Managing Transport Processes in Thermal Cracking to Produce High-Quality Fuel from Extra-Heavy Waste Crude Oil Using a Semi-Batch Reactor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
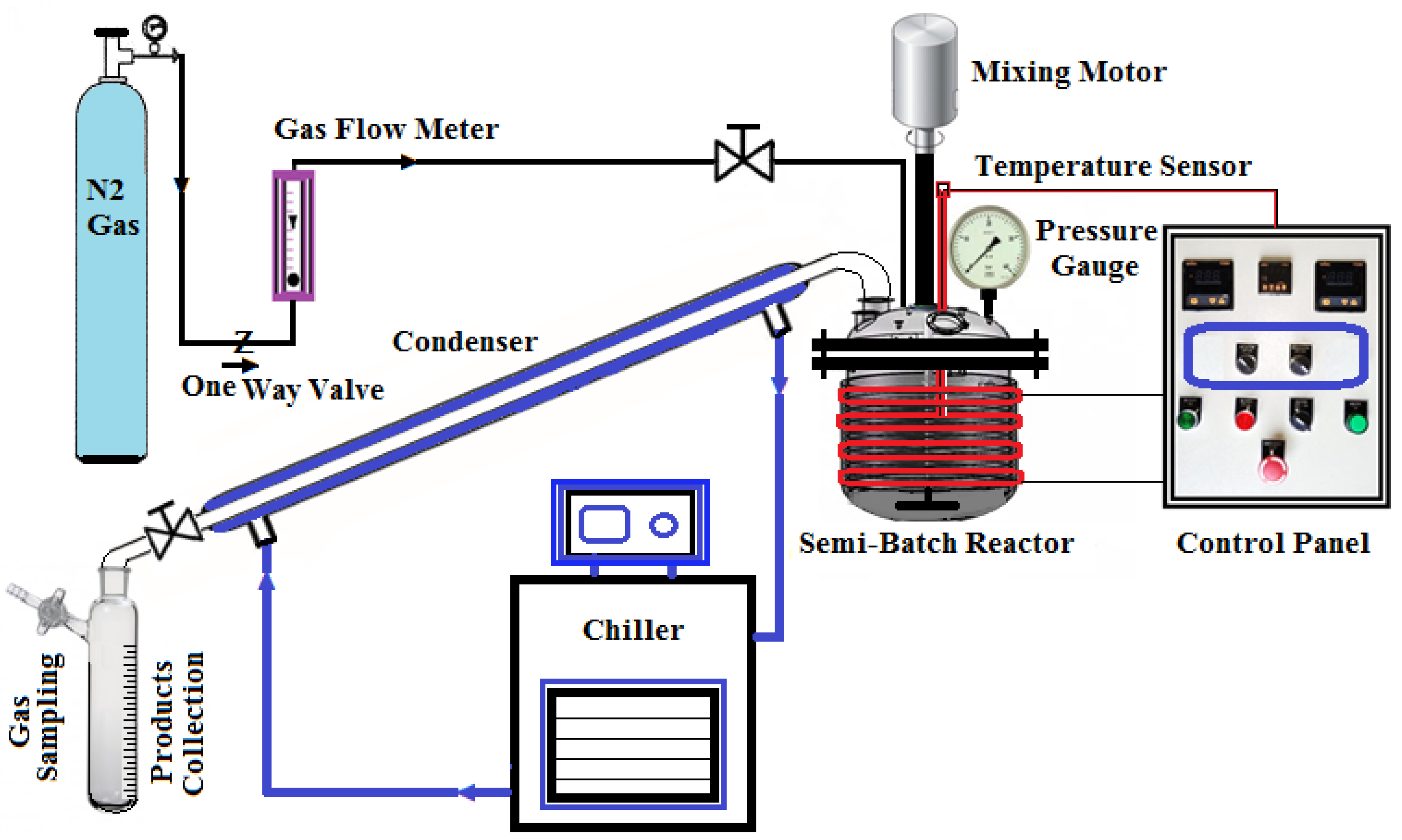
2.2. Thermal Cracking Apparatus
2.3. Cracking Reaction Procedure
3. Results and Discussion
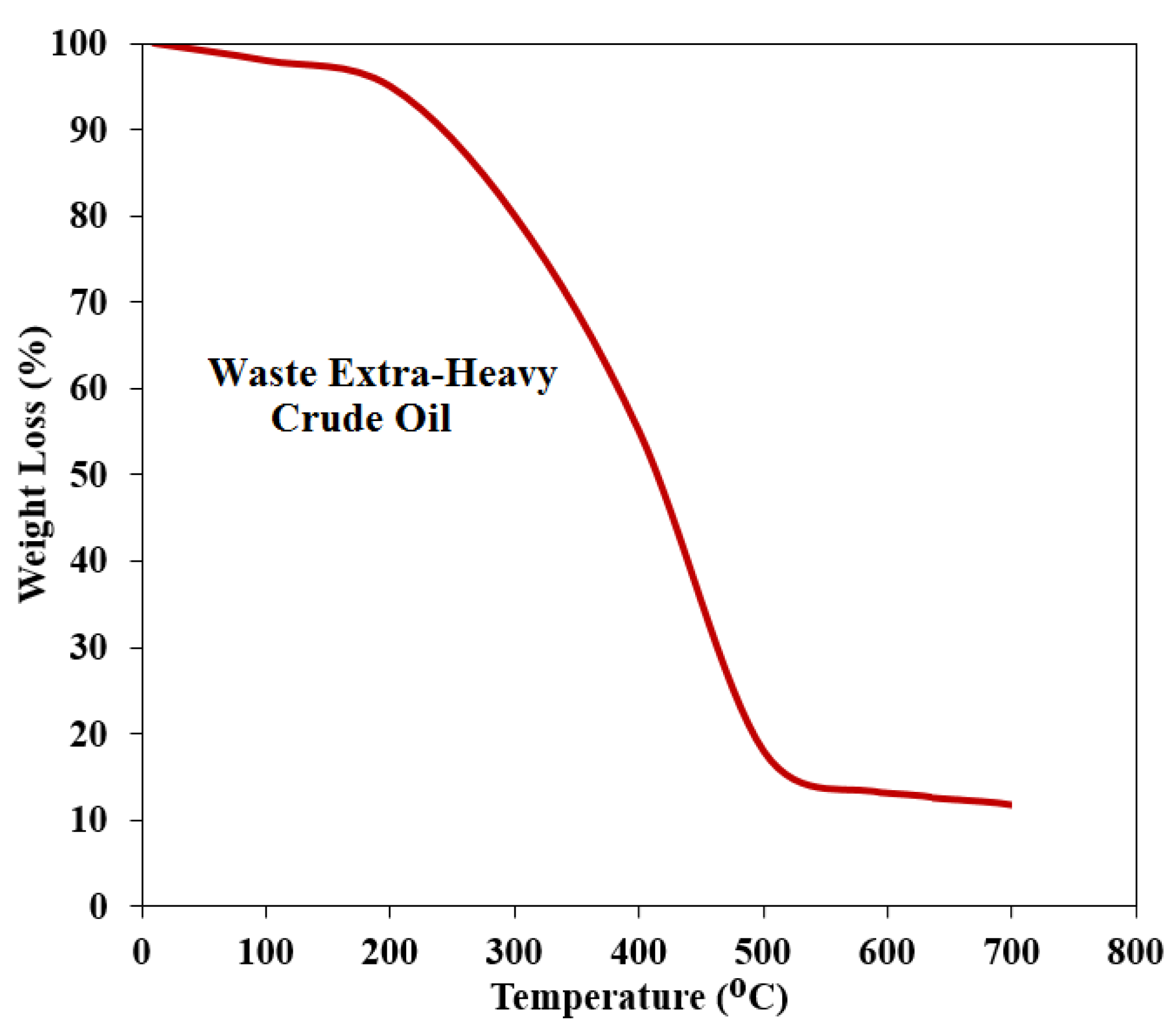
3.1. Thermogravimetric Analysis (TGA)
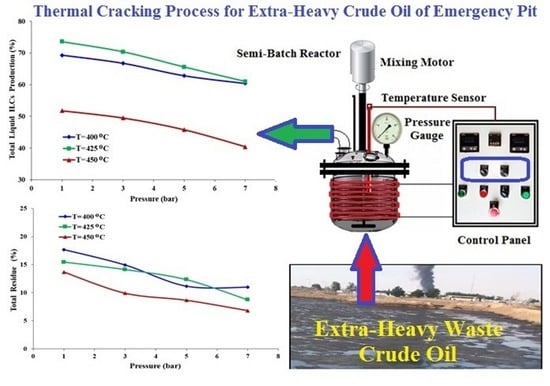
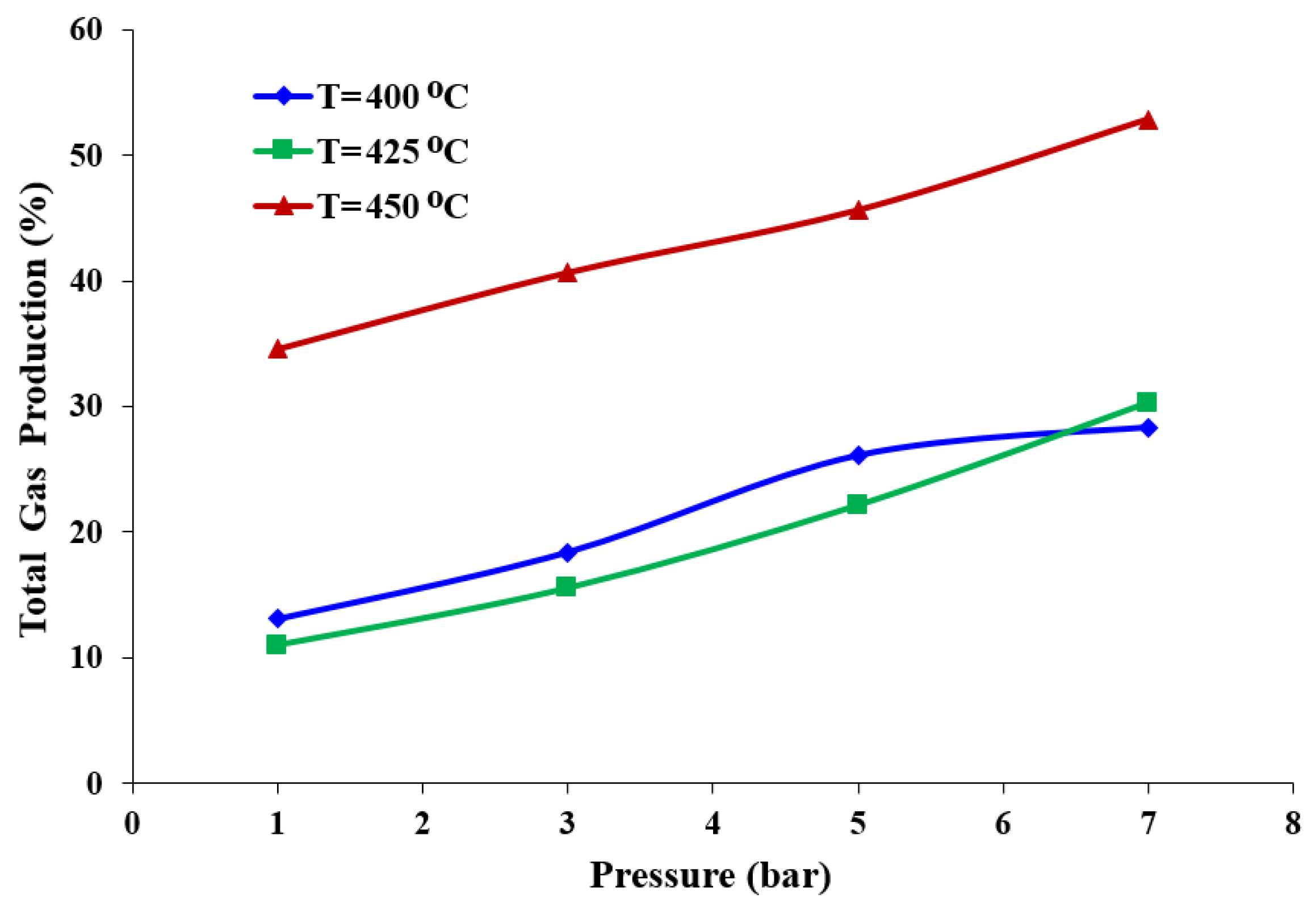
3.2. Influence of the Temperature on the Cracking Reaction
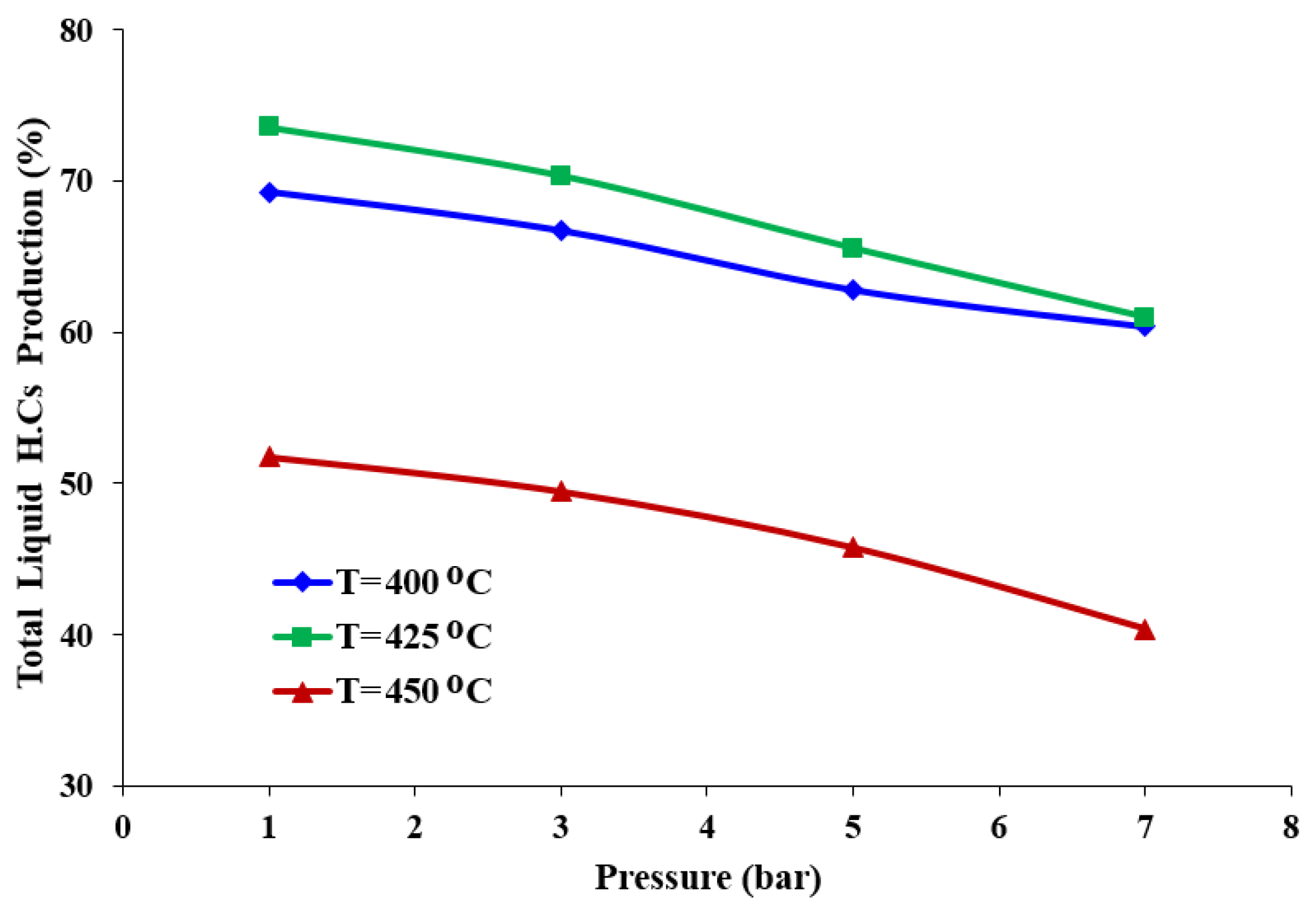
3.3. Effect of the Pressure and Temperature on the Quality of the Liquid Product
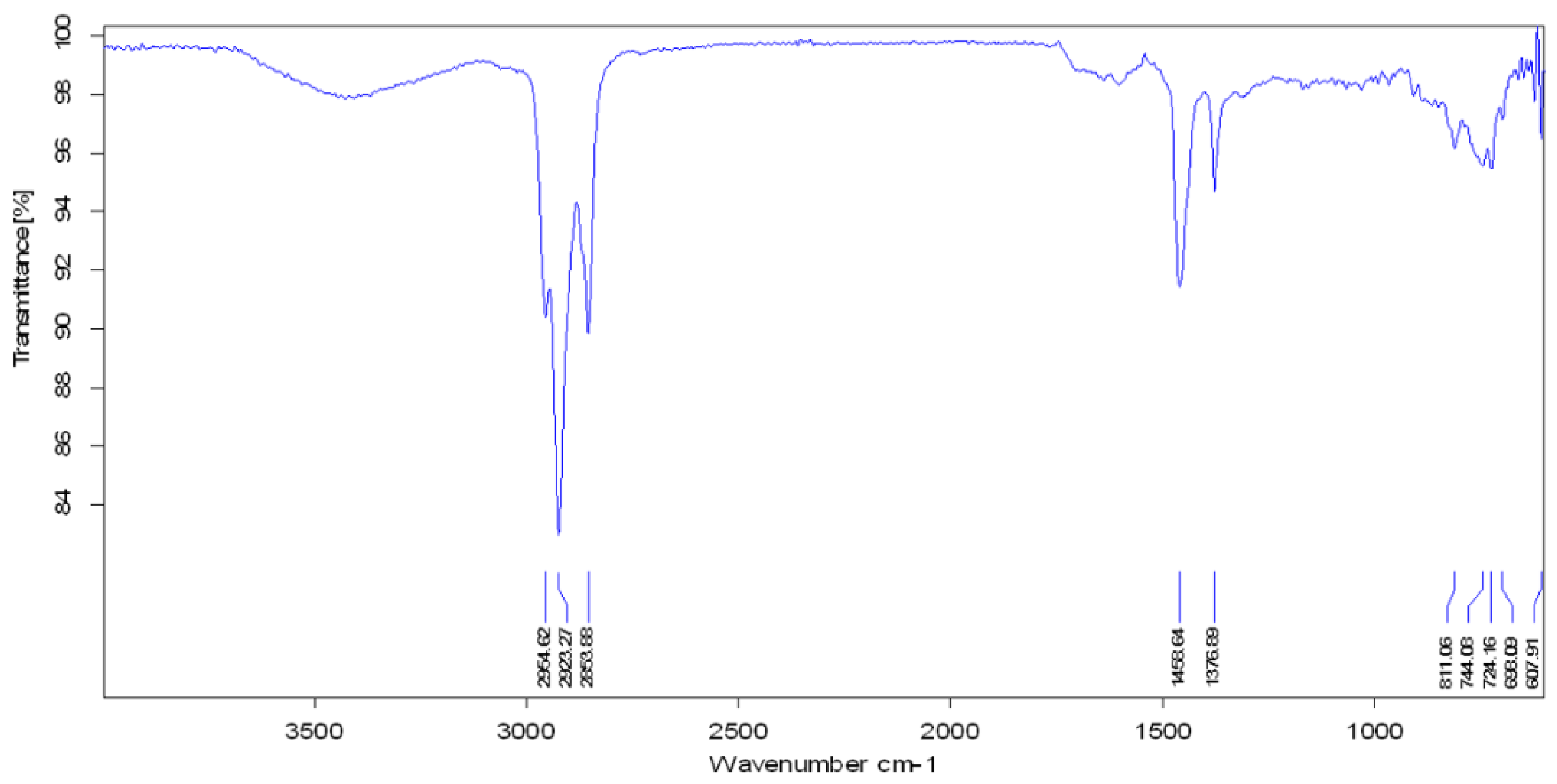
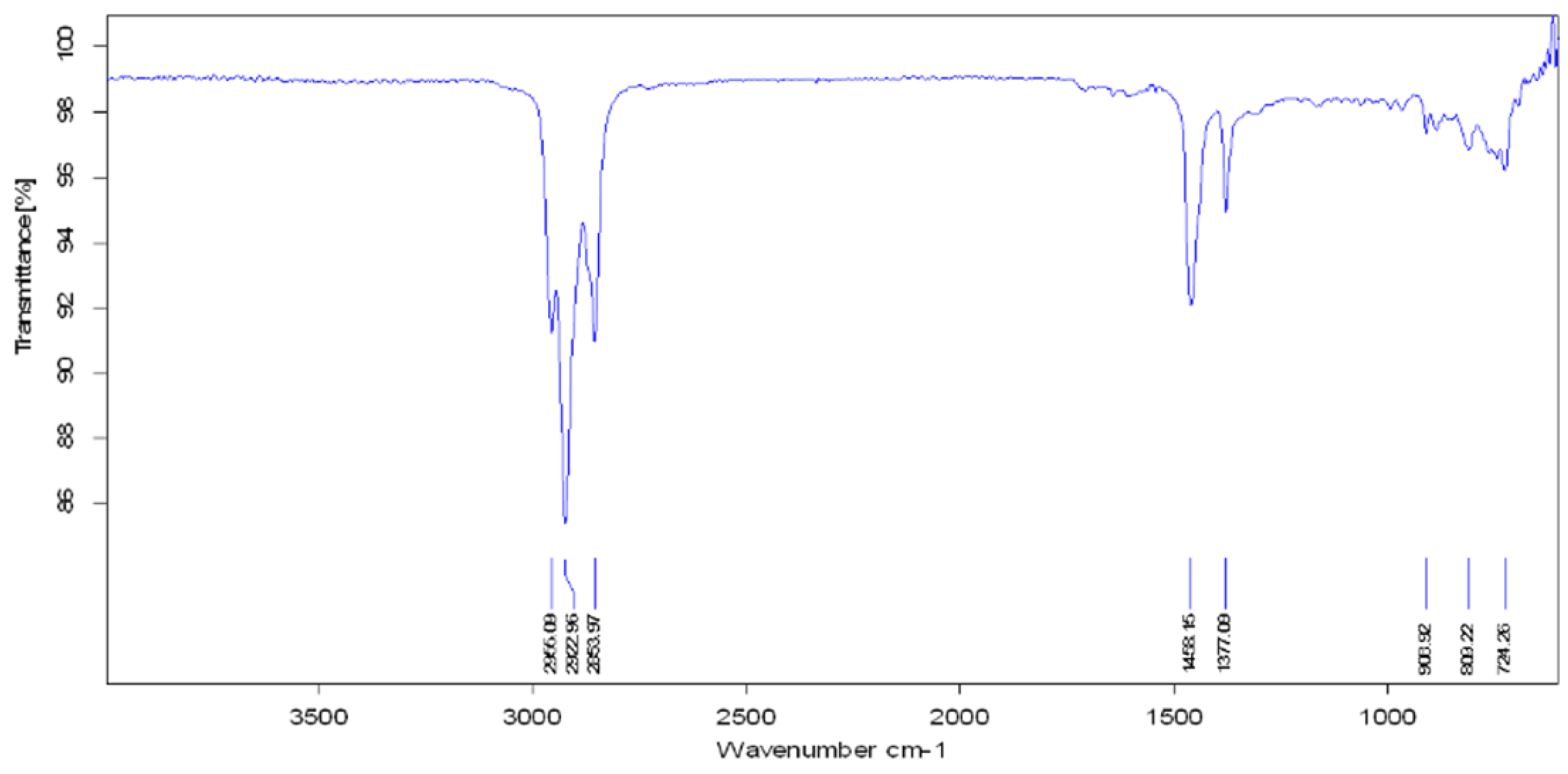
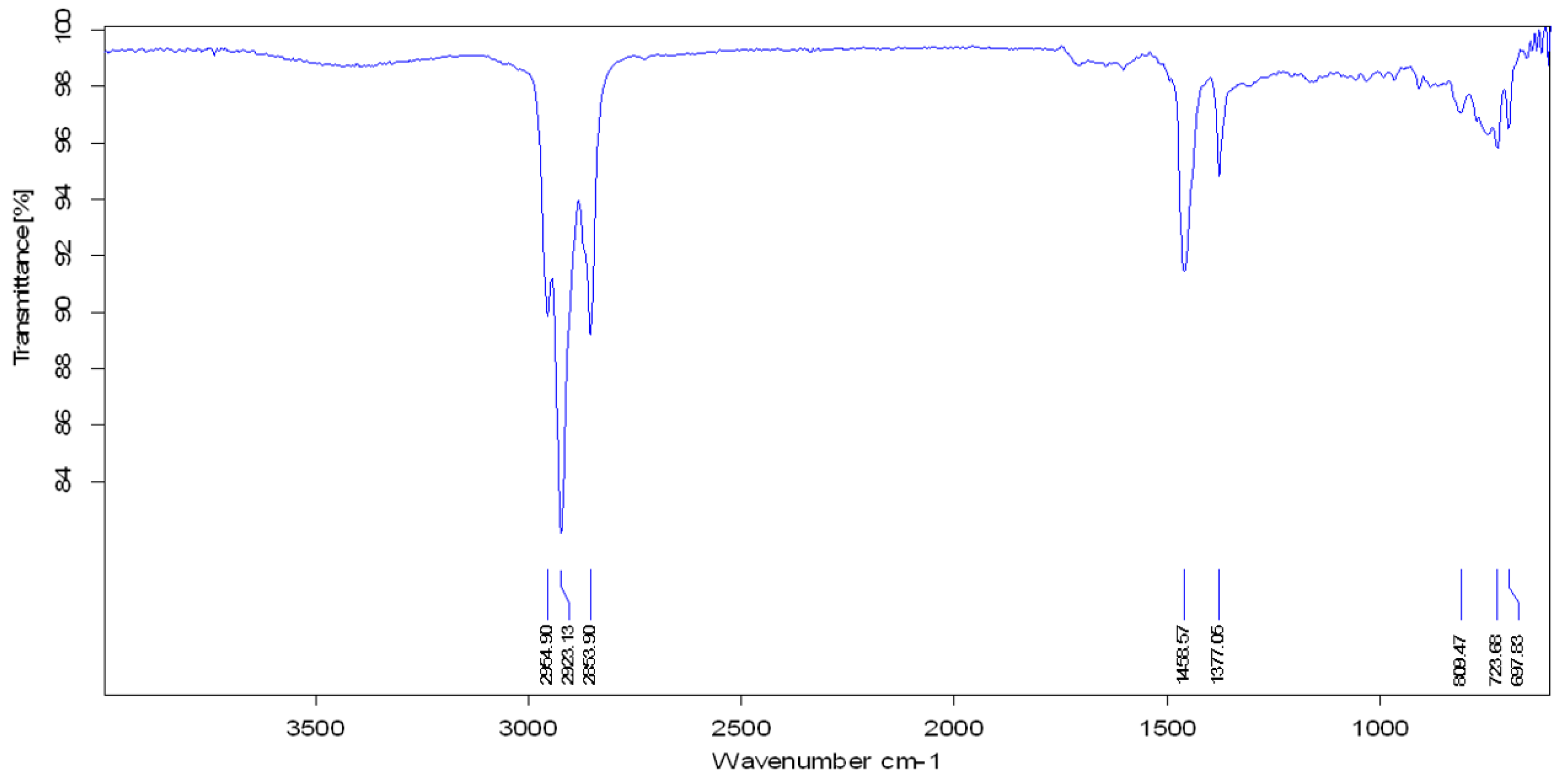
3.4. FTIR Analysis
3.5. Transport Processes and Reaction Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Absi, A.A.; Aitani, A.M.; Al-Khattaf, S.S. Thermal and catalytic cracking of whole crude oils at high severity. J. Anal. Appl. Pyrolysis 2020, 145, 104705. [Google Scholar] [CrossRef]
- Lozano-Navarro, J.I.; Palacio-Pérez, A.; Suárez-Domínguez, E.J.; Pérez-Sánchez, J.F.; Díaz-Zavala, N.P.; Melo-Banda, J.A.; Rodríguez-Valdés, A. Modification of the viscosity of extra-heavy crude oil using aqueous extracts of common geranium (Pelargonium hortorum). J. Petrol. Sci. Eng. 2022, 215, 110583. [Google Scholar] [CrossRef]
- Ghashghaee, M.; Shirvani, S. Two-step thermal cracking of an extra-heavy fuel oil: Experimental evaluation, characterization, and kinetics. Ind. Eng. Chem. Res. 2018, 57, 7421–7430. [Google Scholar] [CrossRef]
- Kok, M.V. Characterization of medium and heavy crude oils using thermal analysis techniques. Fuel Process. Technol. 2011, 92, 1026–1031. [Google Scholar] [CrossRef]
- Nargessi, Z.; Karimzadeh, R. Analysis of heat and mass transfer and parametric sensitivity in an experimental fixed-bed reactor for the catalytic cracking of heavy hydrocarbons based on modeling and experiments. Ind. Eng. Chem. Res. 2021, 60, 4831–4846. [Google Scholar] [CrossRef]
- Gabbar, H.A.; Aboughaly, M. Conceptual process design, energy and economic analysis of solid waste to hydrocarbon fuels via thermochemical processes. Processes 2021, 9, 2149. [Google Scholar] [CrossRef]
- Wang, F.; Yu, Y.; Biney, B.W.; Zhang, Z.; Liu, H.; Chen, K.; Wang, Z.; Guo, A. Relationship between olefins and coking propensity of heavy residual oil derived from vacuum residue thermal cracking products. Fuel 2022, 331, 125737. [Google Scholar] [CrossRef]
- Eklund, R.L.; Knapp, L.C.; Sandifer, P.A.; Colwell, R.C. Oil spills and human health: Contributions of the Gulf of Mexico Research Initiative. GeoHealth 2019, 3, 391–406. [Google Scholar] [CrossRef] [PubMed]
- Paulauskiene, T.; Uebe, J.; Kryzevicius, Z.; Kaskova, V.; Katarzyte, M.; Overlingė, D. Removal of petroleum hydrocarbons from brackish water by natural and modified sorbents. J. Mar. Sci. Eng. 2022, 10, 597. [Google Scholar] [CrossRef]
- Subramanian, D.; Wu, K.; Firoozabadi, A. Ionic liquids as viscosity modifiers for heavy and extra-heavy crude oils. Fuel 2015, 143, 519–526. [Google Scholar] [CrossRef]
- Dim, P.; Hart, A.; Wood, J.; Macnaughtan, B.; Rigby, S.P. Characterization of pore coking in catalyst for thermal down-hole upgrading of heavy oil. Chem. Eng. Sci. 2015, 131, 138–145. [Google Scholar] [CrossRef]
- Yu, J.; Quan, H.; Huang, Z.; Li, P.; Chang, S. Synthesis of a heavy-oil viscosity reducer containing a benzene ring and its viscosity reduction mechanism. ChemistrySelect 2022, 7, 202102694. [Google Scholar] [CrossRef]
- Salehzadeh, M.; Kaminski, T.; Husein, M.M. An optimized thermal cracking approach for onsite upgrading of bitumen. Fuel 2022, 307, 121885. [Google Scholar] [CrossRef]
- Li, X.; You, L.; Kang, Y.; Liu, J.; Chen, M.; Zeng, T.; Hao, Z. Investigation on the thermal cracking of shale under different cooling modes. J. Nat. Gas Sci. Eng. 2022, 97, 104359. [Google Scholar] [CrossRef]
- Goncharov, A.V.; Krivtsov, E.B. Calculation of the rate constants of thermal cracking and condensation reactions of high-sulfur tar asphaltenes. Solid Fuel Chem. 2022, 56, 108–115. [Google Scholar] [CrossRef]
- Ishiyama, E.M.; Kennedy, J.; Pugh, S.J. Fouling management of thermal cracking units. Heat Transf. Eng. 2017, 38, 694–702. [Google Scholar] [CrossRef]
- Almukhtar, R.S.; Abduallah, S.I.H. Characterization of liquid produced from catalytic pyrolysis of mixed polystyrene and polyethylene terephthalate plastic. Eng. Technol. J. 2018, 36, 27–32. [Google Scholar]
- Corma, A.; Sauvanaud, L.; Mathieu, Y.; Al-Bogami, S.; Bourane, A.; Al-Ghrami, M. Direct crude oil cracking for producing chemicals: Thermal cracking modeling. Fuel 2018, 211, 726–736. [Google Scholar] [CrossRef]
- Alsobaai, A.M. Thermal cracking of petroleum residue oil using three level factorial design. J. King Saud Univ.-Eng. Sci. 2013, 25, 21–28. [Google Scholar] [CrossRef] [Green Version]
- Hao, J.; Che, Y.; Tian, Y.; Li, D.; Zhang, J.; Qiao, Y. Thermal cracking characteristics and kinetics of oil sand bitumen and its SARA fractions by TG–FTIR. Energy Fuels 2017, 31, 1295–1309. [Google Scholar] [CrossRef]
- Kumar, S.; Mahto, V. Emulsification of Indian heavy crude oil in water for its efficient transportation through offshore pipelines. Chem. Eng. Res. Des. 2016, 115, 34–43. [Google Scholar] [CrossRef]
- Pei, S.; Huang, L.; Zhang, L.; Ren, S. Experimental study on thermal cracking reactions of ultra-heavy oils during air injection assisted in-situ upgrading process. J. Petrol. Sci. Eng. 2020, 195, 107850. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Hu, Z.; Xu, B.; Li, Y.; Pu, W.; Zhao, J. A review of in situ upgrading technology for heavy crude oil. Petroleum 2021, 7, 117–122. [Google Scholar] [CrossRef]
- Al-Khodor, A.; Yusra, A.; Albayati, T.M. Adsorption desulfurization of actual heavy crude oil using activated carbon. Eng. Technol. J. 2020, 38, 1441–1453. [Google Scholar] [CrossRef]
- Cheshkova, T.V.; Grinko, A.A.; Min, R.S.; Sagachenko, T.A. Structural transformations of heavy oil asphaltenes in the course of heat treatment. Petrol. Chem. 2022, 62, 214–221. [Google Scholar] [CrossRef]
- Guerra, A. Modeling Mild Thermal Cracking of Heavy Crude Oil and Bitumen with VLE Calculations. Ph.D. Thesis, Université d’Ottawa (University of Ottawa), Ottawa, ON, Canada, 2018. [Google Scholar]
- Al Darouich, T.; Behar, F.; Largeau, C. Pressure effect on the thermal cracking of the light aromatic fraction of Safaniya crude oil–Implications for deep prospects. Org. Geochem. 2006, 37, 1155–1169. [Google Scholar] [CrossRef]
- Afanasjeva, N.; González-Córdoba, A.; Palencia, M. Mechanistic approach to thermal production of new materials from asphaltenes of Castilla crude oil. Processes 2020, 8, 1644. [Google Scholar] [CrossRef]
- Avendaño-Salazar, C.A.; Ramírez-Jaramillo, E.; De la Cruz, J.L.M.; Albiter, A. Thermogravimetric and differential thermogravimetric analysis of effect of areal compositional gradient on combustion kinetics of Mexican extra-heavy crude oil. Oil Gas Sci. Technol. 2020, 75, 25. [Google Scholar] [CrossRef]
- Khelkhal, M.A.; Lapuk, S.E.; Buzyurov, A.V.; Krapivnitskaya, T.O.; Peskov, N.Y.; Denisenko, A.N.; Vakhin, A.V. Thermogravimetric study on peat catalytic pyrolysis for potential hydrocarbon generation. Processes 2022, 10, 974. [Google Scholar] [CrossRef]
- Hart, A.; Greaves, M.; Wood, J. A comparative study of fixed-bed and dispersed catalytic upgrading of heavy crude oil using-CAPRI. Chem. Eng. J. 2015, 282, 213–223. [Google Scholar] [CrossRef] [Green Version]
- Sukkar, K.A. Evaluation of transport processes in a catalytic reforming reactor with high performance nanocatalysts. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1067, 012149. [Google Scholar] [CrossRef]
- Jin, F.; Jiang, T.; Yuan, C.; Varfolomeev, M.A.; Wan, F.; Zheng, Y.; Li, X. An improved viscosity prediction model of extra heavy oil for high temperature and high pressure. Fuel 2022, 319, 123852. [Google Scholar] [CrossRef]
- Davudov, D.; Moghanloo, R.G. A systematic comparison of various upgrading techniques for heavy oil. J. Petrol. Sci. Eng. 2017, 156, 623–632. [Google Scholar] [CrossRef]
- Fattah, M.Y.; Abdulkhabeer, W.; Hilal, M.M. Characteristics of asphalt binder and mixture modified with waste polypropylene. Eng. Technol. J. 2021, 39, 1224–1230. [Google Scholar] [CrossRef]
- Jaber, T.N.; Sukkar, K.A.; Karamalluh, A.A. Specifications of heavy diesel lubricating oil improved by MWCNTs and CuO as nano-additives. IOP Conf. Ser. Mater. Sci. Eng. 2019, 579, 012014. [Google Scholar] [CrossRef]
- Jiao, S.; Li, S.; Pu, H.; Dong, M.; Shang, Y. Investigation of pressure effect on thermal cracking of n-decane at supercritical pressures. Energy Fuels 2018, 32, 4040–4048. [Google Scholar] [CrossRef]
- Pleyer, O.; Kubičková, I.; Vráblík, A.; Maxa, D.; Pospíšil, M.; Zbuzek, M.; Schlehöfer, D.; Straka, P. Hydrocracking of heavy vacuum gas oil with petroleum wax. Catalysts 2022, 12, 384. [Google Scholar] [CrossRef]
- Hao, J.; Feng, W.; Qiao, Y.; Tian, Y.; Zhang, J.; Che, Y. Thermal cracking behaviors and products distribution of oil sand bitumen by TG-FTIR and Py-GC/TOF-MS. Energy Conver. Manag. 2017, 151, 227–239. [Google Scholar] [CrossRef]
- Kaminski, T.; Husein, M.M. Partial upgrading of Athabasca bitumen using thermal cracking. Catalysts 2019, 9, 431. [Google Scholar] [CrossRef] [Green Version]
- AlHumaidan, F.; Hauser, A.; Al-Rabiah, H.; Lababidi, H.; Bouresli, R. Studies on thermal cracking behavior of vacuum residues in Eureka process. Fuel 2013, 109, 635–646. [Google Scholar] [CrossRef]
- Rueda-Velásquez, R.I.; Gray, M.R. A viscosity-conversion model for thermal cracking of heavy oils. Fuel 2017, 197, 82–90. [Google Scholar] [CrossRef]
- Al-Absi, A.A.; Al-Khattaf, S.S. Conversion of Arabian light crude oil to light olefins via catalytic and thermal cracking. Energy Fuels 2018, 32, 8705–8714. [Google Scholar] [CrossRef]
- Wang, J.X.; Wang, L.L.; Wang, T.F.; Peng, X.Q. Effects of SARA fractions on pyrolysis behavior and kinetics of heavy crude oil. Petrol. Sci. Technol. 2020, 38, 945–954. [Google Scholar] [CrossRef]
- Voronetskaya, N.G.; Pevneva, G.S. Structural transformations of heavy oil resins and asphaltenes upon thermal cracking. Solid Fuel Chem. 2021, 55, 165–170. [Google Scholar] [CrossRef]
- Mateus, L.; Taborda, E.A.; Moreno-Castilla, C.; López-Ramón, M.V.; Franco, C.A.; Cortés, F.B. Extra-heavy crude oil viscosity reduction using and reusing magnetic copper ferrite nanospheres. Processes 2021, 9, 175. [Google Scholar] [CrossRef]
- Hammoodi, S.I.; Almukhtar, R.S. Thermal Pyrolysis of Municipal Solid Waste (MSW). IOP Conf. Ser. Mater. Sci. Eng. 2019, 579, 012018. [Google Scholar] [CrossRef]
- Shakor, Z.M.; AbdulRazak, A.A.; Sukkar, K.A. A detailed reaction kinetic model of heavy naphtha reforming. Arabian J. Sci. Eng. 2020, 45, 7361–7370. [Google Scholar] [CrossRef]
- Jalal, N.I.; Ibrahim, R.I.; Oudah, M.K. Flow improvement and viscosity reduction for crude oil pipelines transportation using dilution and electrical field. Eng. Technol. J. 2022, 40, 66–75. [Google Scholar] [CrossRef]
- Abbas, A.D.; Sukkar, K.A. Rheological behaviour modification of Basrah crude oil by graphene additions at different temperatures in petroleum pipeline. AIP Conf. Proc. 2022, 2443, 030048. [Google Scholar]
- Lababidi, H.M.; Sabti, H.M.; AlHumaidan, F.S. Changes in asphaltenes during thermal cracking of residual oils. Fuel 2014, 117, 59–67. [Google Scholar] [CrossRef]
- Pevneva, G.S.; Voronetskaya, N.G.; Grin’ko, A.A.; Golovko, A.K. Influence of resins and asphaltenes on thermal transformations of hydrocarbons of paraffin-base heavy crude oil. Pet. Chem. 2016, 56, 690–696. [Google Scholar] [CrossRef]






| Property | Standard | Value |
|---|---|---|
| API Gravity at 15 °C | ASTM D5002 | 6.83 |
| Specific Gravity at 15 °C (g/cm3) | ASTM D-1298 | 0.98 |
| Water Content (Vol%) | ASTM D1744 | 8.00 |
| Sediment (Vol%) | ASTM D4807 | 0.50 |
| Sulfur Content (wt%) | ASTM D4294 | 3.60 |
| Salt Content (ppm) | ASTM-D3230 | 400 |
| Temperature (°C) | Pressure (Bar) | API |
|---|---|---|
| 400 | 1 | 28.29 |
| 3 | 30.50 | |
| 5 | 29.29 | |
| 7 | 27.2 | |
| 425 | 1 | 26.91 |
| 3 | 27.34 | |
| 5 | 28.65 | |
| 7 | 27.15 | |
| 450 | 1 | 27.88 |
| 3 | 26.00 | |
| 5 | 26.93 | |
| 7 | 26.88 |
| Functional Group | Parent Waste Crude Oil | Produced Fuel at 400 °C | Produced Fuel at 425 °C | Produced Fuel at 450 °C |
|---|---|---|---|---|
| Aliphatic C−H | 2922.77 cm−1 2853.95 cm−1 | 2923.27 cm−1 2853.88 cm−1 | 2922.96 cm−1 2853.97 cm−1 | 2923.13 cm−1 2853.90 cm−1 |
| C−H Deformation of −CH2 and −CH3 | 1459.74 cm−1 | 1458.64 cm−1 | 1458.15 cm−1 | 1458.57 cm−1 |
| C−H Symmetric Deformation of −CH2 | 1377.07 cm−1 | 1376.89 cm−1 | 1377.09 cm−1 | 1377.06 cm−1 |
| Aromatic Ring | 1641.96 cm−1 | - | - | - |
| O−H Stretching | 3406.37 cm−1 | - | - | - |
| −C−H Bending | 722.28 cm−1 | 724.16 cm−1 | 724.26 cm−1 | 723.68 cm−1 |
| Reaction Type | Description |
|---|---|
| Initiation Stage | Alkane + Heat  2 Radicals 2 Radicals |
| Propagation Stage | Alkane + Radical  (Radical + H) + Radical (Radical + H) + RadicalRadical  Radical + Olefin Radical + Olefin |
| Termination Stage | Radical + Radical  Hydrocarbons Products Hydrocarbons Products |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almukhtar, R.; Hammoodi, S.I.; Majdi, H.S.; Sukkar, K.A. Managing Transport Processes in Thermal Cracking to Produce High-Quality Fuel from Extra-Heavy Waste Crude Oil Using a Semi-Batch Reactor. Processes 2022, 10, 2077. https://doi.org/10.3390/pr10102077
Almukhtar R, Hammoodi SI, Majdi HS, Sukkar KA. Managing Transport Processes in Thermal Cracking to Produce High-Quality Fuel from Extra-Heavy Waste Crude Oil Using a Semi-Batch Reactor. Processes. 2022; 10(10):2077. https://doi.org/10.3390/pr10102077
Chicago/Turabian StyleAlmukhtar, Riyadh, Sally I. Hammoodi, Hasan Shakir Majdi, and Khalid A. Sukkar. 2022. "Managing Transport Processes in Thermal Cracking to Produce High-Quality Fuel from Extra-Heavy Waste Crude Oil Using a Semi-Batch Reactor" Processes 10, no. 10: 2077. https://doi.org/10.3390/pr10102077