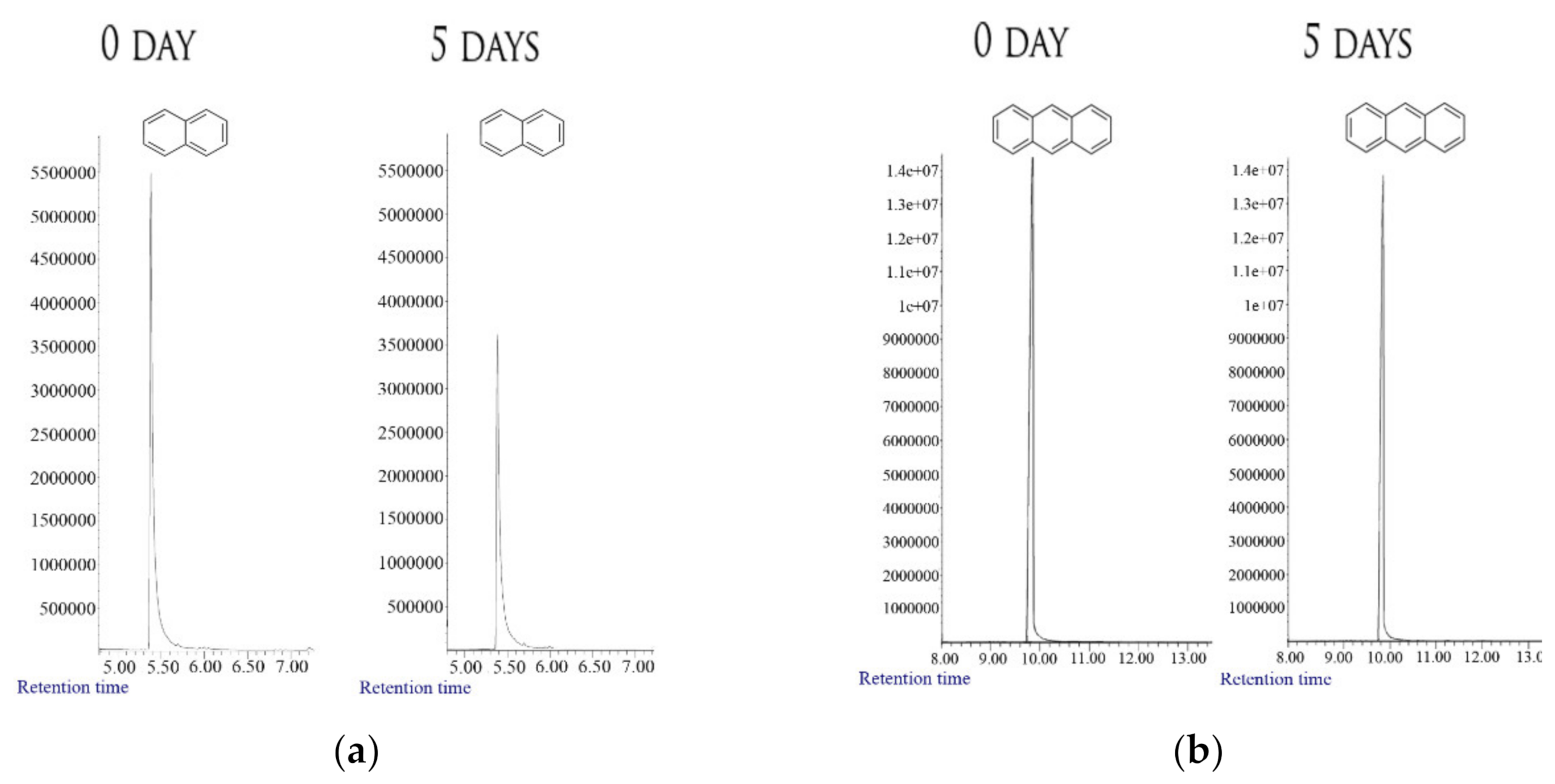




| C-Substrate | Initial Biomass, DW (g/L) | Final Biomass, DW (g/L) | Growth % | C0 * (g/L) | C5 ** (g/L) | Degradation % | C15 *** (g/L) | Degradation % |
|---|---|---|---|---|---|---|---|---|
| Naphthalene C10H8 | 0.012 | 0.05 | 76 | 0.3 | 0.2 | 33.05 | 0.11 | 66.6 |
| Anthracene C14H10 | 0.011 | 0.03 | 64 | 0.32 | 0.24 | 18.75 | 0.18 | 43.75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoyanova, K.; Gerginova, M.; Dincheva, I.; Peneva, N.; Alexieva, Z. Biodegradation of Naphthalene and Anthracene by Aspergillus glaucus Strain Isolated from Antarctic Soil. Processes 2022, 10, 873. https://doi.org/10.3390/pr10050873
Stoyanova K, Gerginova M, Dincheva I, Peneva N, Alexieva Z. Biodegradation of Naphthalene and Anthracene by Aspergillus glaucus Strain Isolated from Antarctic Soil. Processes. 2022; 10(5):873. https://doi.org/10.3390/pr10050873
Chicago/Turabian StyleStoyanova, Katya, Maria Gerginova, Ivayla Dincheva, Nadejda Peneva, and Zlatka Alexieva. 2022. "Biodegradation of Naphthalene and Anthracene by Aspergillus glaucus Strain Isolated from Antarctic Soil" Processes 10, no. 5: 873. https://doi.org/10.3390/pr10050873
APA StyleStoyanova, K., Gerginova, M., Dincheva, I., Peneva, N., & Alexieva, Z. (2022). Biodegradation of Naphthalene and Anthracene by Aspergillus glaucus Strain Isolated from Antarctic Soil. Processes, 10(5), 873. https://doi.org/10.3390/pr10050873






