The Potential of the Oil-Producing Oleaginous Yeast Rhodotorula mucilaginosa for Sustainable Production of Bio-Oil Energy
Abstract
:1. Introduction
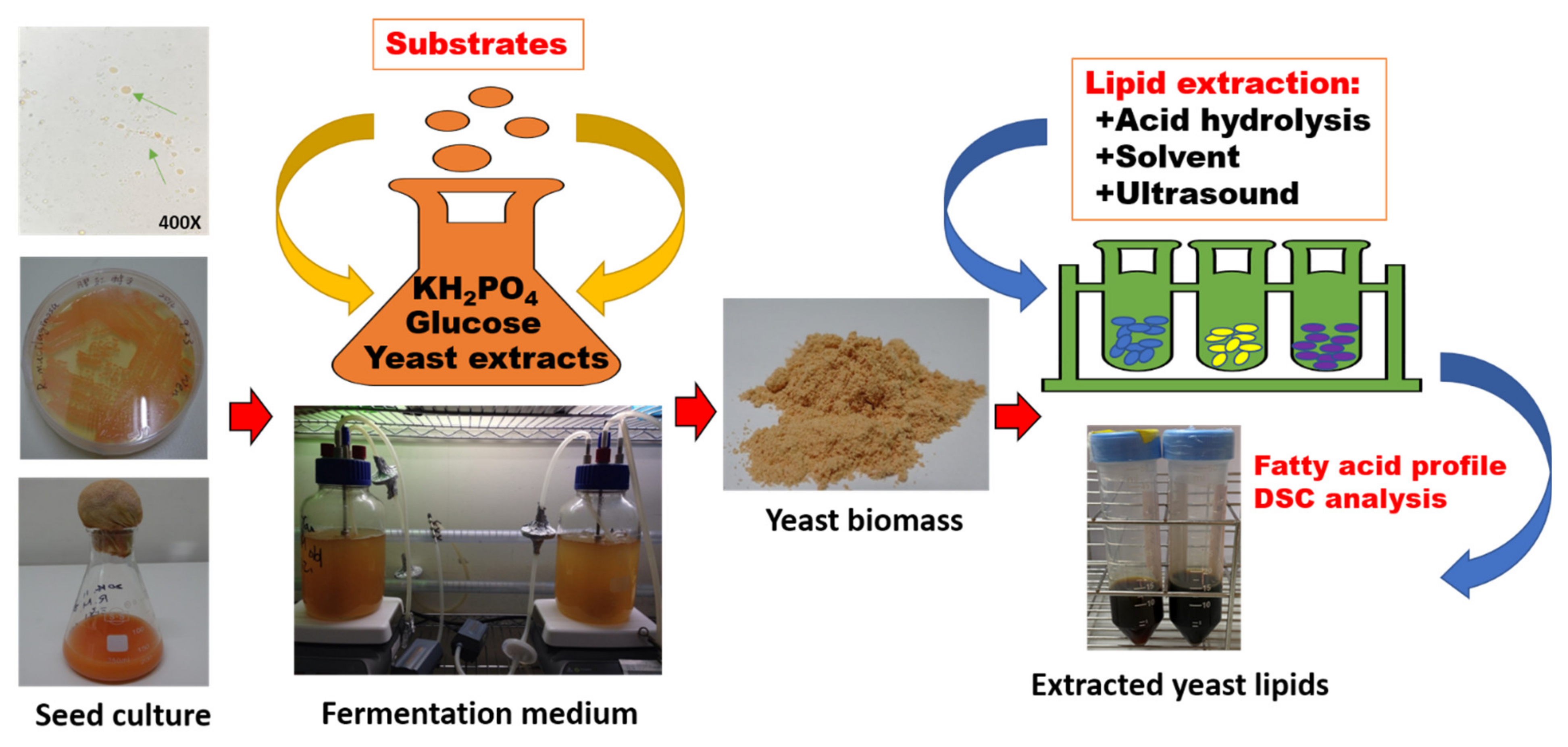
2. Materials and Methods
2.1. Strain and Growth Conditions
2.2. Suitable Liquid Culture Conditions
2.3. Biomass and Lipid Analysis
2.4. Selection of Extraction Conditions and Solvent
2.5. Fatty Acid Composition Analysis
2.6. Differential Scanning Calorimetry (DSC) Thermal Analysis
2.7. Heat of Combustion Measurement
2.8. Measurement of Isothermal 40 °C Kinematic Viscosity
2.9. Statistical Analyses
3. Results and Discussion
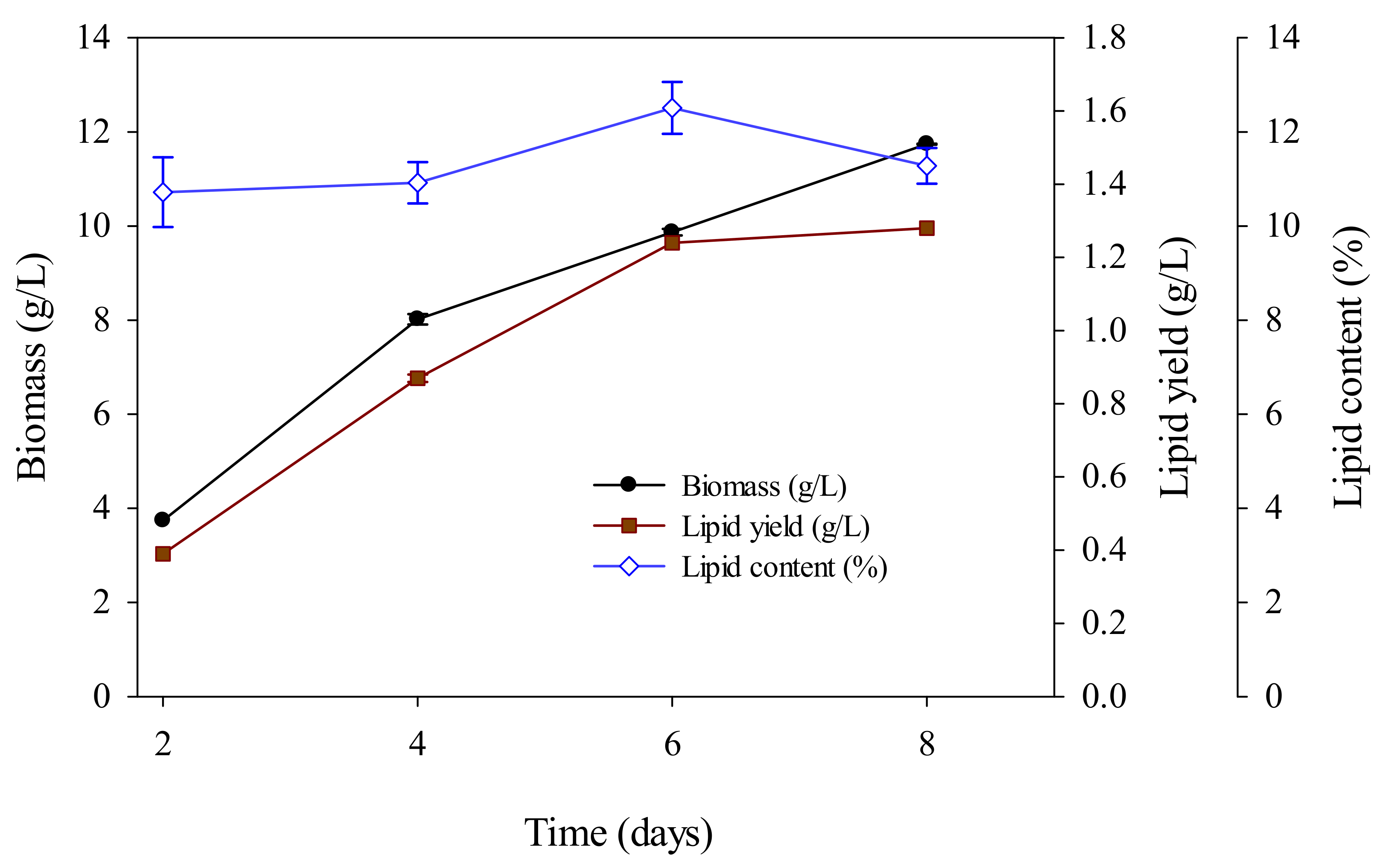
3.1. Rhodotorula mucilaginosa Culture Time and Liquid Medium Composition
3.2. Influence of Liquid Medium Composition on R. mucilaginosa Lipid Production
3.3. Influence of Different Extraction Methods on R. mucilaginosa Lipids
3.4. Analysis of Fatty Acid Composition of Yeast Lipid
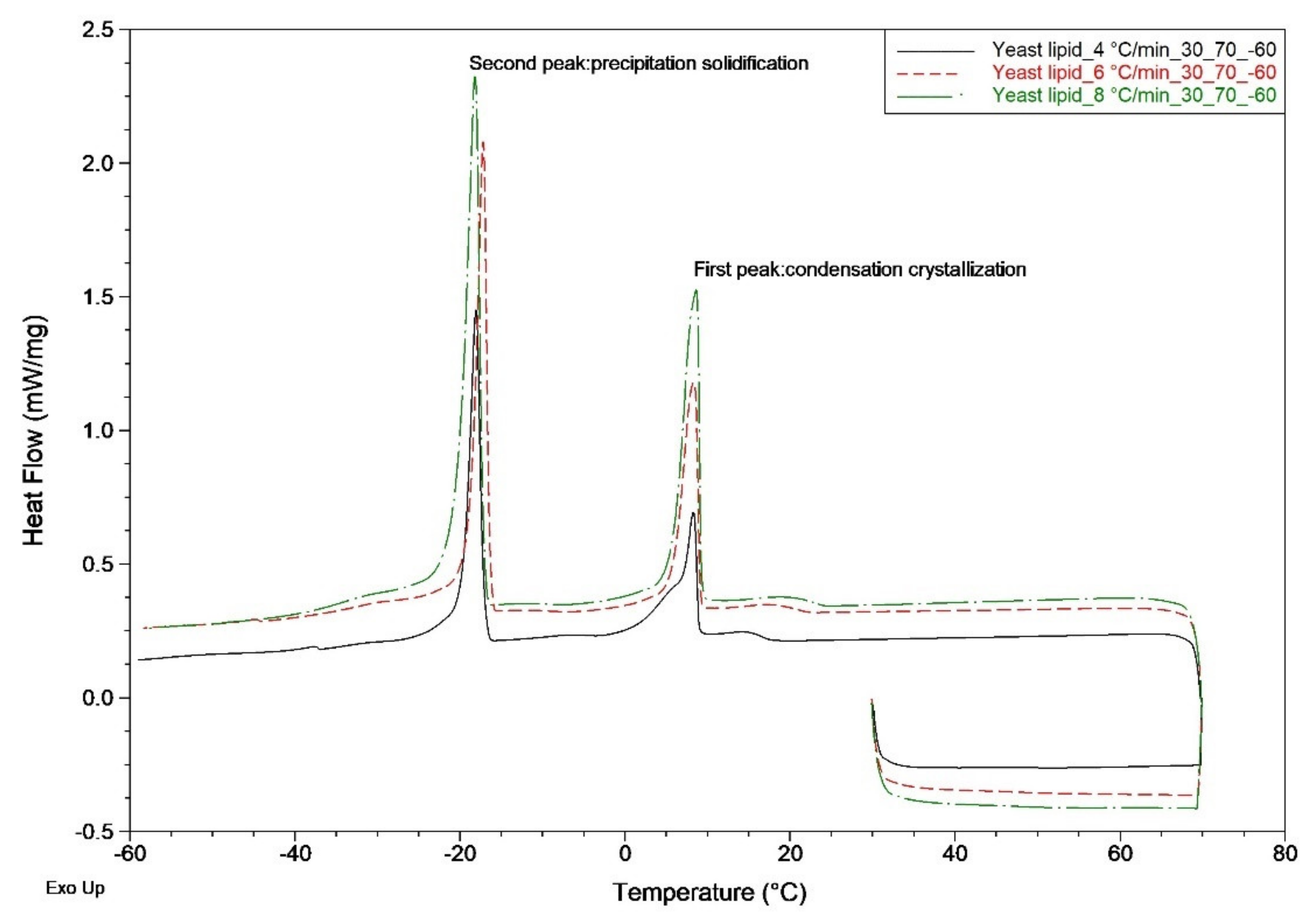
3.5. DSC Thermal Analysis of Yeast Lipid
3.6. Measurement of Yeast Lipid Calorific Value and Isothermal 40 °C Kinematic Viscosity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bandhu, S.; Bansal, N.; Dasgupta, D.; Junghare, V.; Sidana, A.; Kalyan, G.; Hazra, S.; Ghosh, D. Overproduction of single cell oil from xylose rich sugarcane bagasse hydrolysate by an engineered oleaginous yeast Rhodotorula mucilaginosa IIPL32. Fuel 2019, 254, 115653. [Google Scholar] [CrossRef]
- Li, M.; Liu, G.L.; Chi, Z.; Chi, Z.M. Single cell oil production from hydrolysate of cassava starch by marine-derived yeast Rhodotorula mucilaginosa TJY15a. Biomass Bioenergy 2010, 34, 101–107. [Google Scholar] [CrossRef]
- Kitcha, S.; Cheirsilp, B. Screening of oleaginous yeasts and optimization for lipid production using crude glycerol as a carbon source. Energy Procedia 2011, 9, 274–282. [Google Scholar] [CrossRef] [Green Version]
- Bandhu, S.; Dasgupta, D.; Akhter, J.; Kanaujia, P.; Suman, S.K.; Agrawal, D.; Kaul, S.; Adhikari, D.K.; Ghosh, D. Statistical design and optimization of single cell oil production from sugarcane bagasse hydrolysate by an oleaginous yeast Rhodotorula sp. IIP-33 using response surface methodology. SpringerPlus 2014, 3, 691. [Google Scholar] [CrossRef] [Green Version]
- Santamauro, F.; Whiffin, F.M.; Scott, R.J.; Chuck, C.J. Low-cost lipid production by an oleaginous yeast cultured in non-sterile conditions using model waste resources. Biotechnol. Biofuels 2014, 7, 34. [Google Scholar] [CrossRef] [Green Version]
- Annamalai, N.; Sivakumar, N.; Oleskowicz-Popiel, P. Enhanced production of microbial lipids from waste office paper by the oleaginous yeast Cryptococcus curvatus. Fuel 2018, 217, 420–426. [Google Scholar] [CrossRef]
- Saenge, C.; Cheirsilp, B.; Suksaroge, T.T.; Bourtoom, T. Potential use of oleaginous red yeast Rhodotorula glutinis for the bioconversion of crude glycerol from biodiesel plant to lipids and carotenoids. Process Biochem. 2011, 46, 210–218. [Google Scholar] [CrossRef]
- Saran, S.; Mathur, A.; Dalal, J.; Saxena, R.K. Process optimization for cultivation and oil accumulation in an oleaginous yeast Rhodosporidium toruloides A29. Fuel 2017, 188, 324–331. [Google Scholar] [CrossRef]
- Subramaniam, R.; Dufreche, S.; Zappi, M.; Bajpai, R. Microbial lipids from renewable resources: Production and characterization. J. Ind. Microbiol. Biotechnol. 2010, 37, 1271–1287. [Google Scholar] [CrossRef] [PubMed]
- Tkachenko, A.F.; Tigunova, O.A.; Shulga, S.M. Microbial lipids as a source of biofuel. Cytol. Genet. 2013, 47, 343–348. [Google Scholar] [CrossRef] [Green Version]
- Leiva-Candia, D.E.; Pinzi, S.; Redel-Macías, M.D.; Koutinas, A.; Webb, C.; Dorado, M.P. The potential for agro-industrial waste utilization using oleaginous yeast for the production of biodiesel. Fuel 2014, 123, 33–42. [Google Scholar] [CrossRef]
- Prabhu, A.A.; Gadela, R.; Bharali, B.; Deshavath, N.N.; Dasu, V.V. Development of high biomass and lipid yielding medium for newly isolated Rhodotorula mucilaginosa. Fuel 2019, 239, 874–885. [Google Scholar] [CrossRef]
- Pacia, M.Z.; Pukalski, J.; Turnau, K.; Baranska, M.; Kaczor, A. Lipids, hemoproteins and carotenoids in alive Rhodotorula mucilaginosa cells under pesticide decomposition–Raman imaging study. Chemosphere 2016, 164, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Anschau, A. Lipids from oleaginous yeasts: Production and encapsulation. Nutr. Deliv. 2017, 5, 749–794. [Google Scholar]
- Ageitos, J.M.; Vallejo, J.A.; Veiga-Crespo, P.; Villa, T.G. Oily yeasts as oleaginous cell factories. Appl. Microbiol. Biotechnol. 2011, 90, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Song, H.; Cao, Y.; Yang, H.; Hua, S.; Xia, C. The characteristics of biomass production, lipid accumulation and chlorophyll biosynthesis of Chlorella vulgaris under mixotrophic cultivation. Afr. J. Biotechnol. 2011, 10, 11620–11630. [Google Scholar]
- Leong, W.H.; Lim, J.W.; Lam, M.K.; Uemura, Y.; Ho, Y.C. Third generation biofuels: A nutritional perspective in enhancing microbial lipid production. Renew. Sustain. Energy Rev. 2018, 91, 950–961. [Google Scholar] [CrossRef]
- Liang, M.H.; Jiang, J.G. Advancing oleaginous microorganisms to produce lipid via metabolic engineering technology. Prog. Lipid Res. 2013, 52, 395–408. [Google Scholar] [CrossRef]
- Xing, D.; Wang, H.; Pan, A.; Wang, J.; Xue, D. Assimilation of corn fiber hydrolysates and lipid accumulation by Mortierella isabellina. Biomass Bioenergy 2012, 39, 494–501. [Google Scholar] [CrossRef]
- Quigg, A. Micronutrients. In The Physiology of Microalgae; Springer: Cham, Switzerland, 2016; Volume 6, pp. 211–223. [Google Scholar]
- Henderson, R.K.; Jiménez-González, C.; Constable, D.J.; Alston, S.R.; Inglis, G.G.; Fisher, G.; Sherwood, J.; Binks, S.P.; Curzons, A.D. Expanding GSK’s solvent selection guide–embedding sustainability into solvent selection starting at medicinal chemistry. Green Chem. 2011, 13, 854–862. [Google Scholar] [CrossRef]
- Alder, C.M.; Hayler, J.D.; Henderson, R.K.; Redman, A.M.; Shukla, L.; Shuster, L.E.; Sneddon, H.F. Updating and further expanding GSK’s solvent sustainability guide. Green Chem. 2016, 18, 3879–3890. [Google Scholar] [CrossRef]
- Cseri, L.; Razali, M.; Pogany, P.; Szekely, G. Organic solvents in sustainable synthesis and engineering. Green Chem. 2018, 513–553. [Google Scholar]
- Park, J.; Kim, B.; Chang, Y.K.; Lee, J.W. Wet in situ transesterification of microalgae using ethyl acetate as a co-solvent and reactant. Bioresour. Technol. 2017, 230, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Alam, M.A.; Pan, Y.; Nock, W.J.; Wang, Z.; Yuan, Z. Optimization of algal lipid extraction by mixture of ethyl acetate and ethanol via response surface methodology for biodiesel production. Korean J. Chem. Eng. 2016, 33, 2575–2581. [Google Scholar] [CrossRef]
- Lu, W.; Wang, Z.; Yuan, Z. Characteristics of lipid extraction from Chlorella sp. cultivated in outdoor raceway ponds with mixture of ethyl acetate and ethanol for biodiesel production. Bioresour. Technol. 2015, 191, 433–437. [Google Scholar] [CrossRef]
- Fine, F.; Vian, M.A.; Tixier, A.S.F.; Carre, P.; Pages, X.; Chemat, F. Les agro-solvants pour l’extraction des huiles vegetales issues de graines oléagineuses. Oilseeds Fats Crops Lipids 2013, 20, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Lohani, U.C.; Fallahi, P.; Muthukumarappan, K. Comparison of ethyl acetate with hexane for oil extraction from various oilseeds. J. Am. Oil Chem. Soc. 2015, 92, 743–754. [Google Scholar] [CrossRef]
- Jiyane, P.C.; Tumba, K.; Musonge, P. Optimisation of Croton gratissimus oil extraction by n-hexane and ethyl acetate using response surface methodology. J. Oleo Sci. 2018, 67, 369–377. [Google Scholar] [CrossRef]
- Xu, L.; Brilman, D.W.W.; Withag, J.A.; Brem, G.; Kersten, S. Assessment of a dry and a wet route for the production of biofuels from microalgae: Energy balance analysis. Bioresour. Technol. 2011, 102, 5113–5122. [Google Scholar] [CrossRef]
- Teo, C.L.; Idris, A. Enhancing the various solvent extraction method via microwave irradiation for extraction of lipids from marine microalgae in biodiesel production. Bioresour. Technol. 2014, 171, 477–481. [Google Scholar] [CrossRef]
- Deshmukh, S.; Kumar, R.; Bala, K. Microalgae biodiesel: A review on oil extraction, fatty acid composition, properties and effect on engine performance and emissions. Fuel Process. Technol. 2019, 191, 232–247. [Google Scholar] [CrossRef]
- Lee, I.; Han, J.I. The effects of waste-activated sludge pretreatment using hydrodynamic cavitation for methane production. Ultrason. Sonochem. 2013, 20, 1450–1455. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.Y.; Lin, H.Y.; Lu, G.Y.; Lin, C.P. Solid byproducts of Aurantiochytrium sp. oil made into the biodiesel. J. Therm. Anal. Calorim. 2015, 120, 563–572. [Google Scholar] [CrossRef]
- Tsai, S.Y.; Tsay, G.J.; Li, C.Y.; Hung, Y.T.; Lin, C.P. Assessment of melting kinetics of sugar-reduced silver ear mushroom ice cream under various additive models. Appl. Sci. 2020, 10, 2664. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.P.; Tsai, S.Y. Differences in the moisture capacity and thermal stability of Tremella fuciformis polysaccharides obtained by various drying processes. Molecules 2019, 24, 2856. [Google Scholar] [CrossRef] [Green Version]


| Disruption and Extraction Method | Solvent | Conditions | Lipid Yield (g/L) | Lipid Content (%) |
|---|---|---|---|---|
| Soxhlet | Ethyl oxide | 1 g/150 mL, 50 °C, 6 h | 0.40 ± 0.10 e | 7.90 ± 0.15 f |
| Solvent | Dichloromethane: methanol (1:1) | 1 g/10 mL, sonication 30 min | 0.61 ± 0.02 d | 11.38 ± 0.49 e |
| Acid hydrolysis + Solvent | Dichloromethane:methanol (1:1) | 1 g/1 M HCl, 10 min, 60 °C; 10 mL, sonication 30 min | 0.70 ± 0.01 cd | 14.85 ± 0.26 d |
| Acid hydrolysis + Solvent | n-Hexane | 1 g/1 M HCl, 10 min, 60 °C; 10 mL, sonication 30 min | 0.80 ± 0.01 cd | 15.80 ± 0.25 d |
| Acid hydrolysis + Solvent | Ethyl acetate | 1 g/1 M HCl, 10 min, 60 °C; 10 mL, sonication 30 min | 0.91 ± 0.01 c | 18.80 ± 0.03 c |
| Acid hydrolysis + Solvent | Ethyl acetate | 1 g/3 M HCl, 10 min, 60 °C; 10 mL, sonication 30 min | 1.14 ± 0.10 b | 18.29 ± 0.46 c |
| Acid hydrolysis + Solvent | Ethyl acetate | 1 g/4 M HCl, 10 min, 60 °C; 10 mL, sonication 30 min | 1.24 ± 0.01 b | 20.29 ± 0.32 b |
| Acid hydrolysis + Solvent | Ethyl acetate | 1 g/4 M HCl, 10 min, 100 °C; 10 mL, sonication 30 min | 1.50 ± 0.30 a | 25.51 ± 2.07 a |
| Fatty Acids | w/w, % |
|---|---|
| Myristic acid (C14:0) | 1.08 |
| Palmitic acid (C16:0) | 15.85 |
| Palmitoleic acid (C16:1) | 1.92 |
| Stearic acid (C18:0) | 0.86 |
| Oleic acid (C18:1) | 41.44 |
| Linoleic acid (C18:2) | 10.35 |
| Linolenic acid (C18:3) | 0.97 |
| Eicosenoic acid (C20:1) | 0.25 |
| Lignoceric acid (C24:0) | 0.65 |
| Total saturation | 18.45 |
| Total monounsaturated | 43.61 |
| Total polyunsaturated | 11.32 |
| Mass (mg) | Cooling Rate (°C/min) | 1exoTonset (°C) | 1exoTpeak (°C) | 1Enthalpy (J/g) | 2exoTonset (°C) | 2exoTpeak (°C) | 2Enthalpy (J/g) |
|---|---|---|---|---|---|---|---|
| 1.50 | 4 | 8.94 | 8.30 | 24.51 | −17.15 | −18.06 | 38.23 |
| 1.45 | 6 | 9.08 | 8.34 | 21.16 | −16.43 | −17.19 | 37.94 |
| 1.48 | 8 | 9.20 | 8.61 | 26.25 | −17.19 | −18.19 | 42.54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, S.-Y.; Yu, H.-T.; Lin, C.-P. The Potential of the Oil-Producing Oleaginous Yeast Rhodotorula mucilaginosa for Sustainable Production of Bio-Oil Energy. Processes 2022, 10, 336. https://doi.org/10.3390/pr10020336
Tsai S-Y, Yu H-T, Lin C-P. The Potential of the Oil-Producing Oleaginous Yeast Rhodotorula mucilaginosa for Sustainable Production of Bio-Oil Energy. Processes. 2022; 10(2):336. https://doi.org/10.3390/pr10020336
Chicago/Turabian StyleTsai, Shu-Yao, Hsuan-Ti Yu, and Chun-Ping Lin. 2022. "The Potential of the Oil-Producing Oleaginous Yeast Rhodotorula mucilaginosa for Sustainable Production of Bio-Oil Energy" Processes 10, no. 2: 336. https://doi.org/10.3390/pr10020336





