The Impact of Berberine Pharmaceutical Wastewater on Aerobic Granules Formation: Change of Granules’ Size
Abstract
:1. Introduction
2. Materials and Methods
2.1. Inoculum
2.2. Experiment Design and Set-Up
2.3. Medium
2.4. Analytical Methods
3. Results
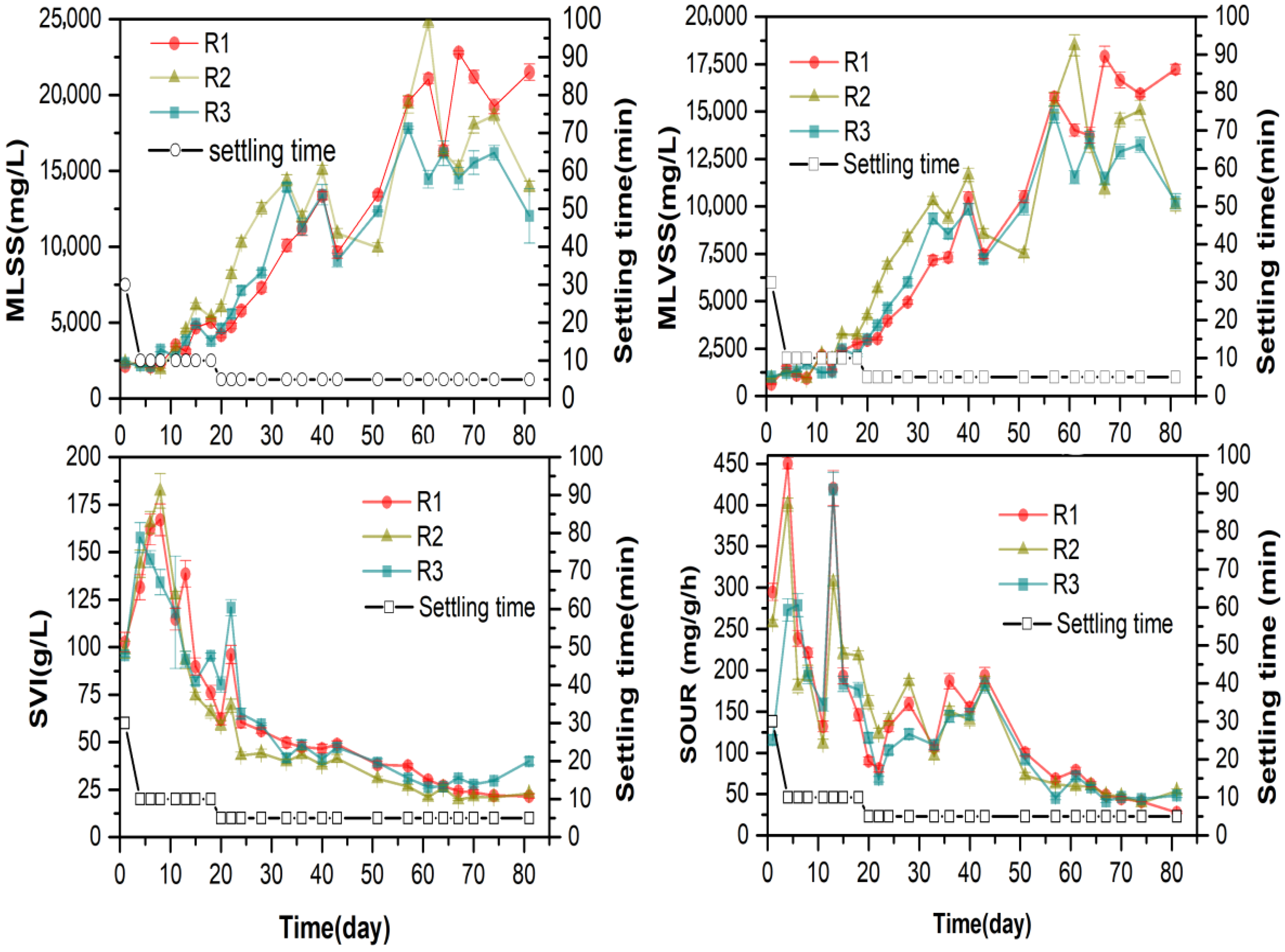
3.1. Biological Treatment Performance during Aerobic Granulation in R1, R2, and R3
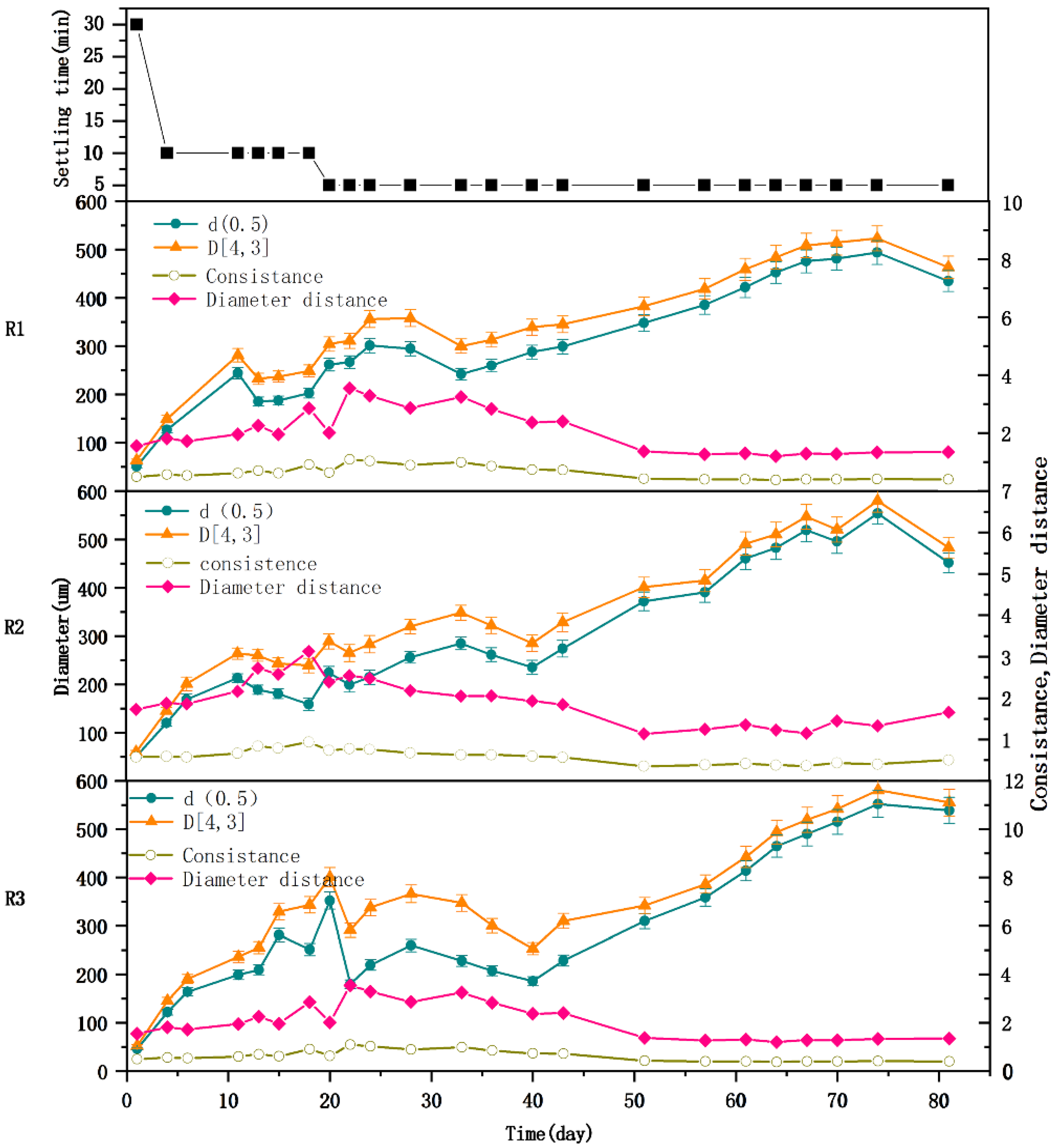
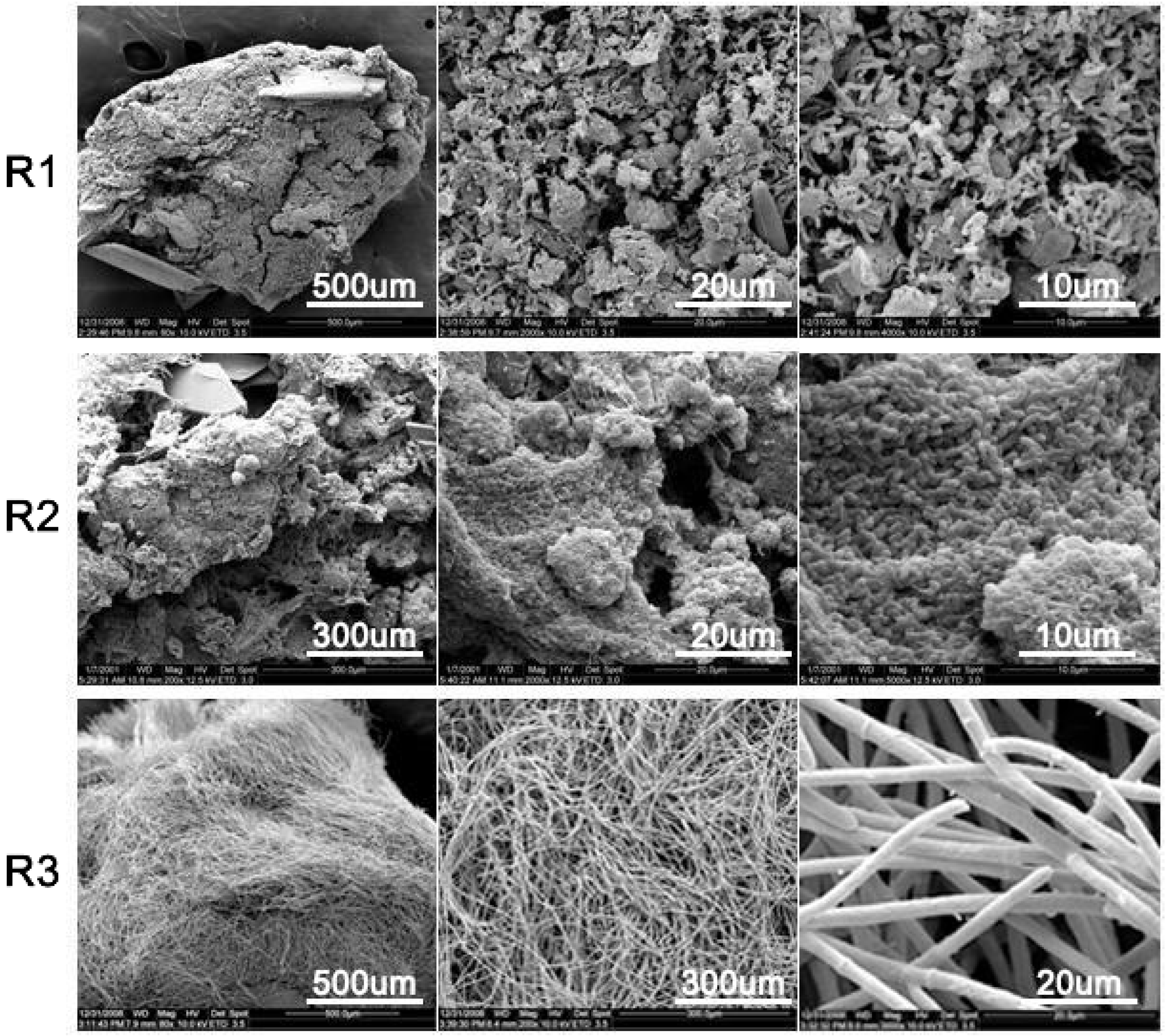
3.2. Effects of Berberine Wastewater on the Morphology of Aerobic Granules
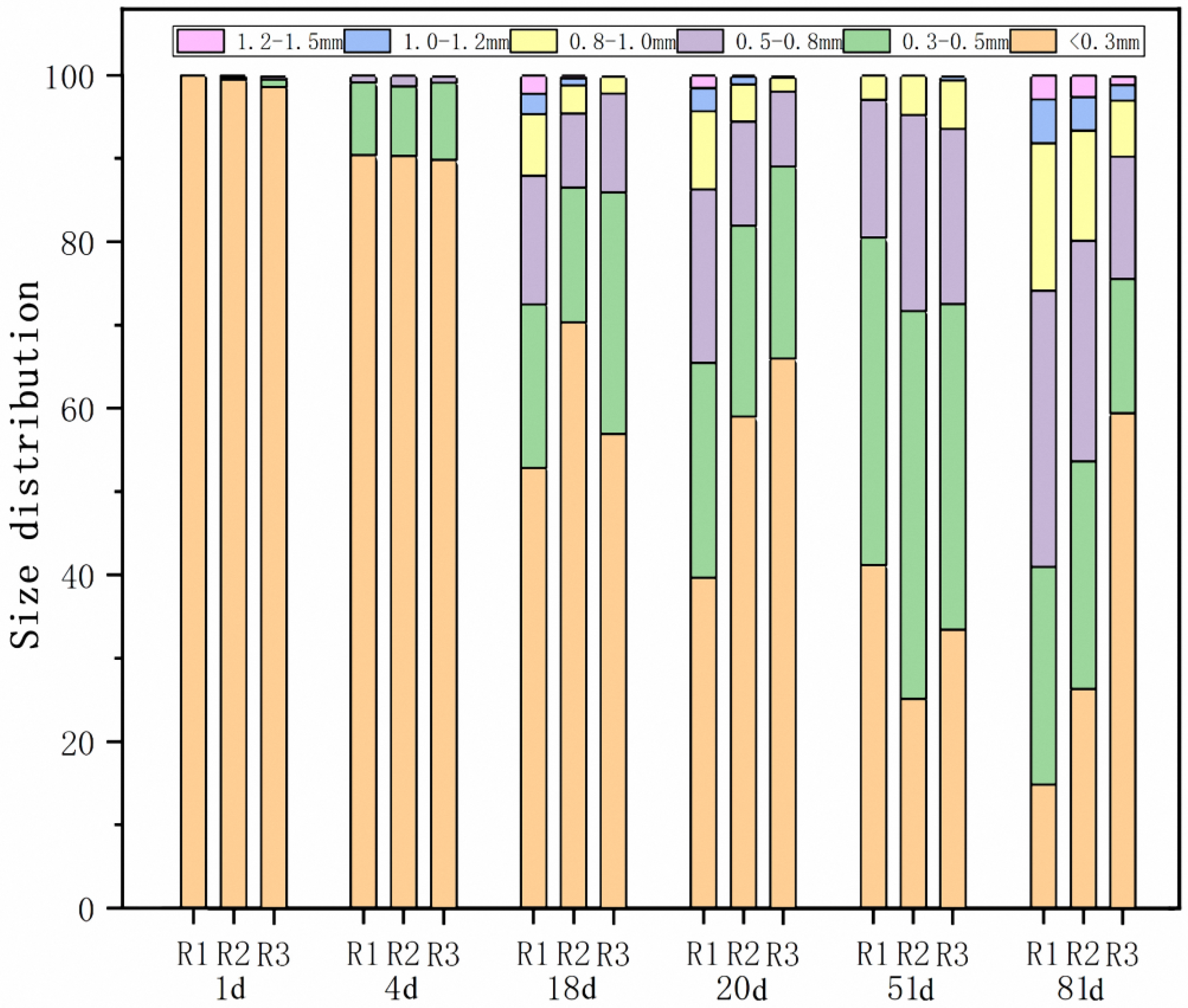
3.3. The Size Distribution of R1, R2, and R3
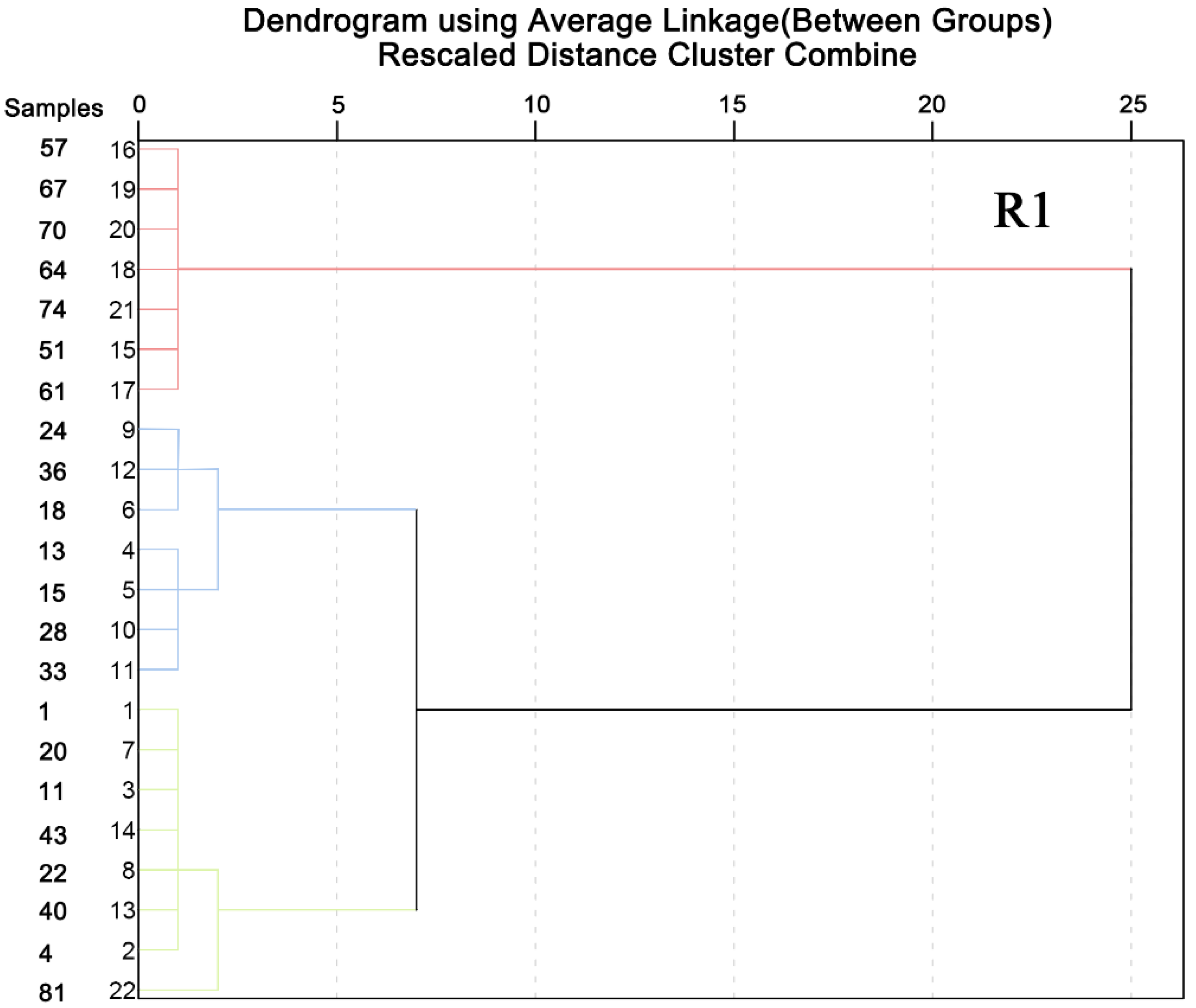
3.4. The Change in the Shape Observation of Granules
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Beun, J.J.; van Loosdrecht, M.C.M.; Heijnen, J.J. Aerobic granulation in a sequencing batch airlift reactor. Water Res. 2002, 36, 702–712. [Google Scholar] [CrossRef]
- Barr, J.J.; Cook, A.E.; Bond, P.L. Granule formation mechanisms within an aerobic wastewater system for phosphorus removal. Appl. Environ. Microb. 2010, 76, 7588–7597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tay, S.T.L.; Zhuang, W.Q.; Tay, J.H. Start-up, microbial community analysis and formation of aerobic granules in a tert-butyl alcohol degrading sequencing batch reactor. Environ. Sci. Technol. 2005, 39, 5774–5780. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, N.; Ujang, Z.; Yahya, A. Aerobic granular sludge formation for high strength agro-based wastewater treatment. Bioresour. Technol. 2011, 102, 6778–6781. [Google Scholar] [CrossRef]
- Beun, J.J.; Hendrik, S.A. Aerobic granulation in a sequencing batch reactor. Water Res. 1999, 33, 2283–2290. [Google Scholar] [CrossRef]
- Sarma, S.J.; Tay, J.H. Aerobic granulation for future wastewater treatment technology: Challenges ahead. Environ. Sci. Water Res. Technol. 2018, 4, 9–15. [Google Scholar] [CrossRef]
- Zhang, Y.; Tay, J.H. Toxic and inhibitory effects of trichloroethylene aerobic co-metabolism on phenol-grown aerobic granules. J. Hazard Mater. 2015, 286, 204–210. [Google Scholar] [CrossRef]
- Adav, S.S.; Lee, D.J.; Ren, N.Q. Degradation of phenol by Acinetobacter strain isolated from aerobic granules. Water Res. 2007, 41, 2903–2910. [Google Scholar] [CrossRef]
- Basheer, F.; Farooqi, H.J. Biodegradation of p-cresol by aerobic granules in sequencing batch reactor. J. Environ. Sci. 2012, 24, 2012–2018. [Google Scholar] [CrossRef]
- Moghaddam, S.S.; Moghaddam, M.R.A. Aerobic granular sludge for dye biodegradation in a sequencing batch reactor with anaerobic/aerobic cycles. Clean-Soil Air Water 2016, 44, 438–443. [Google Scholar] [CrossRef]
- Arrojo, B.; Mosquera-Corral, A.; Garrido, J.; Mendez, M.R. Aerobic granulation with industrial wastewater in sequencing batch reactors. Water Res. 2004, 38, 3389–3399. [Google Scholar] [CrossRef] [PubMed]
- Awang, N.A.; Shaaban, M.G. Effect of reactor height/diameter ratio and organic loading rate on formation of aerobic granular sludge in sewage treatment. Int. Biodeter. Biodegr. 2016, 112, 1–11. [Google Scholar] [CrossRef]
- Hu, L.; Wang, J.; Wen, X.; Yi, Q. Characteristic of the strain of simultaneous denitrification bacteria aerobic granule sludge in SBAR. Process Biochem. 2005, 40, 5–11. [Google Scholar]
- Tsuneda, S.; Ejiri, Y.; Naganom, T.; Hirata, A. Formation mechanism of nitrifying granules observed in an aerobic upflow fluidized bed (AUFB) reactor. Water Sci. Technol. 2004, 49, 27–34. [Google Scholar] [CrossRef]
- Liu, Y.; Tay, J.H. State of the art of biogranulation technology for wastewater treatment. Biotechnol. Adv. 2005, 22, 533–563. [Google Scholar] [CrossRef]
- Wu, K.; Wu, P.; Xu, Y.; Li, Y.; Shen, Y. Formation mechanism of aerobic granular sludge and removal efficiencies in integrated ABR-CSTR reactor. Huanjing Kexue 2015, 36, 2947–2953. [Google Scholar]
- Berberine. Available online: https://www.sciencedirect.com/topics/neuroscience/berberine (accessed on 19 February 2022).
- Cui, X.; Zeng, P.; Qiu, G.L.; Liu, Y.; Song, Y.H.; Xie, X.; Han, L. Pilot-scale treatment of pharmaceutical berberine wastewater by Fenton oxidation. Environ. Earth. Sci. 2015, 73, 4967–4977. [Google Scholar] [CrossRef]
- Qin, W.W.; Song, Y.H.; Dai, Y.; Qiu, G.L.; Ren, M.; Zeng, P. Treatment of berberine hydrochloride pharmaceutical wastewater by O3/UV/H2O2 advanced oxidation process. Environ. Earth Sci. 2015, 73, 4939–4946. [Google Scholar] [CrossRef]
- Li, Y.; Yin, Y.M.; Wang, X.Y.; Wu, H.; Ge, X.Z. Evaluation of berberine as a natural fungicide: Biodegradation and antimicrobial mechanism. J. Asian Nat. Prod. Res. 2017, 20, 148–162. [Google Scholar] [CrossRef]
- Othman, M.S.; Safwat, G.; Aboulkhair, M.; Abdel Moneim, A.E. The potential effect of berberine in mercury-induced hepatorenal toxicity in albino rats. Food Chem. Toxicol. 2014, 69, 175–181. [Google Scholar] [CrossRef]
- Qiu, G.L. Hybrid UASB-MBR Process for Berberine Pharmaceutical Wastewater Treatment and the Process Mechanisms. Ph.D. Thesis, Beijing Normal University, Beijing, China, 2011. [Google Scholar]
- Qiu, G.; Song, Y.H.; Zeng, P.; Duan, L.; Xiao, S.H. Characterization of bacterial communities in hybrid upflow anaerobic sludge blanket (UASB)-membrane bioreactor (MBR) process for berberine antibiotic wastewater treatment. Bioresour. Technol. 2013, 142, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA, 2005.
- Zeng, P.; Moy, B.; Song, Y.H.; Tay, J.W. Biodegradation of dimethyl phthalate by Sphingomonas sp. isolated from phthalic-acid-degrading aerobic granules. Appl. Microbiol. Biotechnol. 2008, 80, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Keerio, H.A.; Bae, W. Experimental investigation of substrate shock and environmental ammonium concentration on the stability of ammonia-oxidizing Bacteria (AOB). Water 2020, 12, 223. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Zhang, Y.; Zeng, P.; Wang, H.; Song, Y.; Li, J. Isolation and characterization of a bacterial strain capable of efficient berberine degradation. Int. J. Environ. Res. Public Health 2019, 16, 646. [Google Scholar] [CrossRef] [Green Version]
- Tay, S.T.L.; Moy, B.Y.P.; Jiang, H.L.; Tay, J.H. Rapid cultivation of stable aerobic phenol-degrading granules using acetate-fed granules as microbial seed. J. Biotechnol. 2005, 115, 387–395. [Google Scholar] [CrossRef]
- Verawaty, M.; Tait, S.; Pijuan, M.; Yuan, Z.G.; Bond, P.L. Breakage and growth towards a stable aerobic granule size during the treatment of wastewater. Water Res. 2013, 47, 5338–5349. [Google Scholar] [CrossRef]
- Shi, R.Q.; Pan, A.D. The application of Mastersizer 2000 in the environmental evolution. J. Heze Univ. 2005, 27, 56–61. [Google Scholar]
- .Shu, X.; Wu, Y.C.; Tao, Q.X.; Cheng, J.G.; Xia, Y.H. An analysis on report of Mastersizer 2000 laser particle size analyzer. Exp. Technol. Manag. 2011, 28, 37–41. [Google Scholar]
- Pronk, M.; Abbas, B.; Al-zuhairy, S.H.K.; Kraan, R.; Kleerebezem, R.; van Loosdrecht, M.C.M. Effect and behaviour of different substrates in relation to the formation of aerobic granular sludge. Appl. Microbiol. Biotechnol. 2015, 99, 5257–5268. [Google Scholar] [CrossRef] [Green Version]
- Tay, J.H.; Pan, S.; Tay, S.T.L.; Ivanov, V.; Liu, Y. The effect of organic loading rate on the aerobic granulation: The development of shear force theory. Water Sci. Technol. 2003, 47, 235–240. [Google Scholar] [CrossRef]
- Moy, B.Y.P.; Tay, J.H.; Toh, S.K.; Liu, Y.; Tay, S.T.L. High organic loading influences the physical characteristics of aerobic sludge granules. Lett. Appl. Microbiol. 2002, 34, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tay, J.H. Co-metabolic degradation activities of trichloroethylene by phenol-grown aerobic granules. J. Biotechnol. 2012, 162, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Franca, R.D.G.; Ortigueira, J.; Pinheiro, H.M.; Lourenco, N.D. Effect of SBR feeding strategy and feed composition on the stability of aerobic granular sludge in the treatment of a simulated textile wastewater. Water Sci. Technol. 2017, 76, 1188–1195. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.L.; Tay, J.H.; Tay, S.T.L. Changes in structure, activity and metabolism of aerobic granules as a microbial response to high phenol loading. Appl. Microbiol. Biotechnol. 2004, 63, 602–608. [Google Scholar] [CrossRef]
- Cai, P.; Chen, Y.; Jiang, S.Y.; Wu, Z.; Xie, J.; Jiang, S. Treating high concentration pharmaceutical wastewater with aerobic granular sludge involves inoculating activated sludge in batch reactor, adding zeolite powder and pumping in pharmaceutical wastewater into batch reactor. Patent Application No. CN 105858873 B, 12 April 2016. [Google Scholar]
- Wang, S.G.; Liu, X.W.; Gong, W.X.; Gao, B.Y.; Zhang, D.H.; Yu, H.Q. Aerobic granulation with brewery wastewater in a sequencing batch reactor. Bioresour. Technol. 2007, 98, 2142–2147. [Google Scholar] [CrossRef]
- Zheng, H. Experimental and Theoretical Studies of Protoberberine Alkaloids on the Mechanism of Multiple Activities. Ph.D. Thesis, Tianjin Medical University, Tianjin, China, 2004. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, P.; Wang, Y.; Liu, Y.; Li, J.; Liu, F.; Chang, M.; Zhang, Y. The Impact of Berberine Pharmaceutical Wastewater on Aerobic Granules Formation: Change of Granules’ Size. Processes 2022, 10, 792. https://doi.org/10.3390/pr10040792
Zeng P, Wang Y, Liu Y, Li J, Liu F, Chang M, Zhang Y. The Impact of Berberine Pharmaceutical Wastewater on Aerobic Granules Formation: Change of Granules’ Size. Processes. 2022; 10(4):792. https://doi.org/10.3390/pr10040792
Chicago/Turabian StyleZeng, Ping, Yan Wang, Yongqiang Liu, Juan Li, Fenghua Liu, Ming Chang, and Yizhang Zhang. 2022. "The Impact of Berberine Pharmaceutical Wastewater on Aerobic Granules Formation: Change of Granules’ Size" Processes 10, no. 4: 792. https://doi.org/10.3390/pr10040792




