Characterization of Streptomyces Species and Validation of Antimicrobial Activity of Their Metabolites through Molecular Docking
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Samples and Isolation of Streptomyces
2.2. Isolation of Streptomyces
2.3. Morphological Characterization
2.4. Molecular Characterization
2.5. Shake Flask Fermentation
2.6. Antimicrobial Assays
2.7. Mass Spectrometry
2.8. Molecular Annotation
2.9. Molecular Docking for the Connection with Antimicrobial Assays
2.9.1. Binding Site Prediction
2.9.2. Ligand Preparation
2.9.3. Receptor Preparation
2.9.4. Molecular Docking and Validation
3. Results
3.1. Morphological Characterization
3.2. Molecular Characterization of Isolates
3.3. Antimicrobial Assays
3.4. Liquid Chromatography–Mass Spectrometry Analysis
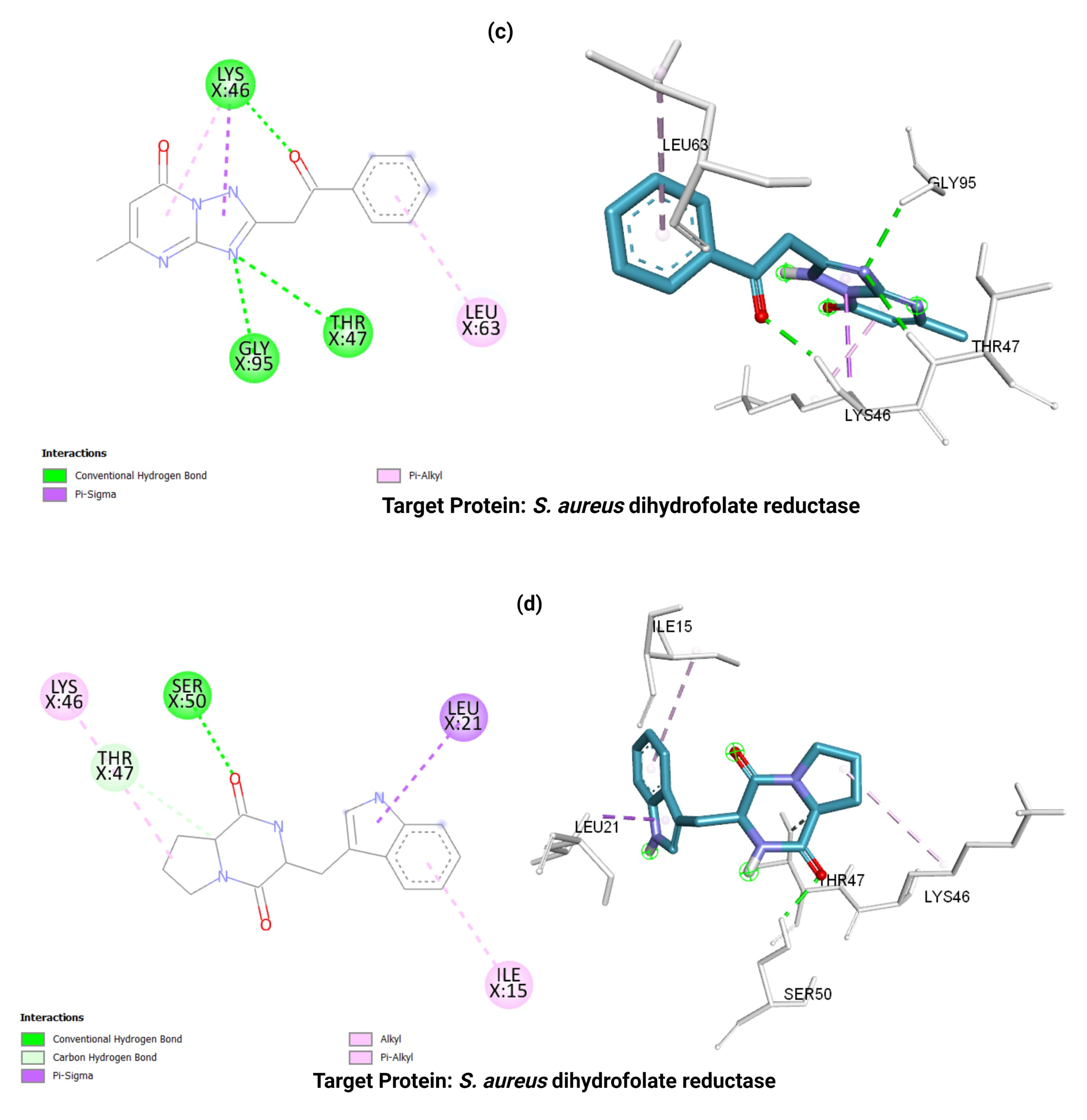
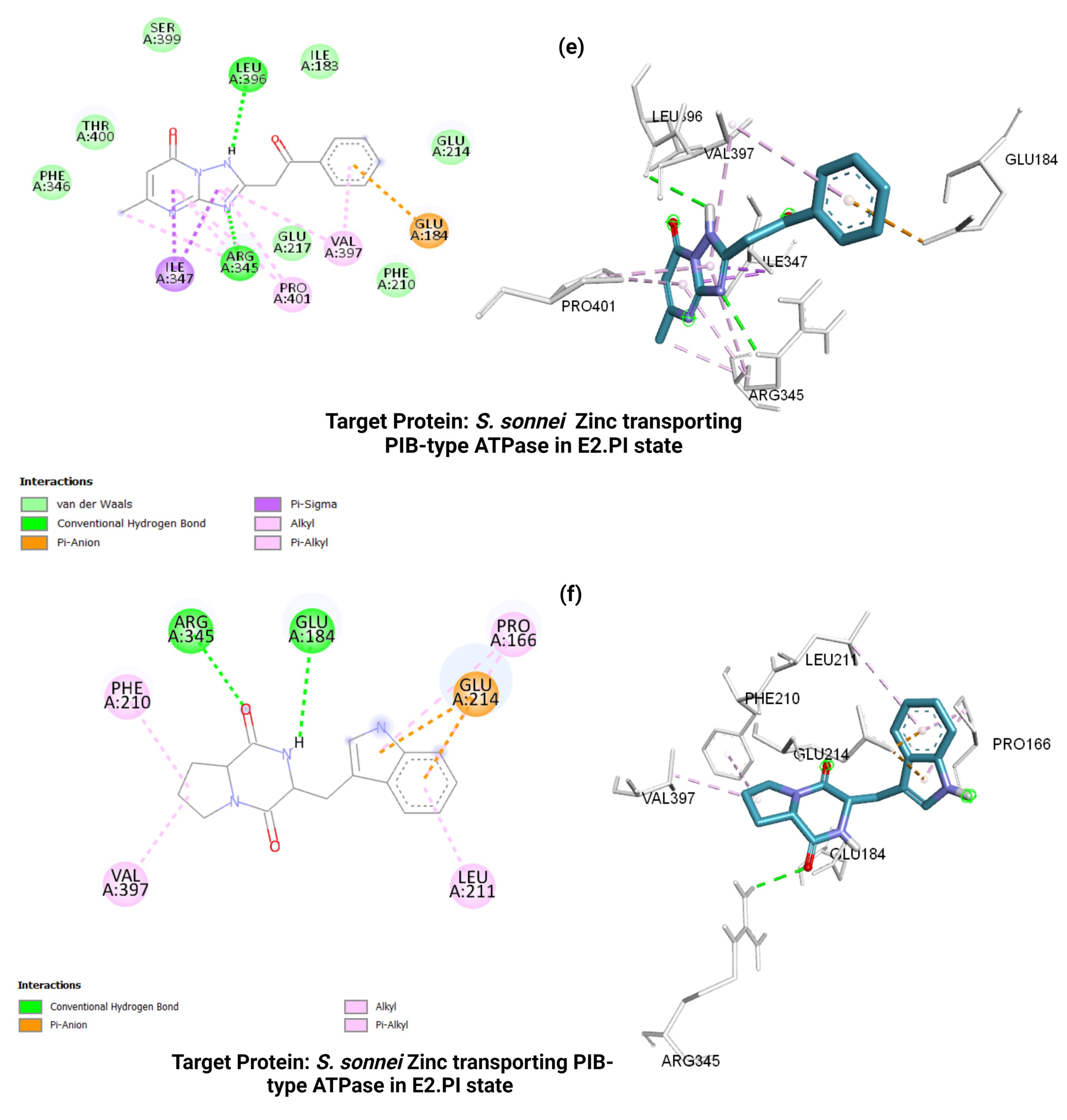
3.5. Molecular Docking Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Uddin, T.M.; Chakraborty, A.J.; Khusro, A.; Zidan, B.R.M.; Mitra, S.; Emran, T.B.; Dhama, K.; Ripon, M.K.H.; Gajdács, M.; Sahibzada, M.U.K.; et al. Antibiotic Resistance in Microbes: History, Mechanisms, Therapeutic Strategies and Future Prospects. J. Infect. Public Health 2021, 14, 1750–1766. [Google Scholar] [CrossRef]
- Baucheron, S.; Monchaux, I.; Le Hello, S.; Weill, F.-X.; Cloeckaert, A. Lack of Efflux Mediated Quinolone Resistance in Salmonella Enterica Serovars Typhi and Paratyphi A. Front. Microbiol. 2014, 5, 12. [Google Scholar] [CrossRef] [Green Version]
- Chuma, T.; Miyasako, D.; Hesham, D.; Takayama, T.; Nakamoto, Y.; Shahada, F.; Akiba, M.; Okamoto, K. Chronological Change of Resistance to β-Lactams in Salmonella Enterica Serovar Infantis Isolated from Broilers in Japan. Front. Microbiol. 2013, 4, 113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frontiers|Prospects for Circumventing Aminoglycoside Kinase Mediated Antibiotic Resistance. Available online: https://www.frontiersin.org/articles/10.3389/fcimb.2013.00022/full (accessed on 5 August 2022).
- Bassetti, M.; Vena, A.; Castaldo, N.; Righi, E.; Peghin, M. New Antibiotics for Ventilator-Associated Pneumonia. Curr. Opin. Infect. Dis. 2018, 31, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Terreni, M.; Taccani, M.; Pregnolato, M. New Antibiotics for Multidrug-Resistant Bacterial Strains: Latest Research Developments and Future Perspectives. Molecules 2021, 26, 2671. [Google Scholar] [CrossRef]
- Al-shaibani, M.M.; Radin Mohamed, R.M.S.; Sidik, N.M.; Enshasy, H.A.E.; Al-Gheethi, A.; Noman, E.; Al-Mekhlafi, N.A.; Zin, N.M. Biodiversity of Secondary Metabolites Compounds Isolated from Phylum Actinobacteria and Its Therapeutic Applications. Molecules 2021, 26, 4504. [Google Scholar] [CrossRef] [PubMed]
- Rajivgandhi, G.N.; Vimala, R.T.V.; Ramachandran, G.; Kanisha, C.C.; Manoharan, N.; Li, W.-J. An Overview on Natural Product from Endophytic Actinomycetes. In Natural Products from Actinomycetes: Diversity, Ecology and Drug Discovery; Rai, R.V., Bai, J.A., Eds.; Springer: Singapore, 2022; pp. 151–165. ISBN 9789811661327. [Google Scholar]
- Arefa, N.; Sarker, A.K.; Rahman, M.A. Resistance-guided Isolation and Characterization of Antibiotic-producing Bacteria from River Sediments. BMC Microbiol. 2021, 21, 116. [Google Scholar] [CrossRef]
- Connell, N.D. Expression Systems for Use in Actinomycetes and Related Organisms. Curr. Opin. Biotechnol. 2001, 12, 446–449. [Google Scholar] [CrossRef]
- Vikineswary, S.; Nadaraj, P.; Wong, W.H.; Balabaskaran, S. Actinomycetes from a Tropical Mangrove Ecosystem- Antifungal Activity of Selected Strains. Asia-Pac. J. Mol. Biol. Biotechnol. 1997, 5, 81–86. [Google Scholar]
- Belknap, K.C.; Park, C.J.; Barth, B.M.; Andam, C.P. Genome Mining of Biosynthetic and Chemotherapeutic Gene Clusters in Streptomyces Bacteria. Sci. Rep. 2020, 10, 2003. [Google Scholar] [CrossRef] [Green Version]
- Chaudhary, K.K.; Mishra, N. A Review on Molecular Docking: Novel Tool for Drug Discovery. Databases 2016, 3, 1029. [Google Scholar]
- Segall, M.D.; Barber, C. Addressing Toxicity Risk When Designing and Selecting Compounds in Early Drug Discovery. Drug Discov. Today 2014, 19, 688–693. [Google Scholar] [CrossRef]
- Pagadala, N.S.; Syed, K.; Tuszynski, J. Software for Molecular Docking: A Review. Biophys. Rev. 2017, 9, 91–102. [Google Scholar] [CrossRef]
- Kurtböke, İ. Bacteriophages; BoD—Books on Demand; InTech: Rijeka, Croatia, 2012; ISBN 978-953-51-0272-4. [Google Scholar]
- Khadayat, K.; Sherpa, D.D.; Malla, K.P.; Shrestha, S.; Rana, N.; Marasini, B.P.; Khanal, S.; Rayamajhee, B.; Bhattarai, B.R.; Parajuli, N. Molecular Identification and Antimicrobial Potential of Streptomyces Species from Nepalese Soil. Int. J. Microbiol. 2020, 2020, 8817467. [Google Scholar] [CrossRef]
- Bhattarai, B.R.; Khadayat, K.; Aryal, N.; Aryal, B.; Lamichhane, U.; Bhattarai, K.; Rana, N.; Regmi, B.P.; Adhikari, A.; Thapa, S.; et al. Untargeted Metabolomics of Streptomyces Species Isolated from Soils of Nepal. Processes 2022, 10, 1173. [Google Scholar] [CrossRef]
- Bergey’s Manual® of Systematic Bacteriology; Goodfellow, M.; Kämpfer, P.; Busse, H.-J.; Trujillo, M.E.; Suzuki, K.; Ludwig, W.; Whitman, W.B. (Eds.) Springer: New York, NY, USA, 2012; ISBN 978-0-387-95043-3. [Google Scholar]
- Sitachitta, N.; Gadepalli, M.; Davidson, B.S. New α-Pyrone-Containing Metabolites from a Marine-Derived Actinomycete. Tetrahedron 1996, 52, 8073–8080. [Google Scholar] [CrossRef]
- Maheshwari, D.K. Practical Microbiology; S. Chand Publishing: New Delhi, India, 2002; ISBN 978-81-219-2153-4. [Google Scholar]
- Nybo, S.E.; Shepherd, M.D.; Bosserman, M.A.; Rohr, J. Genetic Manipulation of Streptomyces Species. Curr. Protoc. Microbiol. 2010, 19, 10E.3.1–10E.3.26. [Google Scholar] [CrossRef]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular Evolutionary Genetics Analysis Using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [Green Version]
- Klausen, C.; Nicolaisen, M.H.; Strobel, B.W.; Warnecke, F.; Nielsen, J.L.; Jørgensen, N.O.G. Abundance of Actinobacteria and Production of Geosmin and 2-Methylisoborneol in Danish Streams and Fish Ponds. FEMS Microbiol. Ecol. 2005, 52, 265–278. [Google Scholar] [CrossRef] [Green Version]
- Aryal, B.; Adhikari, B.; Aryal, N.; Bhattarai, B.R.; Khadayat, K.; Parajuli, N. LC-HRMS Profiling and Antidiabetic, Antioxidant, and Antibacterial Activities of Acacia Catechu (L.f.) Willd. BioMed Res. Int. 2021, 2021, e7588711. [Google Scholar] [CrossRef]
- Aryal, B.; Niraula, P.; Khadayat, K.; Adhikari, B.; Khatri Chhetri, D.; Sapkota, B.K.; Bhattarai, B.R.; Aryal, N.; Parajuli, N. Antidiabetic, Antimicrobial, and Molecular Profiling of Selected Medicinal Plants. Evid.-Based Complement. Altern. Med. 2021, 2021, e5510099. [Google Scholar] [CrossRef]
- Egbert, M.; Porter, K.A.; Ghani, U.; Kotelnikov, S.; Nguyen, T.; Ashizawa, R.; Kozakov, D.; Vajda, S. Conservation of Binding Properties in Protein Models. Comput. Struct. Biotechnol. J. 2021, 19, 2549–2566. [Google Scholar] [CrossRef]
- Belkin, S.; Kundrotas, P.J.; Vakser, I.A. Inhibition of Protein Interactions: Co-Crystalized Protein-Protein Interfaces Are Nearly as Good as Holo Proteins in Rigid-Body Ligand Docking. J. Comput. Aided Mol. Des. 2018, 32, 769–779. [Google Scholar] [CrossRef]
- PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 23 July 2022).
- Kim, S. Exploring Chemical Information in PubChem. Curr. Protoc. 2021, 1, e217. [Google Scholar] [CrossRef]
- PyMOL|Pymol.Org. Available online: https://pymol.org/2/ (accessed on 14 August 2022).
- Yuan, S.; Chan, H.C.S.; Hu, Z. Using PyMOL as a Platform for Computational Drug Design. WIREs Comput. Mol. Sci. 2017, 7, e1298. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Chen, G.; Bai, J.; Jing, Y.-K.; Pei, Y.-H. A Bisamide and Four Diketopiperazines from a Marine-Derived Streptomyces Sp. J. Asian Nat. Prod. Res. 2011, 13, 1146–1150. [Google Scholar] [CrossRef]
- Fang, Q.; Maglangit, F.; Wu, L.; Ebel, R.; Kyeremeh, K.; Andersen, J.H.; Annang, F.; Pérez-Moreno, G.; Reyes, F.; Deng, H. Signalling and Bioactive Metabolites from Streptomyces Sp. RK44. Molecules 2020, 25, 460. [Google Scholar] [CrossRef] [Green Version]
- Sunaryanto, R.; Marwoto, B.; Irawadi, T.T.; Mas’ud, Z.A.; Hartoto, L. Antibiotic Compound from Marine Actinomycetes (Streptomyces Sp. A11): Isolation and Structure Elucidaton. Indones. J. Chem. 2010, 10, 219–225. [Google Scholar] [CrossRef]
- Alshaibani, M.M.; MohamadZin, N.; Jalil, J.; Sidik, N.M.; Ahmad, S.J.; Kamal, N.; Edrada-Ebel, R. Isolation, Purification, and Characterization of Five Active Diketopiperazine Derivatives from Endophytic Streptomyces SUK 25 with Antimicrobial and Cytotoxic Activities. J. Microbiol. Biotechnol. 2017, 27, 1249–1256. [Google Scholar] [CrossRef] [Green Version]
- Tan, L.T.-H.; Chan, K.-G.; Pusparajah, P.; Yin, W.-F.; Khan, T.M.; Lee, L.-H.; Goh, B.-H. Mangrove Derived Streptomyces Sp. MUM265 as a Potential Source of Antioxidant and Anticolon-Cancer Agents. BMC Microbiol 2019, 19, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Gendy, M.M.A.; Shaaban, M.; Shaaban, K.A.; El-Bondkly, A.M.; Laatsch, H. Essramycin: A First Triazolopyrimidine Antibiotic Isolated from Nature†. J. Antibiot. 2008, 61, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Li, J.-J.; Li, Z.; Wang, J.-J. Production and Characterization of Biocontrol Fertilizer from Brewer’s Spent Grain via Solid-State Fermentation. Sci. Rep. 2019, 9, 480. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Dai, S.; Chen, M.; Wu, H.; Xie, L.; Luo, X.; Li, X. Two Diketopiperazine Cyclo(pro-Phe) Isomers from Marine Bacteria Bacillus Subtilis Sp. 13-2. Chem. Nat. Compd. 2010, 46, 583–585. [Google Scholar] [CrossRef]
- Pérez-Picaso, L.; Olivo, H.F.; Argotte-Ramos, R.; Rodríguez-Gutiérrez, M.; Rios, M.Y. Linear and Cyclic Dipeptides with Antimalarial Activity. Bioorganic Med. Chem. Lett. 2012, 22, 7048–7051. [Google Scholar] [CrossRef]
- Řezanka, T.; Spížek, J.; Přikrylová, V.; Dembitsky, V.M. Four New Derivatives of Trihomononactic Acids from Streptomyces Globisporus. Eur. J. Org. Chem. 2004, 2004, 4239–4244. [Google Scholar] [CrossRef]
- Takamatsu, S.; Lin, X.; Nara, A.; Komatsu, M.; Cane, D.E.; Ikeda, H. Characterization of a Silent Sesquiterpenoid Biosynthetic Pathway in Streptomyces Avermitilis Controlling Epi -Isozizaene Albaflavenone Biosynthesis and Isolation of a New Oxidized Epi -Isozizaene Metabolite: Characterization of a Silent Sesquiterpenoid Biosynthetic Pathway. Microb. Biotechnol. 2011, 4, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Bae, M.; Park, S.; Kwon, Y.; Lee, S.; Shin, J.; Nam, J.-W.; Oh, D.-C. QM-HiFSA-Aided Structure Determination of Succinilenes A–D, New Triene Polyols from a Marine-Derived Streptomyces Sp. Mar. Drugs 2017, 15, 38. [Google Scholar] [CrossRef] [Green Version]
- Dickschat, J.S.; Martens, T.; Brinkhoff, T.; Simon, M.; Schulz, S. Volatiles Released by a Streptomyces Species Isolated from the North Sea. CB 2005, 2, 837–865. [Google Scholar] [CrossRef]
- Cho, J.Y.; Kang, J.Y.; Hong, Y.K.; Baek, H.H.; Shin, H.W.; Kim, M.S. Isolation and Structural Determination of the Antifouling Diketopiperazines from Marine-Derived Streptomyces Praecox 291-11. Biosci. Biotechnol. Biochem. 2012, 76, 1116–1121. [Google Scholar] [CrossRef] [Green Version]
- Hazato, T.; Kumagai, M.; Naganawa, H.; Aoyagi, T.; Umezawa, H. p-Hydroxyphenylacetaldoxime, an Inhibitor of β-Galactosidase, Produced by Actinomycetes. J. Antibiot. 1979, 32, 91–93. [Google Scholar] [CrossRef] [PubMed]
- Vaden, R.; Oswald, N.; Potts, M.; MacMillan, J.; White, M. FUSION-Guided Hypothesis Development Leads to the Identification of N6,N6-Dimethyladenosine, a Marine-Derived AKT Pathway Inhibitor. Mar. Drugs 2017, 15, 75. [Google Scholar] [CrossRef]
- Huang, C.; Yang, C.; Zhang, W.; Zhu, Y.; Ma, L.; Fang, Z.; Zhang, C. Albumycin, a New Isoindolequinone from Streptomyces Albus J1074 Harboring the Fluostatin Biosynthetic Gene Cluster. J. Antibiot. 2019, 72, 311–315. [Google Scholar] [CrossRef]
- Thissera, B.; Alhadrami, H.A.; Hassan, M.H.A.; Hassan, H.M.; Behery, F.A.; Bawazeer, M.; Yaseen, M.; Belbahri, L.; Rateb, M.E. Induction of Cryptic Antifungal Pulicatin Derivatives from Pantoea Agglomerans by Microbial Co-Culture. Biomolecules 2020, 10, 268. [Google Scholar] [CrossRef] [Green Version]
- Elleuch, L.; Shaaban, M.; Smaoui, S.; Mellouli, L.; Karray-Rebai, I.; Fourati-Ben Fguira, L.; Shaaban, K.A.; Laatsch, H. Bioactive Secondary Metabolites from a New Terrestrial Streptomyces Sp. TN262. Appl. Biochem. Biotechnol. 2010, 162, 579–593. [Google Scholar] [CrossRef] [Green Version]
- Lee, L.-H.; Chan, K.-G.; Stach, J.; Wellington, E.M.H.; Goh, B.-H. Editorial: The Search for Biological Active Agent(s) From Actinobacteria. Front. Microbiol. 2018, 9, 824. [Google Scholar] [CrossRef] [Green Version]
- De Lima Procópio, R.E.; da Silva, I.R.; Martins, M.K.; de Azevedo, J.L.; de Araújo, J.M. Antibiotics Produced by Streptomyces. Braz. J. Infect. Dis. 2012, 16, 466–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandra, N.; Kumar, S. Antibiotics Producing Soil Microorganisms. In Antibiotics and Antibiotics Resistance Genes in Soils; Hashmi, M.Z., Strezov, V., Varma, A., Eds.; Soil Biology; Springer International Publishing: Cham, Switzerland, 2017; Volume 51, pp. 1–18. ISBN 978-3-319-66259-6. [Google Scholar]
- Martins, M.B.; Carvalho, I. Diketopiperazines: Biological Activity and Synthesis. Tetrahedron 2007, 63, 9923–9932. [Google Scholar] [CrossRef]
- Fdhila, F.; Vázquez, V.; Sánchez, J.L.; Riguera, R. dd -Diketopiperazines: Antibiotics Active against Vibrio a Nguillarum Isolated from Marine Bacteria Associated with Cultures of Pecten m Aximus. J. Nat. Prod. 2003, 66, 1299–1301. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Srivastava, R.; De Clercq, E.; Singh, R.K. Synthesis and Antiviral Properties of Arabino and Ribonucleosides of 1,3-Dideazaadenine, 4-Nitro-1, 3-dideazaadenine and Diketopiperazine. Nucleosides Nucleotides Nucleic Acids 2004, 23, 1815–1824. [Google Scholar] [CrossRef]
- Byun, H.-G.; Zhang, H.; Mochizuki, M.; Adachi, K.; Shizuri, Y.; Lee, W.-J.; Kim, S.-K. Novel Antifungal Diketopiperazine from Marine Fungus. J. Antibiot. 2003, 56, 102–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, O.S.; Park, S.H.; Yun, B.-S.; Pyun, Y.R.; Kim, C.-J. cyclo(Dehydroala-L-Leu), an α-Glucosidase Inhibitor from Penicillium Sp. F70614. J. Antibiot. 2000, 53, 954–958. [Google Scholar] [CrossRef]
- Nicholson, B.; Lloyd, G.K.; Miller, B.R.; Palladino, M.A.; Kiso, Y.; Hayashi, Y.; Neuteboom, S.T.C. NPI-2358 Is a Tubulin-Depolymerizing Agent: In-Vitro Evidence for Activity as a Tumor Vascular-Disrupting Agent. Anti-Cancer Drugs 2006, 17, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Preciado, S.; Mendive-Tapia, L.; Torres-García, C.; Zamudio-Vázquez, R.; Soto-Cerrato, V.; Pérez-Tomás, R.; Albericio, F.; Nicolás, E.; Lavilla, R. Synthesis and Biological Evaluation of a Post-Synthetically Modified Trp-Based Diketopiperazine. Med. Chem. Commun. 2013, 4, 1171–1174. [Google Scholar] [CrossRef]
- Anderle, C.; Stieger, M.; Burrell, M.; Reinelt, S.; Maxwell, A.; Page, M.; Heide, L. Biological Activities of Novel Gyrase Inhibitors of the Aminocoumarin Class. Antimicrob. Agents Chemother. 2008, 52, 1982–1990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadhum, A.A.H.; Mohamad, A.B.; Al-Amiery, A.A.; Takriff, M.S. Antimicrobial and Antioxidant Activities of New Metal Complexes Derived from 3-Aminocoumarin. Molecules 2011, 16, 6969–6984. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Fu, A.; Zhang, L. Progress in Molecular Docking. Quant. Biol. 2019, 7, 83–89. [Google Scholar] [CrossRef] [Green Version]
- Hasan, M.R.; Chowdhury, S.M.; Aziz, M.A.; Shahriar, A.; Ahmed, H.; Khan, M.A.; Mahmud, S.; Emran, T.B. In Silico Analysis of Ciprofloxacin Analogs as Inhibitors of DNA Gyrase of Staphylococcus Aureus. Inform. Med. Unlocked 2021, 26, 100748. [Google Scholar] [CrossRef]
- Dolgonosov, A.M. The Universal Relationship between the Energy and Length of a Covalent Bond Derived from the Theory of Generalized Charges. Russ. J. Inorg. Chem. 2017, 62, 344–350. [Google Scholar] [CrossRef]
- Han, S.; Caspers, N.; Zaniewski, R.P.; Lacey, B.M.; Tomaras, A.P.; Feng, X.; Geoghegan, K.F.; Shanmugasundaram, V. Distinctive Attributes of β-Lactam Target Proteins in Acinetobacter Baumannii Relevant to Development of New Antibiotics. J. Am. Chem. Soc. 2011, 133, 20536–20545. [Google Scholar] [CrossRef]
- Wang, K.; Sitsel, O.; Meloni, G.; Autzen, H.E.; Andersson, M.; Klymchuk, T.; Nielsen, A.M.; Rees, D.C.; Nissen, P.; Gourdon, P. Structure and Mechanism of Zn2+-Transporting P-Type ATPases. Nature 2014, 514, 518–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skupińska, M.; Stępniak, P.; Łętowska, I.; Rychlewski, L.; Barciszewska, M.; Barciszewski, J.; Giel-Pietraszuk, M. Natural Compounds as Inhibitors of Tyrosyl-TRNA Synthetase. Microb. Drug Resist. 2017, 23, 308–320. [Google Scholar] [CrossRef] [PubMed]
- Wadapurkar, R.M.; Shilpa, M.D.; Katti, A.K.S.; Sulochana, M.B. In Silico Drug Design for Staphylococcus Aureus and Development of Host-Pathogen Interaction Network. Inform. Med. Unlocked 2018, 10, 58–70. [Google Scholar] [CrossRef]
- Watve, M.; Tickoo, R.; Jog, M.; Bhole, B. How Many Antibiotics Are Produced by the Genus Streptomyces? Arch. Microbiol. 2001, 176, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Antibiotic Discovery from Actinomycetes: Will a Renaissance Follow the Decline and Fall?|CiNii Research. Available online: https://cir.nii.ac.jp/crid/1570291226223159168 (accessed on 14 August 2022).


| Soil Samples | Location | Habitats | Altitude (m) | Geographical Coordinates |
|---|---|---|---|---|
| SB1 | Halesi, Khotang | Bare land | 3100 | 27.1846° N, 86.5938° E |
| SB2 | Muchchok, Gorkha | Forest | 1300 | 28.1371° N, 84.6584° E |
| SB3 | Shigash, Baitadi | Forest | 2800 | 29.5174° N, 80.5938° E |
| SB4 | Pame, Kaski | Agriculture land | 822 | 28.2256° N, 83.9466° E |
| SB5 | Nagarkot, Bhaktapur | Forest | 2175 | 27.7107° N, 85.5023° E |
| SB6 | Simbhanjyang, Makawanpur | Rhizosphere | 2310 | 27.5921° N, 85.0855° E |
| SB7 | Tatopani, Myagdi | Hot spring | 2180 | 28.4949° N, 83.6194° E |
| SB8 | Swargadawari, Pyuthan | Rhizosphere | 2100 | 28.1214° N, 82.6744° E |
| SB9 | Betini, Okhaldhunga | Forest | 1500 | 27.2866° N, 86.4733° E |
| SB10 | Muktinath, Mustang | Bare land | 3710 | 28.8190° N, 83.8716° E |
| Organisms | Zone of Inhibition (mm) | |||
|---|---|---|---|---|
| Streptomyces Species_SB1 | Streptomyces Species_SB3 | Streptomyces Species_SB10 | Neomycin (Control) | |
| S. aureus | 11 | 19 | 30 | 22 |
| E. coli | - | - | 18 | 18 |
| K. pneumoniae | - | 10 | - | 16 |
| S. typhi | - | - | - | 15 |
| S. sonnei | 10 | 17 | 30 | 23 |
| A. baumannii | 10 | 10 | 18 | 20 |
| S.N. | Annotated Compounds | Calculated Mass | Observed Mass | Molecular Formula | Double Bond Equivalence | Absolute Error (ppm) | Retention Time (min) | Source | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 1. | Cyclo-(L-Pro-4-OH-L-Leu) | 227.13 | 226.13 | C11H18N2O3 | 4.0 | 4.87 | 5.63 | SB1/SB3 | [34] |
| 2. | cyclo-(L-Pro-L-Val) | 196.12 | 197.12 | C10H16N2O2 | 4 | 3.76 | 5.39 | SB1/SB3 | [35] |
| 3. | cyclo (Tyr-Pro)/Maculosin | 260.11 | 261.12 | C14H16N2O3 | 8 | 1.17 | 6.09 | SB1/SB3 | [36] |
| 4. | Cyclo-(L-Leu-L-Pro) | 210.13 | 211.14 | C11H18N2O2 | 4 | 1.92 | 6.71 | SB1/SB3 | [37] |
| 5. | (3R,8aS)-3-Methyl-1,2,3,4,6,7,8, 8a-octahydropyrrolo [1,2-a]pyrazine1,4-dione | 168.08 | 169.09 | C8H12N2O2 | 4 | 3.79 | 3.24 | SB3 | [38] |
| 6. | Essramycin | 268.09 | 268.103 | C14H12N4O2 | 11 | 3.38 | 4.82 | SB3 | [39] |
| 7. | 2-Piperidinone | 99.06 | 100.07 | C5H9NO | 2 | 0.47 | 3.66 | SB1/SB3 | [40] |
| 8. | cyclo-[Pro-Phe] | 244.12 | 245.12 | C14H16N2O2 | 8 | 0.62 | 7.65 | SB1/SB3 | [41] |
| 9. | cyclo-(L-Pro-L-Val) | 196.12 | 197.12 | C10H16N2O2 | 4 | 3.76 | 5.42 | SB1/SB3 | [35] |
| S.N. | Annotated Compound | Calculated Mass | Observed Mass | Adduct Type | Molecular Formula | Retention Time (Min) | RDB | Error ppm | Spectral Match (Sirius Score) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. | (3S,6S)-3-benzyl-6-isopropylpiperazine-2,5-dione [Cyclo (L-Val-L-Phe)] | 246.14 | 247.14 | [M + H]+ | C14H18N2O2 | 16.0 | 7.0 | −1.1 | 32.59% | [42] |
| 2. | Nonactic acid-trihomononactic acid dilactone | 410.27 | 411.27 | [M + H]+ | C23H38O6 | 35.1 | 5.0 | 3.1 | 50.26% | [43] |
| 3. | Albaflavenol | 220.18 | 221.18 | [M + H]+ | C15H24O | 16.5 | 4.0 | −2.4 | 56.10% | [44] |
| 4. | Succinilene D | 338.25 | 339.25 | [M + H]+ | C20H34O4 | 33.7 | 4.0 | 4.1 | 45.32% | [45] |
| 5. | Benzyl acetate | 150.07 | 151.07 | [M + H]+ | C9H10O2 | 13.2 | 5.0 | −1.2 | 27.38% | [46] |
| 6. | (6S,3S)-6-benzyl-3-methyl-2,5-diketopiperazine [Cyclo(L-Phe-L-Ala)] | 218.11 | 219.11 | [M + H]+ | C12H14N2O2 | 12.7 | 7.0 | −1.5 | 66.22% | [47] |
| 7. | P-hydroxyphenylacetaldoxime | 151.06 | 152.06 | [M + H]+ | C8H9NO2 | 10.1 | 5.0 | −1.0 | 46.36% | [48] |
| 8. | N6,N6-dimethyladenosine | 295.23 | 296.23 | [M + H]+ | C12H17N5O4 | 9.3 | 7.0 | −1.2 | 75.56% | [49] |
| 9. | Cyclo(leucylprolyl) | 210.14 | 211.14 | [M + H]+ | C11H18N2O2 | 13.0 | 4.0 | −3.3 | 49.77% | [37] |
| 10 | Albumycin | 190.07 | 191.07 | [M + H]+ | C10H10N2O2 | 8.8 | 7.0 | 4.1 | 18.59% | [50] |
| 11. | Cyclo (L-Tyr-L-Leu) | 276.15 | 277.15 | [M + H]+ | C15H20N2O3 | 12.8 | 7.0 | −1.9 | 69.25% | [51] |
| 12. | Brevianamide F[Cyclo (L-Trp-L-Leu)] | 283.13 | 284.13 | [M + H]+ | C16H17N3O2 | 15.6 | 10.0 | −1.2 | 81.80% | [52] |
| 13. | Maculosin[cyclo (Tyr-Pro)] | 260.11 | 261.12 | [M + H]+ | C14H16N2O3 | 14..4 | 8.0 | −2.5 | - | [40] |
| 14. | Cyclo-(L-Pro-4-OH-L-Leu) | 226.13 | 227.13 | [M + H]+ | C11H18N2O3 | 5.6 | 4.0 | 4.87 | - | [34] |
| 15. | cyclo-(L-Pro-L-Val) | 196.12 | 197.12 | [M + H]+ | C10H16N2O2 | 5.3 | 4.0 | 3.76 | - | [35] |
| 16. | Cyclo-(L-Leu-L-Pro) | 210.13 | 211.14 | [M + H]+ | C11H18N2O2 | 6.0 | 4.0 | 1.92 | - | [37] |
| 17. | cyclo-[Pro-Phe] | 244.12 | 245.12 | [M + H]+ | C14H16N2O2 | 7.6 | 8.0 | 0.6 | - | [41] |
| Target Proteins (PDB ID) | Binding Energy (kcal/mol) | Interacting Residues | ||||||
|---|---|---|---|---|---|---|---|---|
| Brevianamide F | Essramycin | Cyclo(L-Phe-L-Ala) | Cyclo (L-Val-L-Phe) | Brevianamide F | Essramycin | Cyclo(L-Phe-L-Ala) | Cyclo (L-Val-L-Phe) | |
| S. aureus TyrRS (1JIJ) | −9.0 | −9.1 | −8.0 | −7.8 | Gly 38 Asp 40 Tyr 170 Gly 193 Gln 196 His 50 Pro 53 | Asp 40 Tyr 170 Gln 174 Gln 196 Ala 39 His 50 Leu 70 Asp 195 | Gly 38 Gln 174 Leu 70 | Gly 38 Asp 80 Tyr 170, Gln 174 Gln 196 Lys 84 |
| K. pneumonia PulA (6J33) | −8.7 | −7.6 | −7.3 | −7.0 | His 607 Leu 678 Asp 560 Tyr 892 | Asp 834 Glu 706 Asn 835 Arg 675 Tyr 559 Trp 557 Trp 708 Pro 745 | Glu 706 His 833 Arg 675 Tyr 559 Cys 643 | Glu 706 His 833 Arg 675 Asp 834 Tyr 559 Cys 643 Trp 708 Leu 678 |
| S. aureus topoisomerase ATPase inhibitor (3TTZ) | −8.7 | −8.5 | −6.2 | −6.3 | Asp 81 Ser 55 Glu 58 Ile 86 Ile 51 Ile 175 Arg 84 Pro 87 Leu 103 | Asp 81 Ser 55 Gly 85 Asn 54 Ile 86 Pro 87 Ile 51 Ile 175 Leu 103 | Asp 81 Ser 55 Glu 58 | Gln 66 His143 Lys 170 |
| S. aureus dihydrofolate reductase (3SRW) | −9.1 | −8.7 | −7.5 | −8.0 | Leu 21 Ile 15 Thr 47 Lys 46 | Gly 95 Thr 47 Lys 46 Leu63 | Leu 21 Phe 93 Gly 95 | Ala 8 Ile 15 Thr 47 Phe 93 Leu 21 Val 32 |
| S. sonneiZinc transporting p-type ATPase (4UMW) | −8.1 | −7.0 | −7.3 | −5.5 | Arg 345 Glu 184 Phe 210 Leu211 Pro 166 Glu214 Val397 | Arg 345 Leu 396 Ile 347 Pro 401 Val 397 Glu 184 | Arg 345 Glu 184 Phe 210 Ile 167 Pro 166 Glu 214 | Gly 694 Lys 693 Leu 69 Tyr 354 Phe 350 Leu 701 Val 361 Leu 697 |
| A. baumannii PBP1a (3UDI) | −7.5 | −7.7 | −6.2 | −6.6 | Ser 434 Gly 708, Tyr 707 Thr 672 | Ser 434 Gly 708 Tyr 707 | Ser 434 Thr 672 Leu 486 Asn 489 | Ser 434 Thr 672 Leu 48 Asn 489 Tyr 707 |
| A. baumannii FabG (7KRK) | −7.1 | −7.4 | −6.6 | −6.5 | Ile 19 Asn 88 Gly 90 Ala 89 Gly18 Ser 15 Leu 39 Val 111 | Asp 38 Gly 90 Ala 89 Leu 39 Val 111 Val 62 | Gly 12 Ile 19 Gly 20 Asn 88 Gly 90 Gly 18 Ala 89 | Ile 19 Gly 20 Asn 88 Gly 90 Ala 89 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhandari, S.; Bhattarai, B.R.; Adhikari, A.; Aryal, B.; Shrestha, A.; Aryal, N.; Lamichhane, U.; Thapa, R.; Thapa, B.B.; Yadav, R.P.; et al. Characterization of Streptomyces Species and Validation of Antimicrobial Activity of Their Metabolites through Molecular Docking. Processes 2022, 10, 2149. https://doi.org/10.3390/pr10102149
Bhandari S, Bhattarai BR, Adhikari A, Aryal B, Shrestha A, Aryal N, Lamichhane U, Thapa R, Thapa BB, Yadav RP, et al. Characterization of Streptomyces Species and Validation of Antimicrobial Activity of Their Metabolites through Molecular Docking. Processes. 2022; 10(10):2149. https://doi.org/10.3390/pr10102149
Chicago/Turabian StyleBhandari, Sobika, Bibek Raj Bhattarai, Ashma Adhikari, Babita Aryal, Asmita Shrestha, Niraj Aryal, Uttam Lamichhane, Ranjita Thapa, Bijaya B. Thapa, Ram Pramodh Yadav, and et al. 2022. "Characterization of Streptomyces Species and Validation of Antimicrobial Activity of Their Metabolites through Molecular Docking" Processes 10, no. 10: 2149. https://doi.org/10.3390/pr10102149
APA StyleBhandari, S., Bhattarai, B. R., Adhikari, A., Aryal, B., Shrestha, A., Aryal, N., Lamichhane, U., Thapa, R., Thapa, B. B., Yadav, R. P., Khadayat, K., Adhikari, A., Regmi, B. P., & Parajuli, N. (2022). Characterization of Streptomyces Species and Validation of Antimicrobial Activity of Their Metabolites through Molecular Docking. Processes, 10(10), 2149. https://doi.org/10.3390/pr10102149









