Optimization of Phycobiliprotein Solubilization from a Thermotolerant Oscillatoria sp.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strain
2.2. Experimental Design
2.3. Culture Conditions
2.4. Biomass and PBPs Quantification
3. Results
3.1. Effect of Multiple Parameters on the Concentration of PBPs
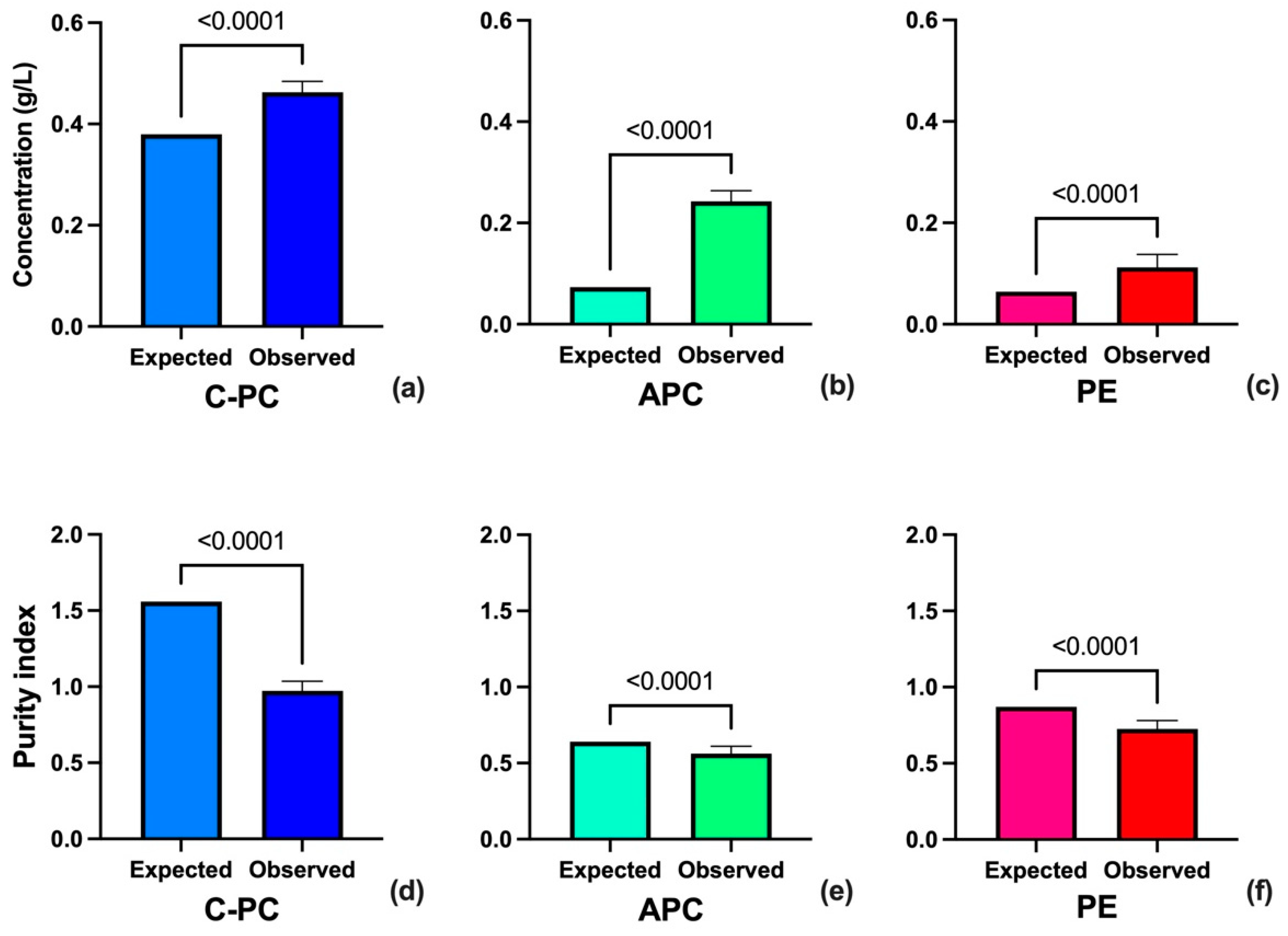
3.2. Effect of Multiple Parameters on the Purity of PBPs
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pez Jaeschke, D.; Rocha Teixeira, I.; Damasceno Ferreira Marczak, L.; Domeneghini Mercali, G. Phycocyanin from Spirulina: A Review of Extraction Methods and Stability. Food Res. Int. 2021, 143, 110314. [Google Scholar] [CrossRef] [PubMed]
- Seghiri, R.; Legrand, J.; Hsissou, R.; Essamri, A. Comparative Study of the Impact of Conventional and Unconventional Drying Processes on Phycobiliproteins from Arthrospira Platensis. Algal Res. 2021, 53, 102165. [Google Scholar] [CrossRef]
- Mehariya, S.; Goswami, R.K.; Verma, P.; Lavecchia, R.; Zuorro, A. Integrated Approach for Wastewater Treatment and Biofuel Production in Microalgae Biorefineries. Energies 2021, 14, 2282. [Google Scholar] [CrossRef]
- Rani, A.; Saini, K.C.; Bast, F.; Mehariya, S.; Bhatia, S.K.; Lavecchia, R.; Zuorro, A. Microorganisms: A Potential Source of Bioactive Molecules for Antioxidant Applications. Molecules 2021, 26, 1142. [Google Scholar] [CrossRef] [PubMed]
- Mehariya, S.; Fratini, F.; Lavecchia, R.; Zuorro, A. Green Extraction of Value-Added Compounds Form Microalgae: A Short Review on Natural Deep Eutectic Solvents (NaDES) and Related Pre-Treatments. J. Environ. Chem. Eng. 2021, 9, 105989. [Google Scholar] [CrossRef]
- Zuorro, A.; García-Martínez, J.B.; Barajas-Solano, A.F. The Application of Catalytic Processes on the Production of Algae-Based Biofuels: A Review. Catalysts 2021, 11, 22. [Google Scholar] [CrossRef]
- Galetović, A.; Seura, F.; Gallardo, V.; Graves, R.; Cortés, J.; Valdivia, C.; Núñez, J.; Tapia, C.; Neira, I.; Sanzana, S.; et al. Use of Phycobiliproteins from Atacama Cyanobacteria as Food Colorants in a Dairy Beverage Prototype. Foods 2020, 9, 244. [Google Scholar] [CrossRef] [Green Version]
- Chittapun, S.; Jonjaroen, V.; Khumrangsee, K.; Charoenrat, T. C-Phycocyanin Extraction from Two Freshwater Cyanobacteria by Freeze Thaw and Pulsed Electric Field Techniques to Improve Extraction Efficiency and Purity. Algal Res. 2020, 46, 101789. [Google Scholar] [CrossRef]
- Ayekpam, C.; Hamsavi, G.K.; Raghavarao, K.S.M.S. Efficient Extraction of Food Grade Natural Blue Colorant from Dry Biomass of Spirulina Platensis Using Eco-Friendly Methods. Food Bioprod. Process. 2021, 129, 84–93. [Google Scholar] [CrossRef]
- Ferreira-Santos, P.; Nunes, R.; De Biasio, F.; Spigno, G.; Gorgoglione, D.; Teixeira, J.A.; Rocha, C.M.R. Influence of Thermal and Electrical Effects of Ohmic Heating on C-Phycocyanin Properties and Biocompounds Recovery from Spirulina Platensis. LWT 2020, 128, 109491. [Google Scholar] [CrossRef]
- Sintra, T.E.; Bagagem, S.S.; Ghazizadeh Ahsaie, F.; Fernandes, A.; Martins, M.; Macário, I.P.E.; Pereira, J.L.; Gonçalves, F.J.M.; Pazuki, G.; Coutinho, J.A.P.; et al. Sequential Recovery of C-Phycocyanin and Chlorophylls from Anabaena Cylindrica. Sep. Purif. Technol. 2021, 255, 117538. [Google Scholar] [CrossRef]
- Tavanandi, H.A.; Raghavarao, K.S.M.S. Ultrasound-Assisted Enzymatic Extraction of Natural Food Colorant C-Phycocyanin from Dry Biomass of Arthrospira Platensis. LWT 2020, 118, 108802. [Google Scholar] [CrossRef]
- Sekar, S.; Chandramohan, M. Phycobiliproteins as a Commodity: Trends in Applied Research, Patents and Commercialization. J. Appl. Phycol. 2008, 20, 113–136. [Google Scholar] [CrossRef]
- İlter, I.; Akyıl, S.; Demirel, Z.; Koç, M.; Conk-Dalay, M.; Kaymak-Ertekin, F. Optimization of Phycocyanin Extraction from Spirulina Platensis Using Different Techniques. J. Food Compos. Anal. 2018, 70, 78–88. [Google Scholar] [CrossRef]
- Khandual, S.; Sanchez, E.O.L.; Andrews, H.E.; de la Rosa, J.D.P. Phycocyanin Content and Nutritional Profile of Arthrospira Platensis from Mexico: Efficient Extraction Process and Stability Evaluation of Phycocyanin. BMC Chem. 2021, 15, 24. [Google Scholar] [CrossRef]
- Vega-Gálvez, A.; Miranda, M.; Clavería, R.; Quispe, I.; Vergara, J.; Uribe, E.; Paez, H.; Di Scala, K. Effect of Air Temperature on Drying Kinetics and Quality Characteristics of Osmo-Treated Jumbo Squid (Dosidicus Gigas). LWT Food Sci. Technol. 2011, 44, 16–23. [Google Scholar] [CrossRef]
- Yoshida, C.; Murakami, M.; Niwa, A.; Takeya, M.; Osanai, T. Efficient Extraction and Preservation of Thermotolerant Phycocyanins from Red Alga Cyanidioschyzon Merolae. J. Biosci. Bioeng. 2021, 131, 161–167. [Google Scholar] [CrossRef]
- Tavanandi, H.A.; Mittal, R.; Chandrasekhar, J.; Raghavarao, K.S.M.S. Simple and Efficient Method for Extraction of C-Phycocyanin from Dry Biomass of Arthospira Platensis. Algal Res. 2018, 31, 239–251. [Google Scholar] [CrossRef]
- Devi, A.C.; Tavanandi, H.A.; Govindaraju, K.; Raghavarao, K.S.M.S. An Effective Method for Extraction of High Purity Phycocyanins (C-PC and A-PC) from Dry Biomass of Arthrospira Maxima. J. Appl. Phycol. 2020, 32, 1141–1151. [Google Scholar] [CrossRef]
- Carullo, D.; Pataro, G.; Donsì, F.; Ferrari, G. Pulsed Electric Fields-Assisted Extraction of Valuable Compounds From Arthrospira Platensis: Effect of Pulse Polarity and Mild Heating. Front. Bioeng. Biotechnol. 2020, 8, 551272. [Google Scholar] [CrossRef]
- Pereira, T.; Barroso, S.; Mendes, S.; Amaral, R.A.; Dias, J.R.; Baptista, T.; Saraiva, J.A.; Alves, N.M.; Gil, M.M. Optimization of Phycobiliprotein Pigments Extraction from Red Algae Gracilaria Gracilis for Substitution of Synthetic Food Colorants. Food Chem. 2020, 321, 126688. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Bhunia, B.; Mondal, A.; Kanti Bandyopadhyay, T.; Devi, I.; Oinam, G.; Prasanna, R.; Abraham, G.; Nath Tiwari, O. Statistical Optimization of Process Parameters for Improvement of Phycobiliproteins (PBPs) Yield Using Ultrasound-Assisted Extraction and Its Kinetic Study. Ultrason. Sonochem. 2020, 60, 104762. [Google Scholar] [CrossRef] [PubMed]
- Sommer, M.C.; Balazinski, M.; Rataj, R.; Wenske, S.; Kolb, J.F.; Zocher, K. Assessment of Phycocyanin Extraction from Cyanidium Caldarium by Spark Discharges, Compared to Freeze-thaw Cycles, Sonication and Pulsed Electric Fields. Microorganisms 2021, 9, 1452. [Google Scholar] [CrossRef]
- Falkeborg, M.F.; Roda-Serrat, M.C.; Burnæs, K.L.; Nielsen, A.L.D. Stabilising Phycocyanin by Anionic Micelles. Food Chem. 2018, 239, 771–780. [Google Scholar] [CrossRef]
- Fratelli, C.; Burck, M.; Amarante, M.C.A.; Braga, A.R.C. Antioxidant Potential of Nature’s “Something Blue”: Something New in the Marriage of Biological Activity and Extraction Methods Applied to C-Phycocyanin. Trends Food Sci. Technol. 2021, 107, 309–323. [Google Scholar] [CrossRef]
- Pott, R.W.M. The Release of the Blue Biological Pigment C-Phycocyanin through Calcium-Aided Cytolysis of Live Spirulina sp. Color Technol. 2019, 135, 17–21. [Google Scholar] [CrossRef] [Green Version]
- Andersen, R.A.; Berges, J.A.; Harrison, P.J.; Watanabe, M.M. Appendix A—Recipes for Freshwater and Seawater Media. In Algal Culturing Techniques; Andersen, R.A., Ed.; Elsevier Academic Press: Burlington, MA, USA, 2005; pp. 429–538. [Google Scholar]
- Zuorro, A.; Leal-Jerez, A.G.; Morales-Rivas, L.K.; Mogollón-Londoño, S.O.; Sanchez-Galvis, E.M.; García-Martínez, J.B.; Barajas-Solano, A.F. Enhancement of Phycobiliprotein Accumulation in Thermotolerant Oscillatoria sp. through Media Optimization. ACS Omega 2021, 6, 10527–10536. [Google Scholar] [CrossRef]
- Bennett, A.; Bogorad, L. Complementary Chromatic Adaptation in a Filamentous Blue-Green Alga. J. Cell Biol. 1973, 58, 419–435. [Google Scholar] [CrossRef]
- Patil, G.; Chethana, S.; Sridevi, A.S.; Raghavarao, K.S.M.S. Method to Obtain C-Phycocyanin of High Purity. J. Chromatogr. A 2006, 1127, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Antelo, F.; Anschau, A.; Costa, J.; Kalil, S. Extraction and Purification of C-Phycocyanin from Spirulina Platensis in Conventional and Integrated Aqueous Two-Phase Systems. J. Braz. Chem. Soc. 2010, 21, 921–926. [Google Scholar] [CrossRef] [Green Version]
- Zuorro, A. Optimization of Polyphenol Recovery from Espresso Coffee Residues Using Factorial Design and Response Surface Methodology. Sep. Purif. Technol. 2015, 152, 64–69. [Google Scholar] [CrossRef]
- Sarasini, F.; Tirillò, J.; Zuorro, A.; Maffei, G.; Lavecchia, R.; Puglia, D.; Dominici, F.; Luzi, F.; Valente, T.; Torre, L. Recycling Coffee Silverskin in Sustainable Composites Based on a Poly(Butylene Adipate-Co-Terephthalate)/Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) Matrix. Ind. Crops Prod. 2018, 118, 311–320. [Google Scholar] [CrossRef]
- Zuorro, A.; Malavasi, V.; Cao, G.; Lavecchia, R. Use of Cell Wall Degrading Enzymes to Improve the Recovery of Lipids from Chlorella Sorokiniana. Chem. Eng. J. 2019, 377, 120325. [Google Scholar] [CrossRef]
- Sanchez-Galvis, E.M.; Cardenas-Gutierrez, I.Y.; Contreras-Ropero, J.E.; García-Martínez, J.B.; Barajas-Solano, A.F.; Zuorro, A. An Innovative Low-Cost Equipment for Electro-Concentration of Microalgal Biomass. Appl. Sci. 2020, 10, 4841. [Google Scholar] [CrossRef]
- Zuorro, A. Enhanced Lycopene Extraction from Tomato Peels by Optimized Mixed-Polarity Solvent Mixtures. Molecules 2020, 25, 2038. [Google Scholar] [CrossRef] [PubMed]
- Zuorro, A.; Lavecchia, R. Polyphenols and Energy Recovery from Spent Coffee Grounds. Chem. Eng. Trans. 2011, 25, 285–290. [Google Scholar] [CrossRef]
- Zuorro, A.; Iannone, A.; Natali, S.; Lavecchia, R. Green Synthesis of Silver Nanoparticles Using Bilberry and Red Currant Waste Extracts. Processes 2019, 7, 193. [Google Scholar] [CrossRef] [Green Version]
- Montanaro, D.; Lavecchia, R.; Petrucci, E.; Zuorro, A. UV-Assisted Electrochemical Degradation of Coumarin on Boron-Doped Diamond Electrodes. Chem. Eng. J. 2017, 323, 512–519. [Google Scholar] [CrossRef]
- Zuorro, A.; Maffei, G.; Lavecchia, R. Kinetic Modeling of Azo Dye Adsorption on Non-Living Cells of Nannochloropsis Oceanica. J. Environ. Chem. Eng. 2017, 5, 4121–4127. [Google Scholar] [CrossRef]
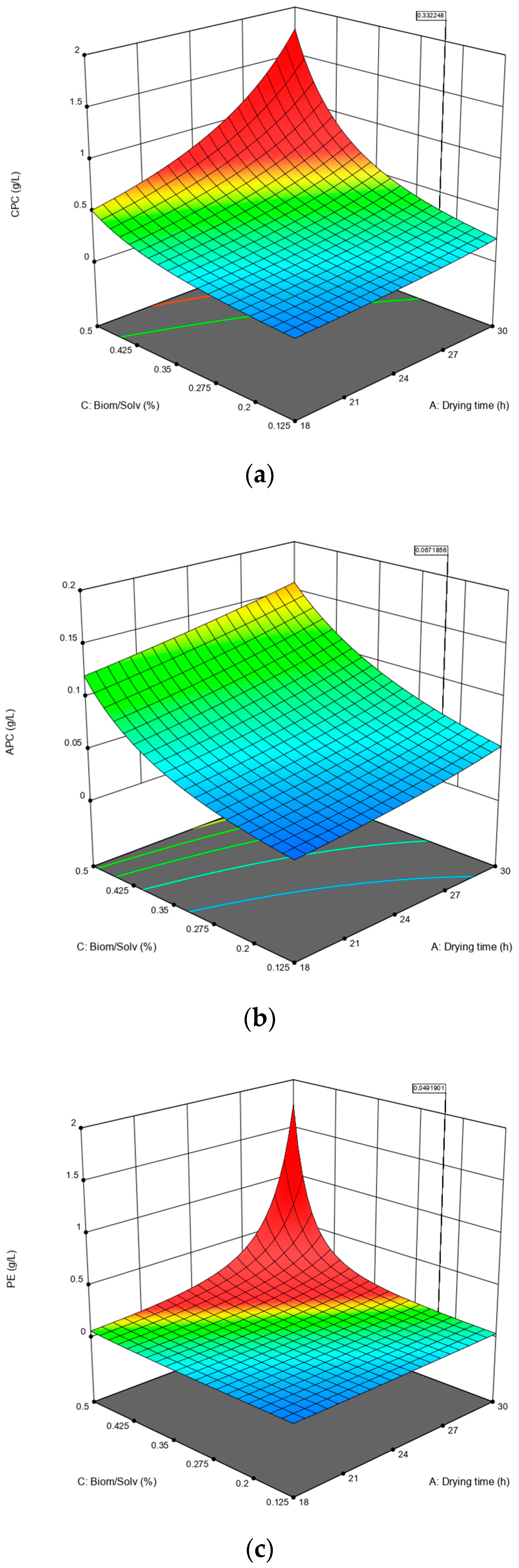

| Variables | Coded Name | Low Level (−1) | High Level (+1) |
|---|---|---|---|
| Drying time (h) | A | 18 | 30 |
| Drying temperature (°C) | B | 40 | 60 |
| Biomass/solvent ratio (%) | C | 0.125 | 0.5 |
| Glass beads/biomass ratio (%) | D | 5 | 15 |
| Extraction time (min) | E | 10 | 30 |
| Extraction speed (rpm) | F | 1000 | 1500 |
| Sum of Squares | Df | Mean Square | F-Value | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| C-PC (g/L) | Model | 47.83 | 27 | 1.77 | 85.78 | <0.0001 * | ||
| Curvature | 1.48 | 1 | 1.48 | 71.73 | <0.0001 * | |||
| Residual | 0.1859 | 9 | 0.0207 | |||||
| Lack of Fit | 0.0956 | 4 | 0.0239 | 1.32 | 0.3760 ** | |||
| Pure Error | 0.0903 | 5 | 0.0181 | |||||
| Cor Total | 49.49 | 37 | ||||||
| APC (g/L) | Model | 92.28 | 23 | 4.01 | 13.38 | <0.0001 * | ||
| Curvature | 1.21 | 1 | 1.21 | 4.03 | 0.0658 | |||
| Residual | 3.90 | 13 | 0.2997 | |||||
| Lack of Fit | 1.51 | 8 | 0.1886 | 0.3948 | 0.8834 ** | |||
| Pure Error | 2.39 | 5 | 0.4776 | |||||
| Cor Total | 97.38 | 37 | ||||||
| PE (g/L) | Model | 302.89 | 11 | 27.54 | 18.54 | <0.0001 * | ||
| Curvature | 16.17 | 1 | 16.17 | 10.89 | 0.0029 | |||
| Residual | 37.14 | 25 | 1.49 | |||||
| Lack of Fit | 34.80 | 20 | 1.74 | 3.73 | 0.0748 ** | |||
| Pure Error | 2.33 | 5 | 0.4666 | |||||
| Cor Total | 356.20 | 37 | ||||||
| R² | Adj R² | Pred R² | Adq Pr | Std. Dev. | Mean | C.V. % | ||
| C-PC g/L | 0.9961 | 0.9845 | 0.8699 | 355.217 | 0.1437 | 2.72 | 5.28 | |
| APC g/L | 0.9595 | 0.8878 | 0.7133 | 135.805 | 0.5475 | 4.81 | 11.39 | |
| PE g/L | 0.8908 | 0.8427 | 0.7281 | 169.951 | 1.22 | 7.56 | 16.11 | |
| Sum of Squares | df | Mean Square | F-Value | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| C-PC Purity | Model | 0.5172 | 29 | 0.0178 | 16.17 | 0.0004 * | ||
| Curvature | 0.0237 | 1 | 0.0237 | 21.50 | 0.0024 | |||
| Residual | 0.0077 | 7 | 0.0011 | |||||
| Lack of Fit | 0.0003 | 2 | 0.0001 | 0.0914 | 0.9141 ** | |||
| Pure Error | 0.0074 | 5 | 0.0015 | |||||
| Cor Total | 0.5486 | 37 | ||||||
| APC Purity | Model | 0.2874 | 26 | 0.0111 | 4.16 | 0.0113 * | ||
| Curvature | 0.0046 | 1 | 0.0046 | 1.73 | 0.2172 | |||
| Residual | 0.0266 | 10 | 0.0027 | |||||
| Lack of Fit | 0.0015 | 5 | 0.0003 | 0.0599 | 0.9961 ** | |||
| Pure Error | 0.0251 | 5 | 0.0050 | |||||
| Cor Total | 0.3187 | 37 | ||||||
| PE Purity | Model | 1.15 | 21 | 0.0550 | 11.24 | <0.0001 * | ||
| Curvature | 0.1099 | 1 | 0.1099 | 22.47 | 0.0003 | |||
| Residual | 0.0734 | 15 | 0.0049 | |||||
| Lack of Fit | 0.0333 | 10 | 0.0033 | 0.4152 | 0.8890 ** | |||
| Pure Error | 0.0401 | 5 | 0.0080 | |||||
| Cor Total | 1.34 | 37 | ||||||
| R² | Adj R² | Pred R² | Adq Pr | Std. Dev. | Mean | C.V. % | ||
| C-PC Purity | 0.9853 | 0.9244 | 0.8468 | 236.165 | 0.0332 | 0.0921 | 36.04 | |
| APC Purity | 0.9153 | 0.6951 | 0.6887 | 115.801 | 0.0516 | 0.3667 | 14.06 | |
| PE Purity | 0.9402 | 0.8565 | 0.6753 | 176.953 | 0.0699 | 1.47 | 4.77 | |
| Coded Name | Variable | Units | Value |
|---|---|---|---|
| A | Drying time | h | 30 |
| B | Drying temperature | °C | 40 |
| C | Biomass/Solvent ratio | % w/v | 0.26 |
| D | Glass beads/Biomass ratio | % w/v | 14.9 |
| E | Extraction time | min | 30 |
| F | Extraction speed | rpm | 1486 |
| Z1 | C-PC | g/L | 0.38 |
| Z2 | APC | 0.073 | |
| Z3 | PE | 0.064 | |
| Z4 | C-PC | Purity Index | 1.56 |
| Z5 | APC | 0.64 | |
| Z6 | PE | 0.87 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barajas-Solano, A.F. Optimization of Phycobiliprotein Solubilization from a Thermotolerant Oscillatoria sp. Processes 2022, 10, 836. https://doi.org/10.3390/pr10050836
Barajas-Solano AF. Optimization of Phycobiliprotein Solubilization from a Thermotolerant Oscillatoria sp. Processes. 2022; 10(5):836. https://doi.org/10.3390/pr10050836
Chicago/Turabian StyleBarajas-Solano, Andrés F. 2022. "Optimization of Phycobiliprotein Solubilization from a Thermotolerant Oscillatoria sp." Processes 10, no. 5: 836. https://doi.org/10.3390/pr10050836
APA StyleBarajas-Solano, A. F. (2022). Optimization of Phycobiliprotein Solubilization from a Thermotolerant Oscillatoria sp. Processes, 10(5), 836. https://doi.org/10.3390/pr10050836






