L-Glutathione-Functionalized Silica Adsorbent for the Removal of Pesticide Malathion from Aqueous Solutions
Abstract
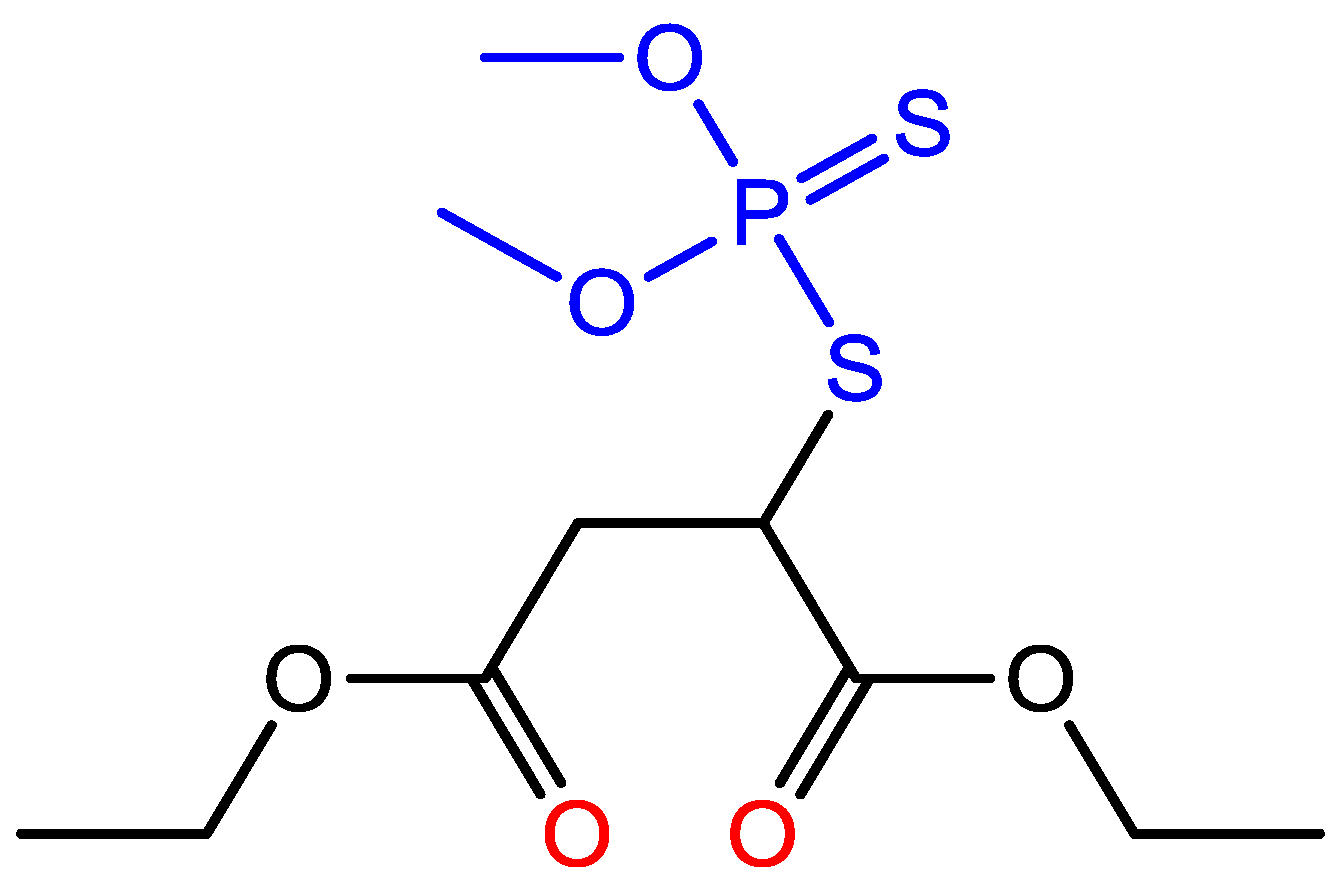
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Adsorbent SG-LGPS
2.3. Malathion Analysis
2.4. Characterization
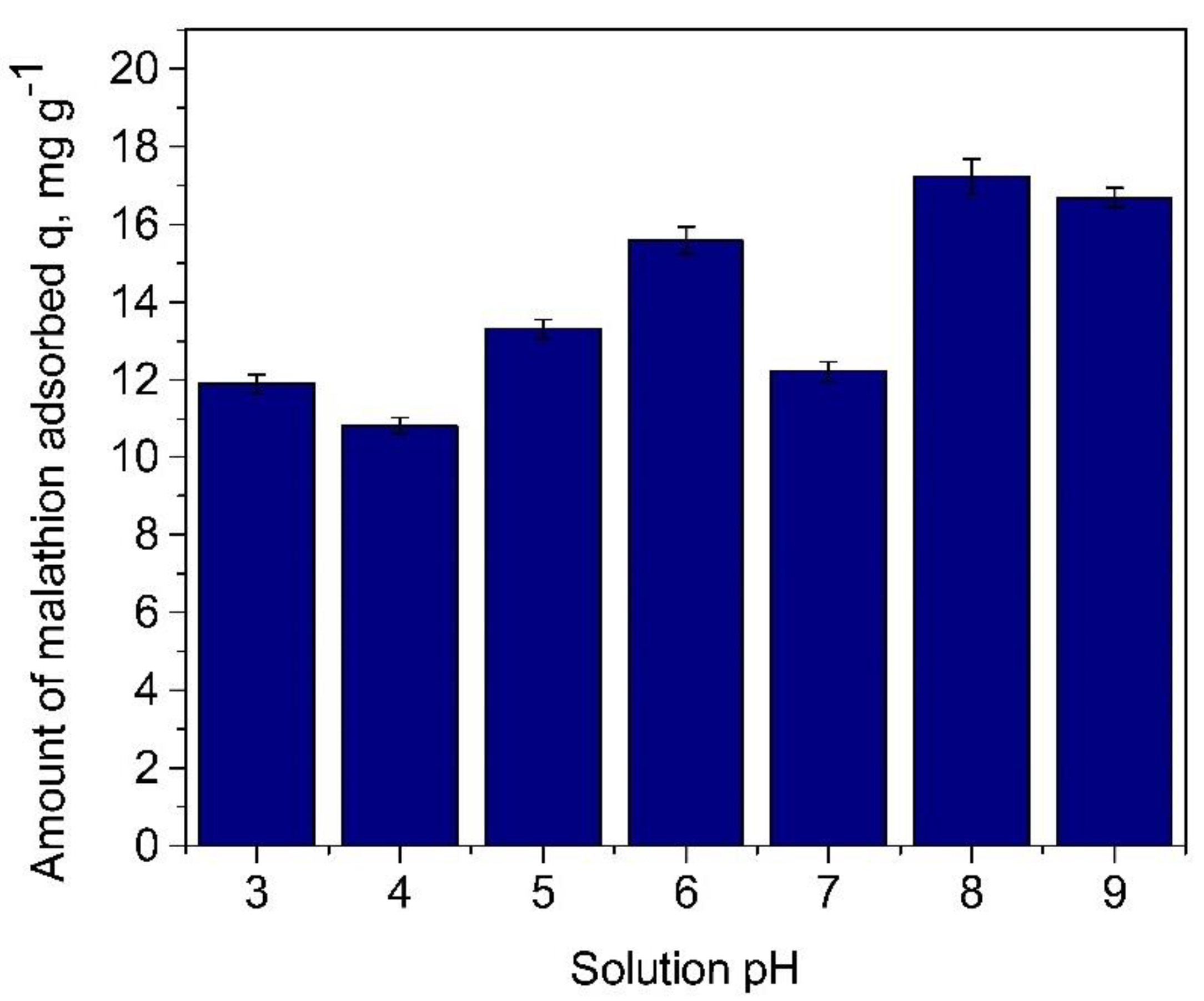
2.4.1. Malathion Adsorption as a Function of Solution pH
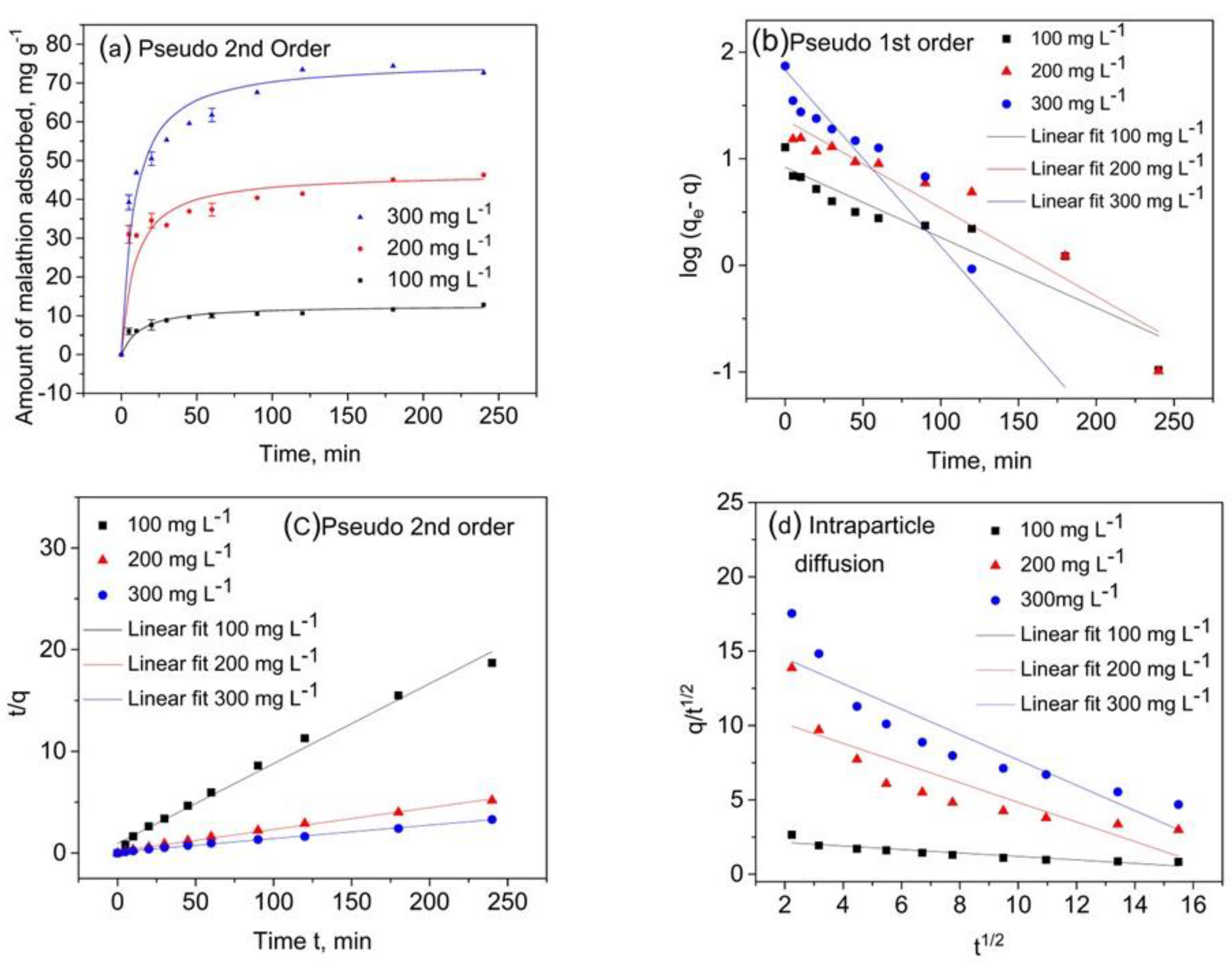
2.4.2. Malathion Adsorption Kinetics
2.4.3. Malathion Adsorption Isotherm Measurements
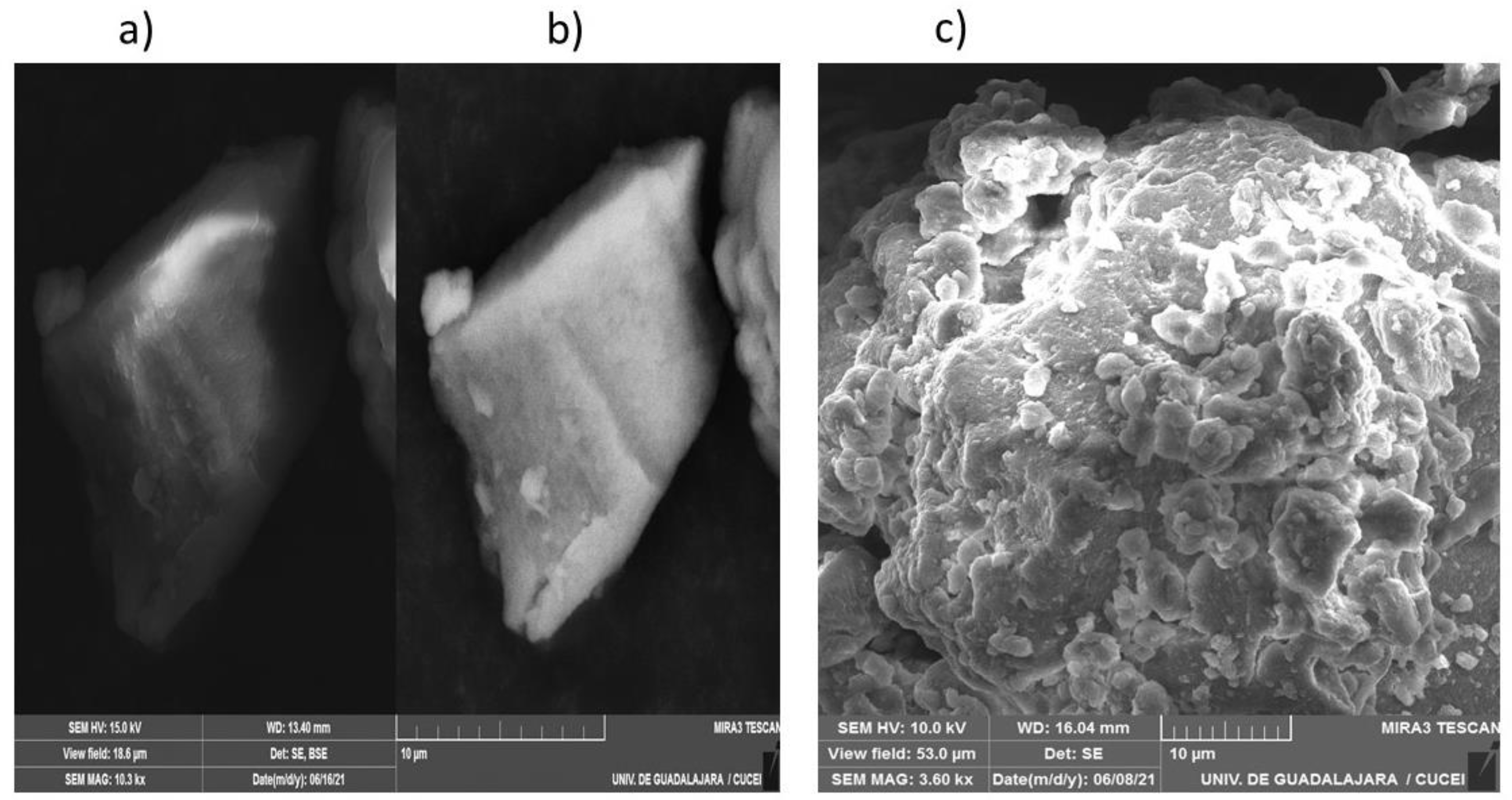
2.4.4. SEM Measurements
2.4.5. TGA Measurements
2.4.6. Elemental Analysis Measurements
3. Results and Discussion
3.1. Characterization
3.1.1. Effect of Solution pH on Malathion Uptake
3.1.2. Malathion Adsorption Kinetic Results
3.1.3. Malathion Adsorption Isotherm Results
3.1.4. Efficiency of Adsorption of Malathion by Various Adsorbents
3.1.5. SEM Results
3.1.6. Elemental Analysis Results
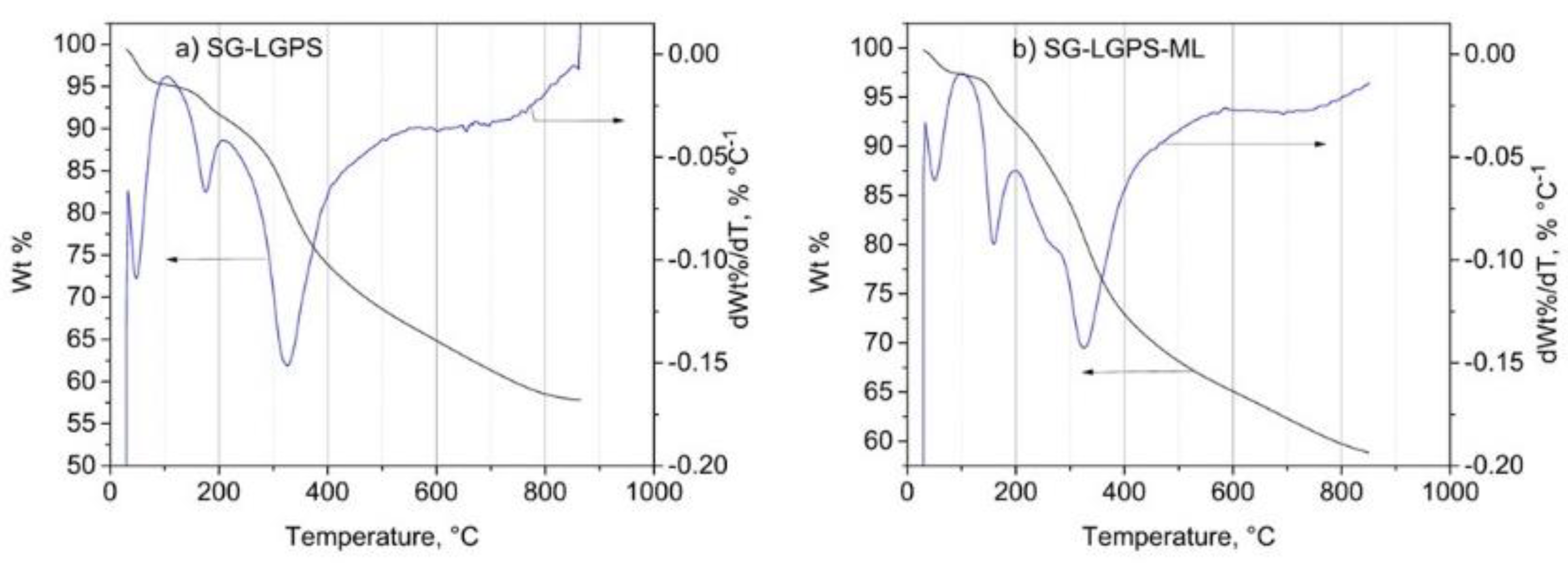
3.1.7. TGA Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tadeo, J.L. Analysis of Pesticides in Food and Environmental Samples; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Naushad, M.; Othman, Z.A.A.L.; Khan, M.R.; ALQahtania, N.J.; ALSohaimi, I.H. Equilibrium, kinetics and thermodynamic studies for the removal of organophosphorus pesticide using Amberlyst-15 resin: Quantitative analysis by liquid chromatography–mass spectrometry. J. Ind. Eng. Chem. 2014, 20, 4393–4400. [Google Scholar] [CrossRef]
- ICAR. Report of the Special Committee on Harmful Effects of Pesticides; ICAR: New Delhi, India, 1967; p. 78. [Google Scholar]
- UN/DESA. Changing Unsustainable Patterns of Consumption and Production, Johannesburg Plan on Implementation of the World Summit on Sustainable Development, Johannesburg, 2002 (Chapter III).
- Casarett, L.J.; Doull’s, J. Toxicology, the Basic Science of Poisons, 6th ed.; MacGraw Hill: New York, NY, USA, 2001. [Google Scholar]
- Lintellman, J.; Katayama, A.; Kurihara, N.; Shore, L.; Wenzel, A. Endocrine disruptors in the environment (IUPAC Technical Report). Pure Appl. Chem. 2003, 75, 631. [Google Scholar] [CrossRef] [Green Version]
- Andersen, H.R.; Vinggaard, A.M.; Rasmussen, T.; Gjermandsen, I.M.; Bonefeld-Jorgensen, E.C. Effects of Currently Used Pesticides in Assays for Estrogenicity, Androgenicity, and Aromatase Activity in VitroToxicol. Appl. Pharmacol. 2002, 179, 1. [Google Scholar] [CrossRef] [PubMed]
- Kojima, H.; Katsura, E.; Takeuchi, S.; Niiyama, K.; Kobayashi, K. Screening for Estrogen and Androgen Receptor Activities in 200 Pesticides by In Vitro Reporter Gene Assays Using Chinese Hamster Ovary Cells Environ. Health Perspect. 2004, 112, 524. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Li, N.; Ma, M.; Giesy, J.P.; Wang, Z. In vitro profiling of the endocrine disrupting potency of organochlorine pesticides. Toxicol. Lett. 2008, 183, 65. [Google Scholar] [CrossRef] [PubMed]
- Drinking Water Directive (DWD) of European Community, Council Directive 98/83/EC, 2007, July.
- Tchounwou, P.B.; Patlolla, A.K.; Yedjou, C.G.; Moore, P.D. Environmental Exposure and Health Effects Associated with Malathion Toxicity. In Toxicity and Hazard of Agrochemicals; IntechOpen: London, UK, 2015. [Google Scholar] [CrossRef] [Green Version]
- Singh, B.K.; Walker, A. Microbial degrading of organophosphorus compounds. FEMS Microbiol. Rev. 2006, 30, 428–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumari, A.R.; Jeevan, G.; Ashok, M.; Rao, C.K.; Vamsi, K.S.K. Malathion degradation by Bacillus spp. isolated from soil. J. Pharm. 2012, 2, 37–42. [Google Scholar]
- Hao, X.; Wang, H.-M. A study of the degradation of organophosphorus pesticides in river waters and the identification of their degradation products by chromatography coupled with mass spectrometry. Arch. Environ. Contam. Toxicol. 2009, 56, 646–653. [Google Scholar]
- Trac, L.N.; Andersen, O.; Palmqvist, A. Deciphering mechanisms of malathion toxicity under pulse exposure of the freshwater cladoceran Daphnia magna. Environ. Toxicol. Chem. 2016, 35, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Key, P.B.; Fulton, M.H.; Scott, G.I.; Layman, S.L.; Wirth, E.F. Lethal and sublethal effects of malathion on three life stages of the grass shrimp, Palaemonetes pugio. Aquat. Toxicol. 1998, 40, 311–322. [Google Scholar] [CrossRef]
- Naushad, M.; ALOthman, Z.A.; Khan, M.R. Removal of malathion from aqueous solution using De-Acidite FF-IP resin and determination by UPLC–MS/MS: Equilibrium, kinetics and thermodynamic studies. Talanta 2013, 115, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yan, X.; Gao, C.; Duan, J.; Beecham, S. A consecutive chlorination and alkaline hydrolysis process for rapid degradation and detoxication of malathion in aqueous solution. Chem. Eng. J. 2020, 392, 123793. [Google Scholar] [CrossRef]
- Thabit, T.M.A.; EL-Naggar, M.A.H. Malathion degradation by soil isolated bacteria and detection of degradation products by GC-MS. Int. J. Environ. Sci. 2013, 3, 1467–1476. [Google Scholar]
- Chelme-Ayala, P.; El-Din, M.G.; Smith, D.W.; Adams, C.D. Oxidation kinetics of two pesticides in natural waters by ozonation and ozone combined with hydrogen peroxide. Water Res. 2011, 45, 2517–2526. [Google Scholar] [CrossRef] [PubMed]
- Sorour, M.H.; Shaalan, H.F. Removal of malathion using ceramic nanofiltration/adsorption system. Desalin. Water Treat. 2013, 51, 5009–5013. [Google Scholar] [CrossRef]
- Kumar, S.S.; Ghosh, P.; Malyan, S.K.; Sharma, J.; Kumar, V. A comprehensive review on enzymatic degradation of the organophosphate pesticide malathion in the environment. J. Environ. Sci. Health Part C Environ. Carcinog. Ecotoxicol. Rev. 2019, 37, 288–329. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, H.; Bagheri, H. Highly efficient Zr-MOF@WO3/graphene oxide photocatalyst: Synthesis, characterization and photodegradation of tetracycline and malathion. Mater. Sci. Semicond. Process. 2020, 107, 104815. [Google Scholar] [CrossRef]
- Topalov, A.; Abramovic, B.; Molnar-Gabor, D.; Csanadi, J.; Arcson, O. Photocatalytic oxidation of the herbicide (4-chloro-2-methylphenoxy)acetic acid (MCPA) over TiO2. J. Photochem. Photobiol. A Chem. 2001, 140, 249–253. [Google Scholar] [CrossRef]
- Martín, M.M.B.; Pérez, J.A.S.; López, J.L.C.; Oller, I.; Rodríguez, S.M. Degradation of a four-pesticide mixture by combined photo-Fenton and biological oxidation. Water Res. 2009, 43, 653–660. [Google Scholar] [CrossRef]
- Behloul, M.; Grib, H.; Drouiche, N.; Abdi, N.; Lounici, H.; Mameri, N. Removal of malathion pesticide from polluted solutions by electrocoagulation: Modeling of experimental results using response surface methodology. Sep. Sci. Technol. 2013, 48, 664–672. [Google Scholar] [CrossRef]
- Upadhyay, A.P.; Mistry, N.J. Feasibility of combined Fenton & coagulation method for the treatment of pesticides waste water Int. J. Eng. Res. Technol. 2012, 1, 115–125. [Google Scholar]
- Yang, Y.; Guo, Y.; Hu, C.; Wang, Y.; Wang, E. Preparation of surface modifications of mesoporous titania with monosubstituted Keggin units and their catalytic performance for organochlorine pesticide and dyes under UV irradiation. Appl. Catal. A 2004, 273, 201–210. [Google Scholar] [CrossRef]
- Mojiri, A.; Zhou, J.L.; Robinson, B.; Ohashi, A.; Ozaki, N.; Kindaichi, T.; Farraji, H.; Vakili, M. Pesticides in aquatic environments and their removal by adsorption methods. Chemosphere 2020, 253, 126646. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.; Hasany, S.M.; Bhager, M.I.; Iqbal, S. Low cost sorbents for the removal of methyl parathion pesticide from aqueous solutions. Chemosphere 2007, 66, 1829–1838. [Google Scholar] [CrossRef]
- Kannan, N.; Sundaram, M.M. Kinetics and mechanism of removal of methylene blue by adsorption on various carbons-a comparative study. Dye. Pigment. 2001, 51, 25–40. [Google Scholar] [CrossRef]
- Naushad, M.; AL-Othman, Z.A.; Islam, M. Adsorption of cadmium ion using a new composite cation-exchanger polyaniline Sn(IV) silicate: Kinetics, thermodynamic and isotherm studies. Int. J. Environ. Sci. Technol. Vol. 2013, 10, 567–578. [Google Scholar] [CrossRef] [Green Version]
- Ali, I. New Generation Adsorbents for Water Treatment. Chem. Rev. 2012, 112, 5073–5091. [Google Scholar] [CrossRef]
- Ioannidou, O.A.; Zabaniotou, A.A.; Stavropoulos, G.G.; Islam, M.A.; Albanis, T.A. Preparation of activated carbons from agricultural residues for pesticide adsorption. Chemosphere 2010, 80, 1328–1336. [Google Scholar] [CrossRef]
- Bouchenafa-Saïb, N.; Mekarzia, A.; Bouzid, B.; Mohammedi, O.; Khelifa, A.; Benrache, N. Removal of malathion from polluted water by adsorption onto chemically activated carbons produced from coffee grounds. Desalin. Water Treat. 2012, 52, 4920–4927. [Google Scholar] [CrossRef]
- Jusoh, A.; Hartini, W.J.H.; Ali, N.; Endut, A. Study on the removal of pesticide in agricultural run off by granular activated carbon. Bioresour. Technol. 2011, 102, 5312–5318. [Google Scholar] [CrossRef]
- Dehghani, M.H.; Niasar, Z.S.; Mehrnia, M.R.; Shayeghi, M.; Al-Ghouti, M.A.; Heibati, B.; McKay, G.; Yetilmezsoy, K. Optimizing the removal of organophosphorus pesticide malathion from water using multi-walled carbon nanotubes. Chem. Eng. J. 2017, 310, 22–32. [Google Scholar] [CrossRef]
- Surendra, B.; Manikanta Raju, B.; Srikanth Onesimus, K.N.; Lavkumar Choudhary, G.; Francis Paul, P.; Vangalapati, M. Synthesis and characterization of Ni doped TiO2 nanoparticles and its application for the degradation of malathion. Mater. Today Proc. 2020, 26, 1091–1095. [Google Scholar] [CrossRef]
- Kumar, P.; Singh, H.; Kapur, M.; Kumar Mondal, M. Comparative study of malathion removal from aqueous solution by agricultural and commercial adsorbents. J. Water Process Eng. 2014, 3, 67–73. [Google Scholar] [CrossRef]
- Donia, A.M.; Atia, A.A.; Hussien, R.A.; Rashad, R.T. Comparative study on the adsorption of malathion pesticide by different adsorbents from aqueous solution. Desalination Water Treat. 2012, 47, 300–309. [Google Scholar] [CrossRef]
- Asadi, M.; Sereshti, H. Magnetic amino-functionalized hollow silica-titania microsphere as an efficient sorbent for extraction of pesticides in green and roasted coffee beans. J. Sep. Sci. 2020, 43, 2115–2124. [Google Scholar] [CrossRef]
- Rodríguez-de-la-Peña, S.; Gómez-Salazar, S.; Gutiérrez-Ortega, J.A.; Badillo-Camacho, J.; Peregrina-Lucano, A.A.; Shenderovich, I.G.; Manríquez-González, R. Novel Silica Hybrid Adsorbent Functionalized with L-Glutathione Used for the Uptake of As(V) from Aqueous Media. Ind. Eng. Chem. Res. 2022, 61, 4348–4362. [Google Scholar] [CrossRef]
- Pergal, M.V.; Kodranov, I.D.; Gašić, M.M.P.; Stanković, D.M.; Petković, B.B.; Manojlović, D.D. Degradation Products, Mineralization, and Toxicity Assessment of Pesticides Malathion and Fenitrothion. Water Air Soil Pollut. 2020, 231, 433. [Google Scholar] [CrossRef]
- Corbett, J.F. Pseudo first-order kinetics. J. Chem. Educ. 1972, 49, 663. [Google Scholar] [CrossRef]
- Chen, H.; Wang, A. Adsorption Characteristics of Cu(II) from Aqueous Solution onto Poly(Acrylamide)/Attapulgite Composite. J. Hazard. Mater. 2009, 165, 223–231. [Google Scholar] [CrossRef]
- Ho, Y.S.; Mckay, G. Pseudo-Second Order Model for Sorption Processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Walter, J.; Weber, J.; Morris, J.C. Kinetics of Adsorption on Carbon from Solution. J. Sanit. Eng. Div. Am. Soc. Civ. Eng. 1963, 89, 31–60. [Google Scholar] [CrossRef]
- Reichenberg, D. Properties of Ion-Exchange Resins in Relation to their Structure. III. Kinetics of Exchange. J. Am. Chem. Soc. 1953, 75, 589–597. [Google Scholar] [CrossRef]
- DOF. Salud Ambiental, Agua Para Uso Y Consumo Humano-Limites Permisibles de Calidad Y Tratamientos a Que Debe Someterse El Agua Para SU Potabilizacion; DOF: Gazette, Mexico, 1994. NOM-SSA-127-1994. (In Spanish) [Google Scholar]
- Giles, C.H.; Macewan, T.H.; Nakhwa, S.N.; Smith, D. Studies in adsorption. Part XI. A system of classification of solution adsorption isotherms, and its use in diagnosis of adsorption mechanisms and in measurement of specific surface areas of solids. J. Chem. Soc. 1960, 2, 3973–3993. [Google Scholar] [CrossRef]
- Atia, A.A.; Donia, A.M.; Hussien, R.A.; Rashad, R.T. Efficient adsorption of malathion from different media using thermally treated kaolinite. Desalination Water Treat. 2011, 30, 178–185. [Google Scholar] [CrossRef] [Green Version]
- Sabbagh, N.; Tahvildari, K.; Sharif, A.A.M. Application of chitosan-alginate bio composite for adsorption of malathion from wastewater: Characterization and response surface methodology. J. Contam. Hydrol. 2021, 242, 103868. [Google Scholar] [CrossRef]
- Wanjeri, V.W.O.; Sheppard, C.J.; Prinsloo, A.R.E.; Ngila, J.C.; Ndungu, P.G. Isotherm and kinetic investigations on the adsorption of organophosphorus pesticides on graphene oxide-based silica coated magnetic nanoparticles functionalized with 2-phenylethylamine. J. Environ. Chem. Eng. 2018, 6, 1333–1346. [Google Scholar] [CrossRef]
- Habila, M.A.; ALOthman, Z.A.; Al-Tamrah, S.A.; Ghafar, A.A.; Soylak, M. Activated carbon from waste as an efficient adsorbent for malathion for detection and removal purposes. J. Ind. Eng. Chem. 2015, 32, 336–344. [Google Scholar] [CrossRef]
- Ju, X.J.; Chu, L.Y.; Liu, L.; Mi, P.; Lee, Y.M. A Novel Thermoresponsive Hydrogel with Ion-Recognition Property through Supramolecular Host−Guest Complexation. J. Phys. Chem. B 2008, 112, 1112–1118. [Google Scholar] [CrossRef] [PubMed]
- Gruenberg, B.; Emmler, T.; Gedat, E.; Shenderovich, I.; Findenegg, G.H.; Limbach, H.-H.; Buntkowsky, G. Hydrogen Bonding of Water Confined in Mesoporous Silica MCM-41 and SBA-15 Studied by 1H Solid-State NMR. Chem. Eur. J. 2004, 10, 5689–5696. [Google Scholar] [CrossRef] [PubMed]
- Gurinov, A.A.; Mauder, D.; Akcakayiran, D.; Findenegg, G.H.; Shenderovich, I.G. Does Water Affect the Acidity of Surfaces? The Proton-Donating Ability of Silanol and Carboxylic Acid Groups at Mesoporous Silica. ChemPhysChem 2012, 13, 2282–2285. [Google Scholar] [CrossRef] [PubMed]
- Mauder, D.; Akcakayiran, D.; Lesnichin, S.B.; Findenegg, G.H.; Shenderovich, I.G. Acidity of Sulfonic and Phosphonic Acid-Functionalized SBA-15 under Almost Water-Free Conditions. J. Phys. Chem. C 2009, 113, 19185–19192. [Google Scholar] [CrossRef]
- Singh, B.K.; Bhagwan, P.M.; Garg, S. Nickel(II) complexes of biologically active glutathione: Spectroscopic, kinetics of thermal decomposition and XRPD studies. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2007, 67, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Ortega, J.A.; Gomez-Salazar, S.; Shenderovich, I.G.; Manríquez-Gonzalez, R. Efficiency and lead uptake mechanism of a phosphonate functionalized mesoporous silica through P/Pb association ratio. Mater. Chem. Phys. 2020, 239, 122037. [Google Scholar] [CrossRef]


| Model Parameters | Malathion Initial Concentration, mg L−1 | ||
|---|---|---|---|
| 100 | 200 | 300 | |
| qe (mg g−1) experimental | 12.8 | 46.3 | 74.4 |
| Pseudo-first-order | |||
| qe (mg g−1) | 8.34 | 23.27 | 67.70 |
| k1 (min−1) | −0.0152 | −0.0191 | −0.0379 |
| R2 | 0.883 | 0.914 | 0.933 |
| Pseudo-second-order | |||
| qe (mg g−1) | 12.72 | 46.72 | 75.75 |
| k2 (g mg min−1) | 0.0065 | 0.0026 | 0.0017 |
| R2 | 0.990 | 0.995 | 0.997 |
| Intraparticle diffusion | |||
| A (mg g−1 min−1/2) | 2.366 | 11.432 | 16.220 |
| B (mg g−1 min−1) | −0.1169 | −0.6597 | −0.8531 |
| R2 | 0.826 | 0.727 | 0.833 |
| Initial pH | Langmuir | Freundlich | Temkin | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| KL (L mg−1) | qmax exp (mg g−1) | qmax calc (mg g−1) | R2 | n | KF (mg1−1/n L1/n g−1) | R2 | b (J mol−1)/(mg g−1) | A (L mg−1) | R2 | |
| 5.00 | −615.41 | 94.66 | 0.082 | 0.991 | 0.681 | 0.012 | 0.963 | 62.21 | 0.014 | 0.778 |
| 6.00 | 126.05 | 71.02 | 0.777 | 0.988 | 1.600 | 2.123 | 0.957 | 130.45 | 0.107 | 0.991 |
| 7.00 | −233.56 | 126.45 | 0.077 | 0.910 | a | 0.004 | 0.969 | 41.84 | 0.017 | 0.738 |
| 8.00 | −204.75 | 130.12 | 0.047 | 0.990 | 0.386 | 9.6 × 10−5 | 0.942 | 31.35 | 0.014 | 0.665 |
| Adsorbent | OPP Removed | qmax, mg g−1 | Removal Efficiency, % | Time, min | Reference |
|---|---|---|---|---|---|
| Chitosan-alginate | Malathion | 98.0 | 82.35 | 20 | [52] |
| MWCNTs | Malathion | --- | ~100 | 30 | [37] |
| De-Acidite FF-IP resin | Malathion | 16.39 | 96 | 40 | [17] |
| Amberlyst-15 resin | Malathion | --- | 96 | 30 | [2] |
| Fe3O4@SiO2@GO-PEA | Chlorpyrifos, malathion, and parathion | 32.6 | --- | 15 | [53] |
| Activated carbon from waste | Malathion | 32.1 | --- | 120 | [54] |
| SG-LGPS material | Malathion | 130.12 | 80 | 90 | This study |
| Sample | N% | C% | H% | S% | C/N | Si% |
|---|---|---|---|---|---|---|
| SG-LGPS | 2.91 | 18.60 | 3.51 | 11.73 | 6.39 | 17.2 a,b |
| SG-LGPS-ML | 2.34 | 19.10 | 3.57 | 13.23 | 8.16 | --- |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vegas-Mendoza, S.M.; Gutierrez-Ortega, J.A.; Moran-Salazar, R.G.; Cortes-Llamas, S.A.; Carbajal-Arizaga, G.G.; Peregrina-Lucano, A.A.; Shenderovich, I.G.; Torres-Santiago, G.; Gómez-Salazar, S. L-Glutathione-Functionalized Silica Adsorbent for the Removal of Pesticide Malathion from Aqueous Solutions. Processes 2022, 10, 2146. https://doi.org/10.3390/pr10102146
Vegas-Mendoza SM, Gutierrez-Ortega JA, Moran-Salazar RG, Cortes-Llamas SA, Carbajal-Arizaga GG, Peregrina-Lucano AA, Shenderovich IG, Torres-Santiago G, Gómez-Salazar S. L-Glutathione-Functionalized Silica Adsorbent for the Removal of Pesticide Malathion from Aqueous Solutions. Processes. 2022; 10(10):2146. https://doi.org/10.3390/pr10102146
Chicago/Turabian StyleVegas-Mendoza, Sonia M., José A. Gutierrez-Ortega, Rene G. Moran-Salazar, Sara A. Cortes-Llamas, Gregorio G. Carbajal-Arizaga, Alejandro A. Peregrina-Lucano, Ilya G. Shenderovich, Gabriela Torres-Santiago, and Sergio Gómez-Salazar. 2022. "L-Glutathione-Functionalized Silica Adsorbent for the Removal of Pesticide Malathion from Aqueous Solutions" Processes 10, no. 10: 2146. https://doi.org/10.3390/pr10102146






