Photocatalytic Treatment of Emerging Contaminants with Ag-Modified Titania—Is There a Risk Arising from the Degradation Products?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Instrumentation
2.3. Preparation of the Ag-TiO2 Photocatalyst
2.4. Experimental Procedure of Photocatalytic Degradation
2.5. Analytical Method for the Determination of Tested Contaminants and Identification of Their Degradation Products
2.6. Toxicity Evaluation
3. Results
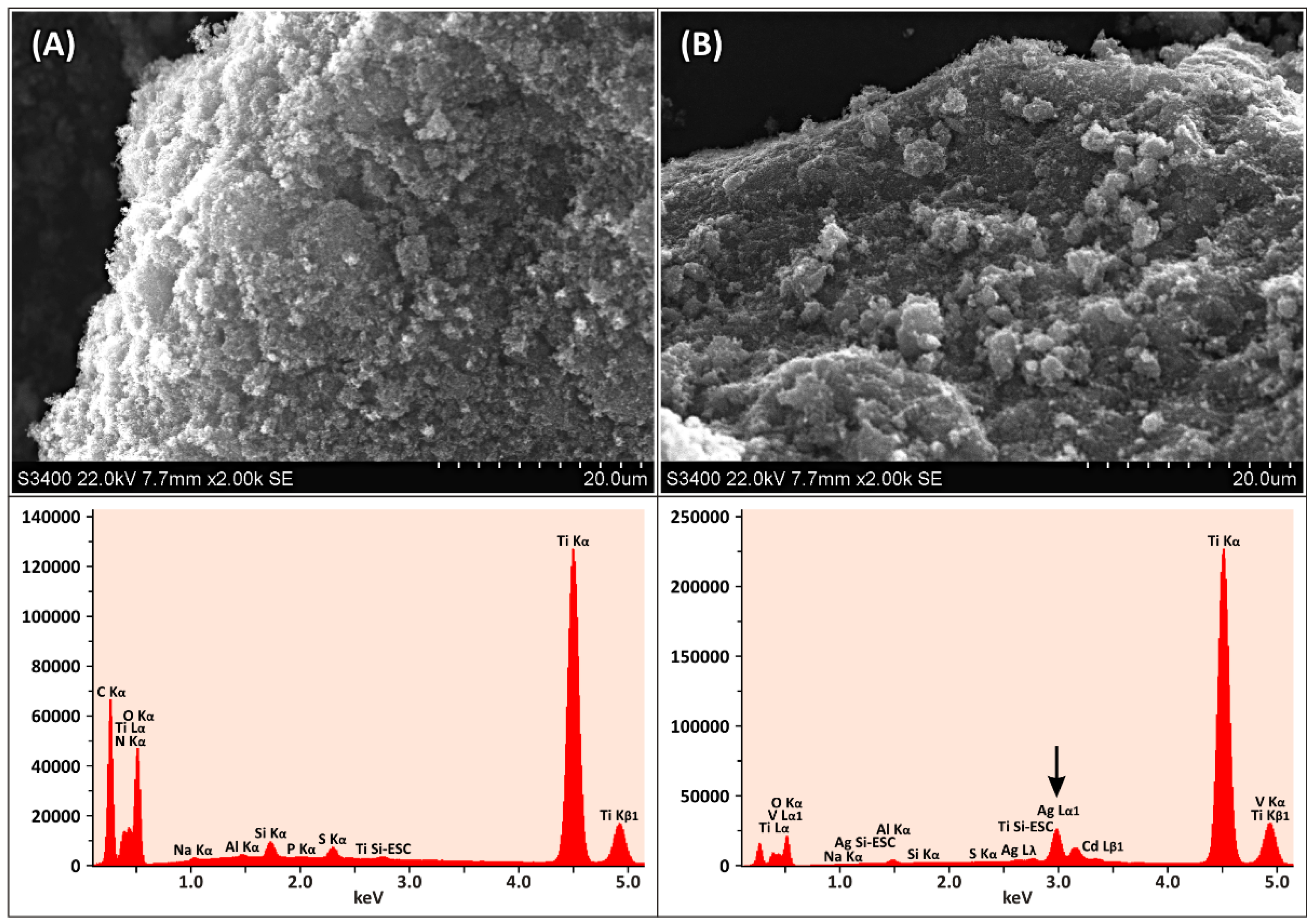
3.1. Characterization of Synthesized Ag-TiO2
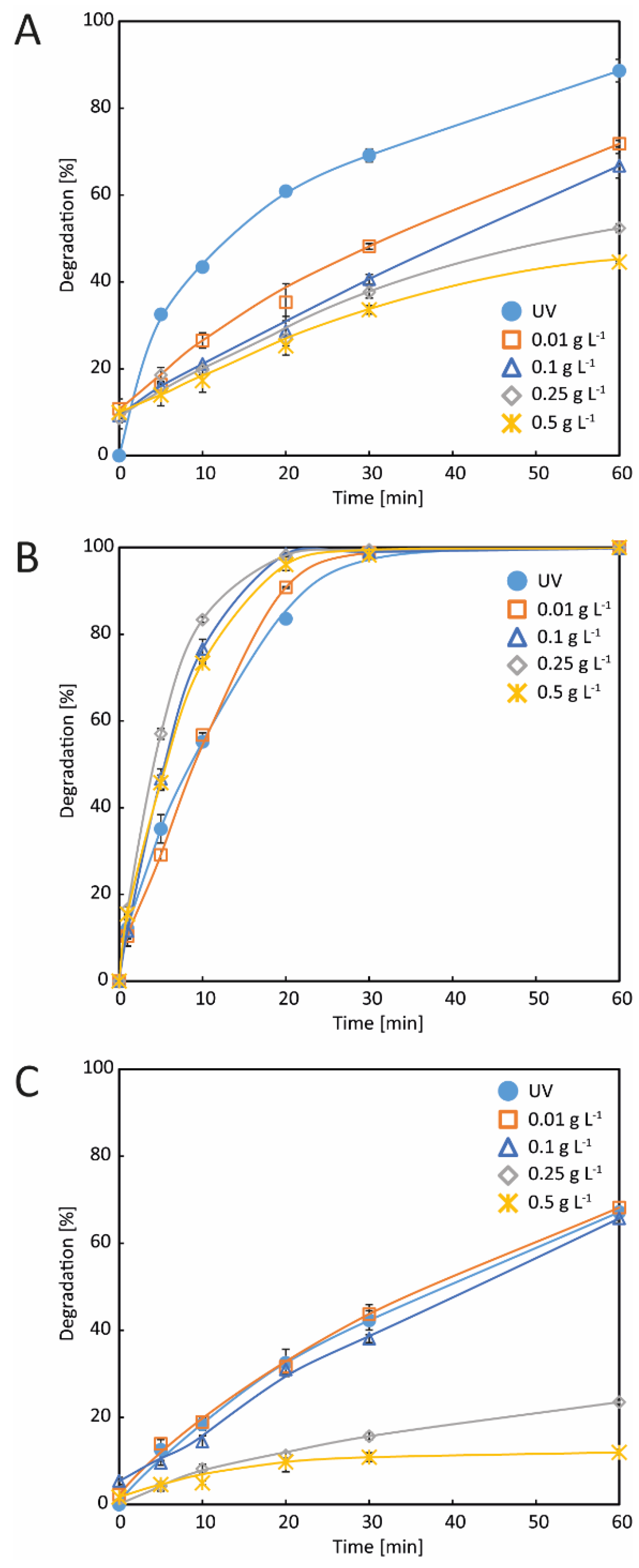
3.2. Degradation Tests
3.2.1. Tests with ZnO
3.2.2. Tests with TiO2
3.2.3. Tests with Ag-TiO2
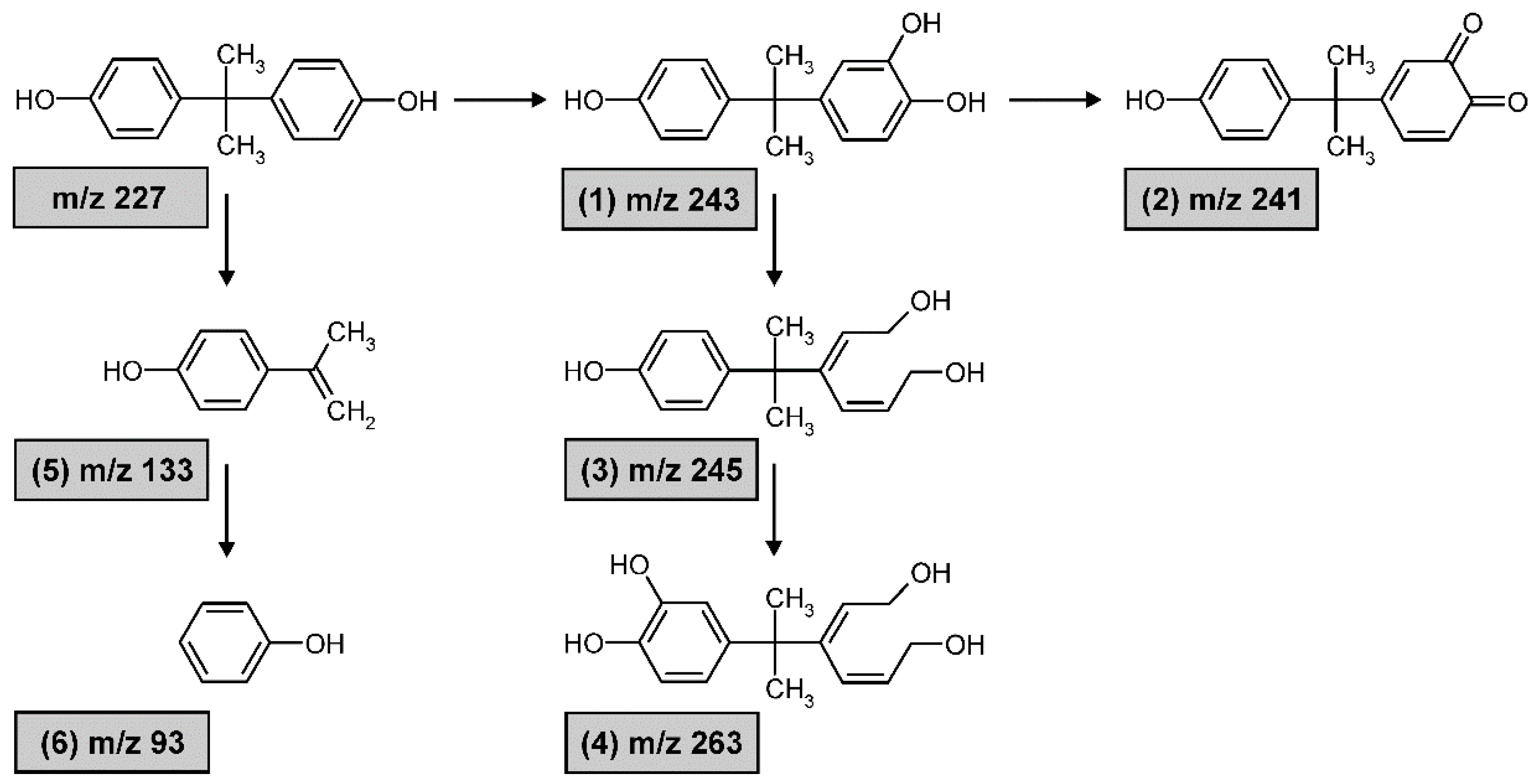
3.3. Degradation Products and Their Toxicity
4. Discussion
4.1. Removal of Tested Compounds during the Degradation
4.2. Comparison with Other Studies
4.3. Formation of the Degradation Products and Their Toxicity
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pignatello, J.J.; Oliveros, E.; MacKay, A. Advanced oxidation processes for organic contaminant destruction based on the Fenton reaction and related chemistry. Crit. Rev. Environ. Sci. Technol. 2006, 36, 1–84. [Google Scholar] [CrossRef]
- Gusain, R.; Gupta, K.; Joshi, P.; Khatri, O.P. Adsorptive removal and photocatalytic degradation of organic pollutants using metal oxides and their composites: A comprehensive review. Adv. Colloid Interface Sci. 2019, 272, 102009. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Khan, M.D.; Shahane, S.; Rai, D.; Chauhan, D.; Kant, C.; Chaudhary, V.K. Emerging pollutants in aquatic environment: Source, effect, and challenges in biomonitoring and bioremediation—A review. Pollution 2020, 6, 99–113. [Google Scholar] [CrossRef]
- Pironti, C.; Ricciardi, M.; Proto, A.; Bianco, P.M.; Montano, L.; Motta, O. Endocrine-disrupting compounds: An overview on their occurrence in the aquatic environment and human exposure. Water 2021, 13, 1347. [Google Scholar] [CrossRef]
- Petrie, B.; Barden, R.; Kasprzyk-Hordern, B. A review on emerging contaminants in wastewater and the environment: Current knowledge, understudied areas and recommendations for future monitoring. Water Res. 2015, 72, 3–27. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xi, H.; Xu, L.; Jin, M.; Zhao, W.; Liu, H. Ecotoxicological effects, environmental fate and risks of pharmaceutical and personal care products in the water environment: A review. Sci. Total Environ. 2021, 788, 147819. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Kurniawan, S.B.; Abdullah, S.R.S.; Othman, A.R.; Hasan, H.A. Contaminants of emerging concern (CECs) in aquaculture effluent: Insight into breeding and rearing activities, alarming impacts, regulations, performance of wastewater treatment unit and future approaches. Chemosphere 2022, 290, 133319. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Anquandah, G.A.K.; Nesnas, N. Kinetics of the oxidation of endocrine disruptor nonylphenol by ferrate(VI). Environ. Chem. Lett. 2009, 7, 115–119. [Google Scholar] [CrossRef]
- Zoeller, R.T.; Brown, T.R.; Doan, L.L.; Gore, A.C.; Skakkebaek, N.E.; Soto, A.M.; Woodruff, T.J.; Vom Saal, F.S. Endocrine-disrupting chemicals and public health protection: A statement of principles from The Endocrine Society. Endocrinology 2012, 153, 4097–4110. [Google Scholar] [CrossRef] [PubMed]
- Kahle, M.; Buerge, I.J.; Hauser, A.; Müller, M.D.; Poiger, T. Azole fungicides: Occurrence and fate in wastewater and surface waters. Environ. Sci. Technol. 2008, 42, 7193–7200. [Google Scholar] [CrossRef] [PubMed]
- Joseph-Horne, T.; Hollomon, D.W. Molecular mechanisms of azole resistance in fungi. FEMS Microbiol. Lett. 1997, 149, 41–149. [Google Scholar] [CrossRef] [PubMed]
- Georgopapadakou, N.H. Antifungals: Mechanism of action and resistance, established and novel drugs. Curr. Opin. Microbiol. 1998, 1, 547–557. [Google Scholar] [CrossRef]
- Cai, W.; Ye, P.; Yang, B.; Shi, Z.; Xiong, Q.; Gao, F.; Liu, J.; Zhao, J.; Ying, G. Biodegradation of typical azole fungicides in activated sludge under aerobic conditions. J. Environ. Sci. 2021, 103, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Peschka, M.; Roberts, P.H.; Knepper, T.P. Analysis, fate studies and monitoring of the antifungal agent clotrimazole in the aquatic environment. Anal. Bioanal. Chem. 2007, 389, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Huang, Q.; Zhang, K.; Yu, Y.; Wang, Z.; Wang, C. Distribution, behavior and fate of azole antifungals during mechanical, biological, and chemical treatments in sewage treatment plants in China. Sci. Total Environ. 2012, 426, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-F.; Ying, G.-G. Occurrence, fate and ecological risk of five typical azole fungicides as therapeutic and personal care products in the environment: A review. Environ. Int. 2015, 84, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhu, Q.; Yan, X.; Liao, C.; Jiang, G. Occurrence, fate and risk assessment of BPA and its substituents in wastewater treatment plant: A review. Environ. Res. 2019, 178, 108732. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Kannan, K. Widespread occurrence of bisphenol A in paper and paper products: Implications for human exposure. Environ. Sci. Technol. 2011, 45, 9372–9379. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.H.; Zhang, X.M.; Wang, F.; Gao, C.J.; Chen, D.; Palumbo, J.R.; Guo, Y.; Zeng, E.Y. Occurrence of bisphenol S in the environment and implications for human exposure: A short review. Sci. Total Environ. 2018, 615, 87–98. [Google Scholar] [CrossRef]
- Cacho, J.I.; Campillo, N.; Viñas, P.; Hernández-Córdoba, M. Stir bar sorptive extraction with EG-Silicone coating for bisphenols determination in personal care products by GC-MS. J. Pharmaceut. Biomed. Anal. 2013, 78–79, 255–260. [Google Scholar] [CrossRef]
- Martín-Pozo, L.; del Carmen Gómez-Regalado, M.; Moscoso-Ruiz, I.; Zafra-Gómez, A. Analytical methods for the determination of endocrine disrupting chemicals in cosmetics and personal care products: A review. Talanta 2021, 234, 122642. [Google Scholar] [CrossRef] [PubMed]
- Lestido-Cardama, A.; Millán Sánchez, B.; Sendón, R.; Rodríguez-Bernaldo de Quirós, A.; Barbosa-Pereira, L. Study on the chemical behaviour of bisphenol S during the in vitro gastrointestinal digestion and its bioaccessibility. Food Chem. 2021, 367, 130758. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Kannan, K.; Tan, H.; Zheng, Z.; Feng, Y.L.; Wu, Y.; Widelka, M. Bisphenol analogues other than BPA: Environmental occurrence, human exposure, and toxicity—A review. Environ. Sci. Technol. 2016, 50, 5438–5453. [Google Scholar] [CrossRef] [PubMed]
- Noszczyńska, M.; Piotrowska-Seget, Z. Bisphenols: Application, occurrence, safety, and biodegradation mediated by bacterial communities in wastewater treatment plants and rivers. Chemosphere 2018, 201, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Frankowski, R.; Zgoła-Grześkowiak, A.; Grześkowiak, T.; Sójka, K. The presence of bisphenol A in the thermal paper in the face of changing European regulations e A comparative global research. Environ. Pollut. 2020, 265, 114879. [Google Scholar] [CrossRef] [PubMed]
- Majumder, A.; Gupta, B.; Gupta, A.K. Pharmaceutically active compounds in aqueous environment: A status, toxicity and insights of remediation. Environ. Res. 2019, 176, 108542. [Google Scholar] [CrossRef] [PubMed]
- Parida, V.K.; Saidulu, D.; Majumder, A.; Srivastava, A.; Gupta, B.; Gupta, A.K. Emerging contaminants in wastewater: A critical review on occurrence, existing legislations, risk assessment, and sustainable treatment alternatives. J. Environ. Chem. Eng. 2021, 9, 105966. [Google Scholar] [CrossRef]
- Rathi, B.S.; Kumar, P.S.; Show, P.L. A review on effective removal of emerging contaminants from aquatic systems: Current trends and scope for further research. J. Hazard. Mater. 2021, 409, 124413. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, K.E.; Dionysiou, D.D. Advanced oxidation processes for water treatment. J. Phys. Chem. Lett. 2012, 3, 2112–2113. [Google Scholar] [CrossRef]
- Fast, S.A.; Gude, V.G.; Truax, D.D.; Martin, J.; Magbanua, B.S. A critical evaluation of advanced oxidation processes for emerging contaminants removal. Environ. Process. 2017, 4, 283–302. [Google Scholar] [CrossRef]
- Andreozzi, R.; Caprio, V.; Insola, A.; Marotta, R. Advanced oxidation processes (AOP) for water purification and recovery. Catal. Today 1999, 53, 51–59. [Google Scholar] [CrossRef]
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman, C.U.; Mohan, D. Pharmaceuticals of emerging concern in aquatic systems: Chemistry, occurrence, effects, and removal methods. Chem. Rev. 2019, 119, 3510–3673. [Google Scholar] [CrossRef] [PubMed]
- Oturan, M.A.; Aaron, J.-J. Advanced oxidation processes in water/wastewater treatment: Principles and applications. A review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2577–2641. [Google Scholar] [CrossRef]
- Ajith, M.P.; Aswath, M.; Priyadarshini, E.; Rajamani, P. Recent innovations of nanotechnology in water treatment: A comprehensive review. Bioresour. Technol. 2021, 342, 126000. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Rasul, M.G.; Brown, R.; Hashib, M.A. Influence of parameters on the heterogeneous photocatalytic degradation of pesticides and phenolic contaminants in wastewater: A short review. J. Environ. Manag. 2011, 92, 311–330. [Google Scholar] [CrossRef] [PubMed]
- Rauf, M.A.; Meetani, M.A.; Hisaindee, S. An overview on the photocatalytic degradation of azo dyes in the presence of TiO2 doped with selective transition metals. Desalination 2011, 276, 13–27. [Google Scholar] [CrossRef]
- Gupta, S.M.; Tripathi, M. A review of TiO2 nanoparticles. Chin. Sci. Bull. 2011, 56, 1639–1657. [Google Scholar] [CrossRef]
- Tahir, M.B.; Nawaz, T.; Nabi, G.; Sagir, M.; Khan, M.I.; Malik, N. Role of nanophotocatalysts for the treatment of hazardous organic and inorganic pollutants in wastewater. Int. J. of Environ. Anal. Chem. 2022, 102, 491–515. [Google Scholar] [CrossRef]
- Sun, C.; Yang, J.; Xu, M.; Cui, Y.; Ren, W.; Zhang, J.; Zhao, H.; Liang, B. Recent intensification strategies of SnO2-based photocatalysts: A review. Chem. Eng. J. 2022, 427, 131564. [Google Scholar] [CrossRef]
- Matusiewicz, H.; Stanisz, E. Evaluation of the catalyzed photo-cold vapour generation for determination of mercury by AAS. J. Braz. Chem. Soc. 2012, 23, 247–257. [Google Scholar] [CrossRef] [Green Version]
- Tan, T.T.Y.; Yip, C.K.; Beydoun, D.; Amal, R. Effects of nano-Ag particles loading on TiO2 photocatalytic reduction of selenate ions. Chem. Eng. J. 2003, 95, 179–186. [Google Scholar] [CrossRef]
- Khaki, M.R.D.; Shafeeyan, M.S.; Raman, A.A.A.; Daud, W.M.A.W. Application of doped photocatalysts for organic pollutant degradation—A review. J. Environ. Manag. 2017, 198, 78–94. [Google Scholar] [CrossRef] [PubMed]
- Majumder, S.; Chatterjee, S.; Basnet, P.; Mukherjee, J. ZnO based nanomaterials for photocatalytic degradation of aqueous pharmaceutical waste solutions—A contemporary review. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100386. [Google Scholar] [CrossRef]
- Yahya, N.; Aziz, F.; Jamaludin, N.A.; Mutalib, M.A.; Ismail, A.F.; Salleh, W.N.W.; Jaafar, J.; Yusof, N.; Ludin, N.A. A review of integrated photocatalyst adsorbents for wastewater treatment. J. Environ. Chem. Eng. 2018, 6, 7411–7425. [Google Scholar] [CrossRef]
- Abraham, A.; Chakraborty, P. A review on sources and health impacts of bisphenol A. Rev. Environ. Health 2020, 35, 201–210. [Google Scholar] [CrossRef]
- Huang, Y.Q.; Wong, C.K.C.; Zheng, J.S.; Bouwman, H.; Barra, R.; Wahlström, B.; Neretin, L.; Wong, M.H. Bisphenol A (BPA) in China: A review of sources, environmental levels, and potential human health impacts. Environ. Int. 2012, 42, 91–99. [Google Scholar] [CrossRef]
- Rotimi, O.A.; Olawole, T.D.; De Campos, O.C.; Adelani, I.B.; Rotimi, S.O. Bisphenol A in Africa: A review of environmental and biological levels. Sci. Total Environ. 2021, 764, 142854. [Google Scholar] [CrossRef]
- An, S.-N.; Choi, N.-C.; Choi, J.-W.; Lee, S. Photodegradation of bisphenol A with ZnO and TiO2: Influence of metal ions and Fenton process. Water Air Soil Pollut. 2018, 229, 43. [Google Scholar] [CrossRef]
- Zacharakis, A.; Chatzisymeon, E.; Binas, V.; Frontistis, Z.; Venieri, D.; Mantzavinos, D. Solar Photocatalytic Degradation of Bisphenol A on Immobilized ZnO or TiO2. Int. J. Photoenergy 2013, 2013, 570587. [Google Scholar] [CrossRef]
- Bechambi, O.; Sayadi, S.; Najjar, W. Photocatalytic degradation of bisphenol A in the presence of C-doped ZnO: Effect of operational parameters and photodegradation mechanism. J. Ind. Eng. Chem. 2015, 32, 201–210. [Google Scholar] [CrossRef]
- Bechambi, O.; Jlaiel, L.; Najjar, W.; Sayadi, S. Photocatalytic degradation of bisphenol A in the presence of Ce-ZnO: Evolution of kinetics, toxicity and photodegradation mechanism. Mater. Chem. Phys. 2016, 173, 95–105. [Google Scholar] [CrossRef]
- Farzadkia, M.; Esrafili, A.; Baghapour, M.A.; Shahamat, Y.D.; Okhovat, N. Degradation of metronidazole in aqueous solution by nano-ZnO/UV photocatalytic process. Desalination Water Treat. 2014, 52, 4947–4952. [Google Scholar] [CrossRef]
- Kryczyk, A.; Żmudzki, P.; Hubicka, U. Determination of bifonazole and identification of its photocatalytic degradation products using UPLC-MS/MS. Biomed. Chromatogr. 2017, 31, e3955. [Google Scholar] [CrossRef] [PubMed]
- Kryczyk, A.; Żmudzki, P.; Koczurkiewicz, P.; Piotrowska, J.; Pękala, E.; Hubicka, U. The impact of ZnO and TiO2 on the stability of clotrimazole under UVA irradiation: Identification of photocatalytic degradation products and in vitro cytotoxicity assessment. J. Pharm. Biomed. Anal. 2017, 145, 283–292. [Google Scholar] [CrossRef]
- Tsai, W.-T.; Lee, M.-K.; Su, T.-Y.; Chang, Y.-M. Photodegradation of bisphenol-A in a batch TiO2 suspension reactor. J. Hazard. Mater. 2009, 168, 269–275. [Google Scholar] [CrossRef]
- Lee, J.-M.; Kim, M.-S.; Kim, B.-W. Photodegradation of bisphenol-A with TiO2 immobilized on the glass tubes including the UV light lamps. Water Res. 2004, 38, 3605–3613. [Google Scholar] [CrossRef]
- Yu, F.; Bai, X.; Yang, C.; Xu, L.; Ma, J. Reduced graphene oxide-P25 nanocomposites as efficient photocatalysts for degradation of bisphenol A in water. Catalysts 2019, 9, 607. [Google Scholar] [CrossRef]
- Jia, J.; Liu, D.; Wang, Q.; Li, H.; Ni, J.; Cui, F.; Tian, J. Comparative study on bisphenols oxidation via TiO2 photocatalytic activation of peroxymonosulfate: Effectiveness, mechanism and pathways. J. Hazard. Mater. 2022, 424, 127434. [Google Scholar] [CrossRef]
- Sousa, M.A.; Gonçalves, C.; Vilar, V.J.P.; Boaventura, R.A.R.; Alpendurada, M.F. Suspended TiO2-assisted photocatalytic degradation of emerging contaminants in a municipal WWTP effluent using a solar pilot plant with CPCs. Chem. Eng. J. 2012, 198–199, 301–309. [Google Scholar] [CrossRef]
- Rengaraj, S.; Li, X.Z. Photocatalytic degradation of bisphenol A as an endocrine disruptor in aqueous suspension using Ag-TiO2 catalysts. Int. J. Environ. Pollut. 2006, 27, 20–37. [Google Scholar] [CrossRef] [Green Version]
- Suwanchawalit, C.; Wongnawa, S.; Sriprang, P.; Meanha, P. Enhancement of the photocatalytic performance of Ag-modified TiO2 photocatalyst under visible light. Ceram. Int. 2012, 38, 5201–5207. [Google Scholar] [CrossRef]
- Shareef, U.; Othman, M.H.D.; Ismail, A.F.; Jilani, A. Facile removal of bisphenol A from water through novel Ag-doped TiO2 photocatalytic hollow fiber ceramic membrane. J. Aust. Ceram. Soc. 2020, 56, 29–39. [Google Scholar] [CrossRef]
- Kondrakov, A.O.; Ignatev, A.N.; Frimmel, F.H.; Bräse, S.; Horn, H.; Revelsky, A.I. Formation of genotoxic quinones during bisphenol A degradation by TiO2 photocatalysis and UV photolysis: A comparative study. Appl. Catal. B Environ. 2014, 160–161, 106–114. [Google Scholar] [CrossRef]
- Frankowski, R.; Płatkiewicz, J.; Stanisz, E.; Grześkowiak, T.; Zgoła-Grześkowiak, A. Biodegradation and photo-Fenton degradation of bisphenol A, bisphenol S and fluconazole in water. Environ. Pollut. 2021, 289, 117947. [Google Scholar] [CrossRef] [PubMed]
- Mehrabani-Zeinabad, M.; Langford, C.H.; Achari, G. Advanced oxidative degradation of bisphenol A and bisphenol S. J. Environ. Eng. Sci. 2015, 10, 92–102. [Google Scholar] [CrossRef]
- Kusvuran, E.; Yildirim, D. Degradation of bisphenol A by ozonation and determination of degradation intermediates by gas chromatography–mass spectrometry and liquid chromatography–mass spectrometry. Chem. Eng. J. 2013, 220, 6–14. [Google Scholar] [CrossRef]
- Hua, Z.; Ma, W.; Bai, X.; Feng, R.; Yu, L.; Zhang, X.; Dai, Z. Heterogeneous Fenton degradation of bisphenol A catalyzed by efficient adsorptive Fe3O4/GO nanocomposites. Environ. Sci. Pollut. Res. 2014, 21, 7737–7745. [Google Scholar] [CrossRef]




| Compound | Acute Toxicity to Fish, LC50 at 96 h (mg mL−1) | Acute Toxicity to Daphnid, LC50 at 96 h (mg mL−1) | Acute Toxicity to Green Algae, LC50 at 96 h (mg mL−1) |
|---|---|---|---|
| BPA | 1.28 | 5.24 | 1.33 |
| (1) | 2.65 | 13.1 | 2.07 |
| (2) | 4.15 | 3.25 | 0.38 |
| (3) | 2.45 | 5.72 | 0.13 |
| (4) | 3.59 | 8.88 | 0.23 |
| (5) | 3.99 | 2.67 | 0.36 |
| (6) | 27.7 | 9.64 | 2.40 |
| BPS | 21.8 | 196 | 6.90 |
| Pollutant/Concentration, mg L−1 | Photocatalyst/Additive/pH | Degradation Efficiency in 1 h (%) | Ref. |
|---|---|---|---|
| BPA/100 | ZnO/H2O2/6.3 | 90 | [48] |
| BPA/0.1 | ZnO/H2O2/- | 85 | [49] |
| BPA/50 | ZnO (C-doped)/H2O2/8–9 | 70 | [50] |
| BPA/50 | ZnO (Ce-doped)/H2O2/9 | 60 | [51] |
| BPA/20 | TiO2/H2O2/5–9 | 100 | [55] |
| BPA/10 | TiO2/-/3 | 15 | [56] |
| BPS/10 | TiO2/KHSO5/3 | 95 | [58] |
| BPA/10 | Ag-TiO2/-/5.3 | 100 | [60] |
| BPA/10 | Ag-TiO2/-/- | 35 | [62] |
| BPA/10 | Ag-TiO2/-/- | 100 | Present study |
| BPS/10 | Ag-TiO2/-/- | 100 (in 10 min) | Present study |
| FLC/10 | Ag-TiO2/-/- | 60 | Present study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frankowski, R.; Zgoła-Grześkowiak, A.; Grześkowiak, T.; Stanisz, E.; Werner, J.; Płatkiewicz, J. Photocatalytic Treatment of Emerging Contaminants with Ag-Modified Titania—Is There a Risk Arising from the Degradation Products? Processes 2022, 10, 2523. https://doi.org/10.3390/pr10122523
Frankowski R, Zgoła-Grześkowiak A, Grześkowiak T, Stanisz E, Werner J, Płatkiewicz J. Photocatalytic Treatment of Emerging Contaminants with Ag-Modified Titania—Is There a Risk Arising from the Degradation Products? Processes. 2022; 10(12):2523. https://doi.org/10.3390/pr10122523
Chicago/Turabian StyleFrankowski, Robert, Agnieszka Zgoła-Grześkowiak, Tomasz Grześkowiak, Ewa Stanisz, Justyna Werner, and Julia Płatkiewicz. 2022. "Photocatalytic Treatment of Emerging Contaminants with Ag-Modified Titania—Is There a Risk Arising from the Degradation Products?" Processes 10, no. 12: 2523. https://doi.org/10.3390/pr10122523