Removal of Dye (Methylene Blue) from Wastewater Using Bio-Char Derived from Agricultural Residues in Palestine: Performance and Isotherm Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Plant-Based Waste Biomass and Chemicals Used
2.2. Preparation of the Natural Adsorbents
2.3. Production of Activated Carbon
2.4. Batch Adsorption Experiments
2.5. Parametric Study
2.6. Adsorption Isotherm
3. Results and Discussions
3.1. Adsorption Kinetics of the Natural, Unprocessed Biomass-Based Adsorbents
3.2. Adsorption Tests Using Biochar and Activated Carbon
3.3. Effect of Adsorption Parameters on the Removal of MB and Kinetic Constants
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Corbin, G.; Vulliet, E.; Lanson, B.; Rimola, A.; Mignon, P. Adsorption of pharmaceuticals onto smectite clay minerals: A combined experimental and theoretical study. Minerals 2021, 11, 62. [Google Scholar] [CrossRef]
- Subratti, A.; Vidal, J.L.; Lalgee, L.J.; Kerton, F.M.; Jalsa, N.K. Preparation and characterization of biochar derived from the fruit seed of Cedrela odorata L. and evaluation of its adsorption capacity with methylene blue. Sustain. Chem. Pharm. 2021, 21, 100421. [Google Scholar] [CrossRef]
- Strezov, V.; Patterson, M.; Zymla, V.; Fisher, K.; Evans, T.J.; Nelson, P.F. Fundamental aspects of biomass carbonisation. J. Anal. Appl. Pyrolysis 2007, 79, 91–100. [Google Scholar] [CrossRef]
- Bridgwater, A.V. Upgrading biomass fast pyrolysis liquids. Environ. Prog. Sustain. Energy 2012, 31, 261–268. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, J.; Li, D.; Liu, C.; Lu, Y.; Lin, X.; Zheng, Z. Insight into the KOH/KMnO4 activation mechanism of oxygen-enriched hierarchical porous biochar derived from biomass waste by in-situ pyrolysis for methylene blue enhanced adsorption. J. Anal. Appl. Pyrolysis 2021, 158, 105269. [Google Scholar] [CrossRef]
- Ismadji, S.; Sudaryanto, Y.; Hartono, S.B.; Setiawan, L.E.K.; Ayucitra, A. Activated carbon from char obtained from vacuum pyrolysis of teak sawdust: Pore structure development and characterization. Bioresour. Technol. 2005, 96, 1364–1369. [Google Scholar] [CrossRef]
- Sawalha, H.; Maghalseh, M.; Qutaina, J.; Junaidi, K.; Rene, E.R. Removal of hydrogen sulfide from biogas using activated carbon synthesized from different locally available biomass wastes—A case study from Palestine. Bioengineered 2020, 11, 607–618. [Google Scholar] [CrossRef]
- Sahu, S.; Pahi, S.; Tripathy, S.; Singh, S.K.; Behera, A.; Sahu, U.K.; Patel, R.K. Adsorption of methylene blue on chemically modified lychee seed biochar: Dynamic, equilibrium, and thermodynamic study. J. Mol. Liq. 2020, 315, 113743. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Li, S.; Zhong, W.; Wei, W. Enhanced methylene blue adsorption onto activated reed-derived biochar by tannic acid. J. Mol. Liq. 2018, 268, 658–666. [Google Scholar] [CrossRef]
- Fan, S.; Tang, J.; Wang, Y.; Li, H.; Zhang, H.; Tang, J.; Wang, Z.; Li, X. Biochar prepared from co-pyrolysis of municipal sewage sludge and tea waste for the adsorption of methylene blue from aqueous solutions: Kinetics, isotherm, thermodynamic and mechanism. J. Mol. Liq. 2016, 220, 432–441. [Google Scholar] [CrossRef]
- Sun, L.; Wan, S.; Luo, W. Biochars prepared from anaerobic digestion residue, palm bark, and eucalyptus for adsorption of cationic methylene blue dye: Characterization, equilibrium, and kinetic studies. Bioresour. Technol. 2013, 140, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Khan, N.; Giri, B.S.; Chowdhary, P.; Chaturvedi, P. Removal of methylene blue dye using rice husk, cow dung and sludge biochar: Characterization, application, and kinetic studies. Bioresour. Technol. 2020, 306, 123202. [Google Scholar] [CrossRef] [PubMed]
- Boonamnuayvitaya, V.; Chaiya, C.; Tanthapanichakoon, W.; Jarudilokkul, S. Removal of heavy metals by adsorbent prepared from pyrolyzed coffee residues and clay. Sep. Purif. Technol. 2004, 35, 11–22. [Google Scholar] [CrossRef]
- Chowdhury, Z.Z.; Abd Hamid, S.B.; Das, R.; Hasan, M.R.; Zain, S.M.; Khalid, K.; Uddin, M.N. Preparation of carbonaceous adsorbents from lignocellulosic biomass and their use in removal of contaminants from aqueous solution. BioResources 2013, 8, 33. [Google Scholar] [CrossRef]
- Kalderis, D.; Bethanis, S.; Paraskeva, P.; Diamadopoulos, E. Production of activated carbon from bagasse and rice husk by a single-stage chemical activation method at low retention times. Bioresour. Technol. 2008, 99, 6809–6816. [Google Scholar] [CrossRef]
- Lgaz, H.; Salghi, R.; Larouj, M.; Elfaydy, M.; Jodeh, S.; Rouifi, Z.; Lakhrissi, B.; Oudda, H. Experimental, theoretical and Monte Carlo simulation of quinoline derivative as effective corrosion inhibitor for mild steel in 1 M HCl. J. Mater. Environ. Sci. 2016, 7, 4471–4488. [Google Scholar]
- Nakhli, A.; Bergaoui, M.; Toumi, K.H.; Khalfaoui, M.; Benguerba, Y.; Balsamo, M.; Soetaredjo, F.E.; Ismadji, S.; Ernst, B.; Erto, A. Molecular insights through computational modeling of methylene blue adsorption onto low-cost adsorbents derived from natural materials: A multi-model’s approach. Comput. Chem. Eng. 2020, 140, 106965. [Google Scholar] [CrossRef]
- Nguyen, P.X.T.; Ho, K.H.; Do, N.H.N.; Nguyen, C.T.X.; Nguyen, H.M.; Tran, K.A.; Le, K.A.; Le, P.K. A comparative study on modification of aerogel-based biosorbents from coconut fibers for treatment of dye- and oil-contaminated water. Mater. Today Sustain. 2022, 19, 100175. [Google Scholar] [CrossRef]
- Behloul, H.; Ferkous, H.; Bougdah, N.; Djellali, S.; Alam, M.; Djilani, C.; Sedik, A.; Lerari, D.; Jeon, B.-H.; Benguerba, Y. New insights on the adsorption of CI-Reactive Red 141 dye using activated carbon prepared from the ZnCl2-treated waste cotton fibers: Statistical physics, DFT, COSMO-RS, and AIM studies. J. Mol. Liq. 2022, 364, 119956. [Google Scholar] [CrossRef]
- Li, H.; Budarin, V.L.; Clark, J.H.; North, M.; Wu, X. Rapid and efficient adsorption of methylene blue dye from aqueous solution by hierarchically porous, activated starbons®: Mechanism and porosity dependence. J. Hazard. Mater. 2022, 436, 129174. [Google Scholar] [CrossRef]
- Xue, H.; Wang, X.; Xu, Q.; Dhaouadi, F.; Sellaoui, L.; Seliem, M.K.; Lamine, A.B.; Belmabrouk, H.; Bajahzar, A.; Bonilla-Petriciolet, A.J.C.E.J. Adsorption of methylene blue from aqueous solution on activated carbons and composite prepared from an agricultural waste biomass: A comparative study by experimental and advanced modeling analysis. Chem. Eng. J. 2022, 430, 132801. [Google Scholar] [CrossRef]
- Nabais, J.V.; Carrott, P.; Carrott, M.R.; Luz, V.; Ortiz, A.L. Influence of preparation conditions in the textural and chemical properties of activated carbons from a novel biomass precursor: The coffee endocarp. Bioresour. Technol. 2008, 99, 7224–7231. [Google Scholar] [CrossRef] [PubMed]
- Geçgel, Ü.; Özcan, G.; Gürpınar, G.Ç. Removal of methylene blue from aqueous solution by activated carbon prepared from pea shells (Pisum sativum). J. Chem. 2013, 2013, 614083. [Google Scholar] [CrossRef] [Green Version]
- Yılmaz, Ş.; Zengin, A.; Ecer, Ü.; Şahan, T. Conversion from a natural mineral to a novel effective adsorbent: Utilization of pumice grafted with polymer brush for methylene blue decolorization from aqueous environments. Colloids Surf. A Physicochem. Eng. Asp. 2019, 583, 123961. [Google Scholar] [CrossRef]
- Sharma, Y.C.; Uma. Optimization of parameters for adsorption of methylene blue on a low-cost activated carbon. J. Chem. Eng. Data 2010, 55, 435–439. [Google Scholar] [CrossRef]
- Gupta, S.A.; Vishesh, Y.; Sarvshrestha, N.; Bhardwaj, A.S.; Kumar, P.A.; Topare, N.S.; Raut-Jadhav, S.; Bokil, S.A.; Khan, A. Adsorption isotherm studies of methylene blue using activated carbon of waste fruit peel as an adsorbent. Mater. Today Proc. 2022, 57, 1500–1508. [Google Scholar] [CrossRef]
- Ramutshatsha-Makhwedzha, D.; Mavhungu, A.; Moropeng, M.L.; Mbaya, R. Activated carbon derived from waste orange and lemon peels for the adsorption of methyl orange and methylene blue dyes from wastewater. Heliyon 2022, 8, e09930. [Google Scholar] [CrossRef]
- Rani, S.; Chaudhary, S. Adsorption of methylene blue and crystal violet dye from waste using Citrus limetta peel as an adsorbent. Mater. Today Proc. 2022, 60, 336–344. [Google Scholar] [CrossRef]
- Geankoplis, C.J. Transport Processes and Separation Process Principles: (Includes Unit Operations), 4th ed.; Prentice-Hall Inc.: Upper Saddle River, NJ, USA, 2003. [Google Scholar]
- Mongioví, C.; Crini, G.; Gabrion, X.; Placet, V.; Blondeau-Patissier, V.; Krystianiak, A.; Durand, S.; Beaugrand, J.; Dorlando, A.; Rivard, C.; et al. Revealing the adsorption mechanism of copper on hemp-based materials through EDX, nano-CT, XPS, FTIR, Raman, and XANES characterization techniques. Chem. Eng. J. Adv. 2022, 10, 100282. [Google Scholar] [CrossRef]
- Temitope Bankole, D.; Peter Oluyori, A.; Abosede Inyinbor, A. Potent adsorbent prepared from Bilghia sapida waste material: Surface chemistry and morphological characterization. Mater. Today Proc. 2022, 65, 3665–3670. [Google Scholar] [CrossRef]
- Maleki, F.; Gholami, M.; Torkaman, R.; Torab-Mostaedi, M.; Asadollahzadeh, M. Cobalt (II) removal from aqueous solution by modified polymeric adsorbents prepared with induced-graft polymerization: Batch and continuous column study with analysis of breakthrough behaviors. Environ. Technol. Innov. 2021, 24, 102054. [Google Scholar] [CrossRef]
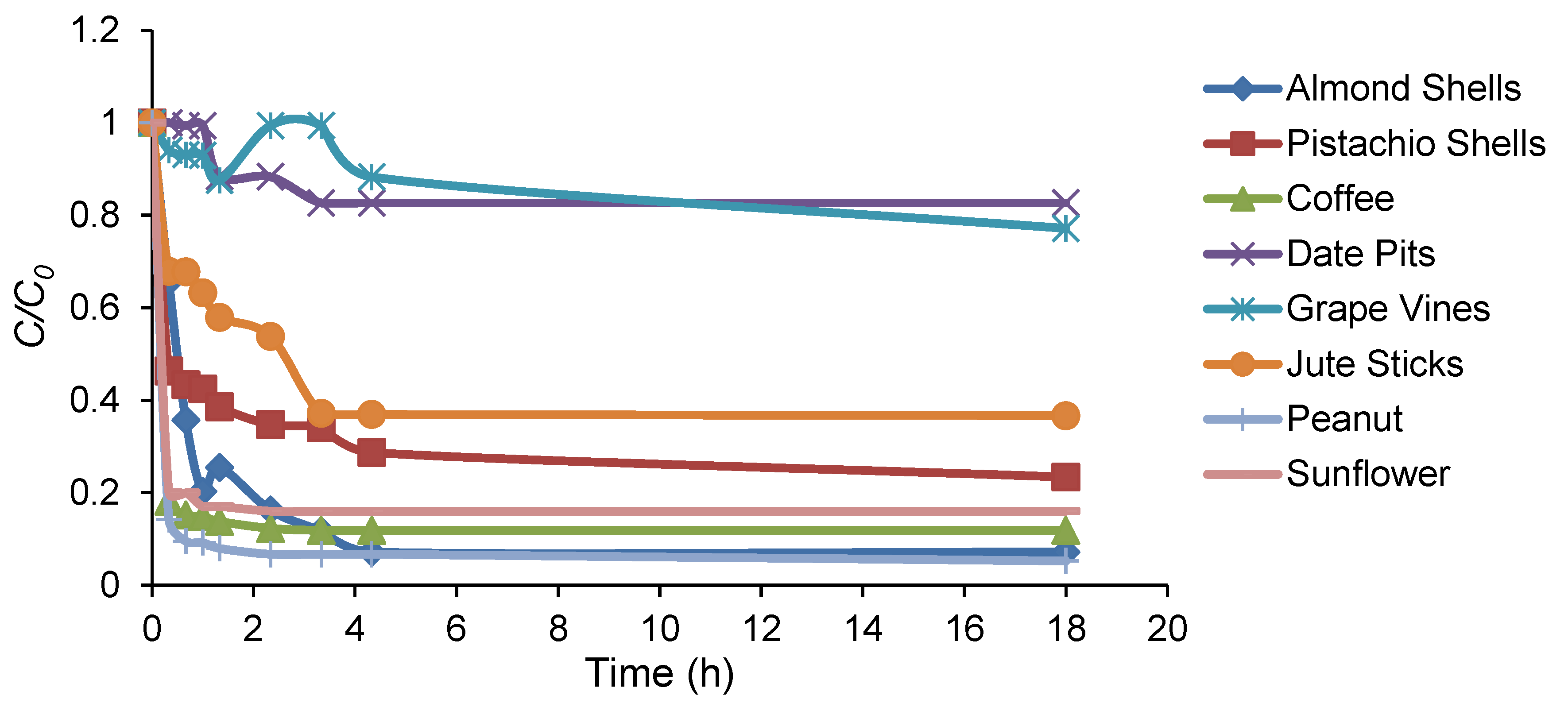


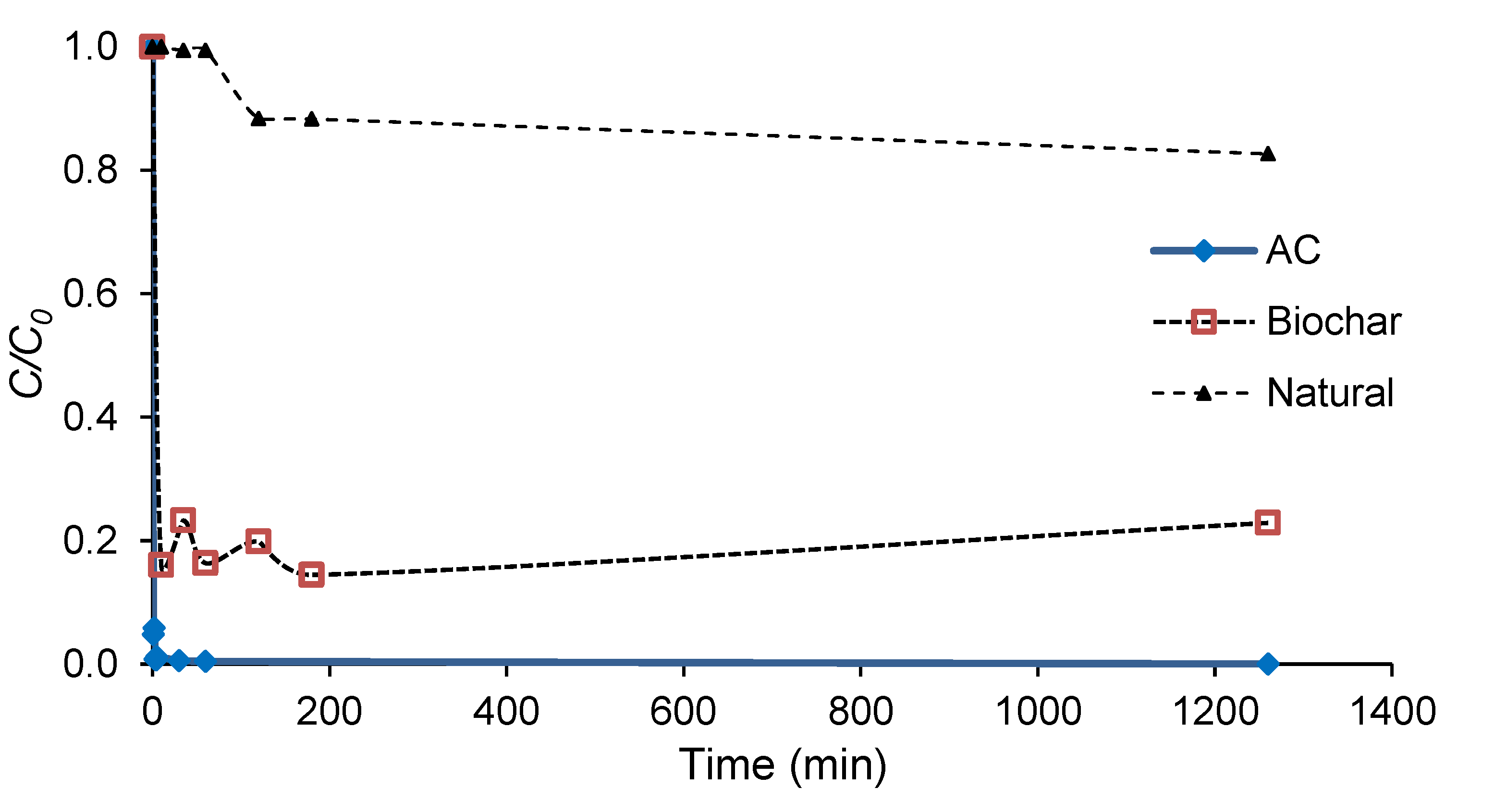

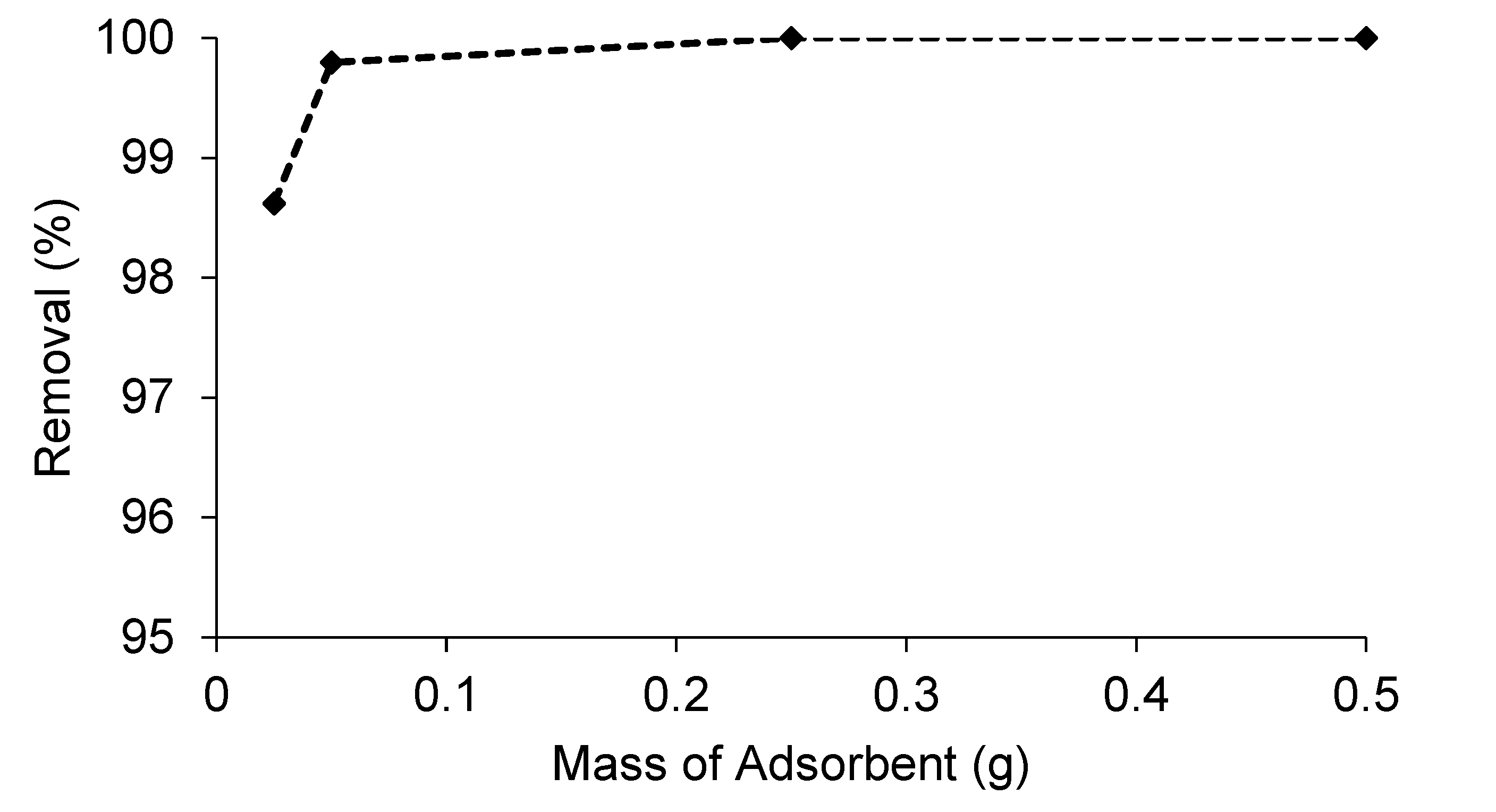

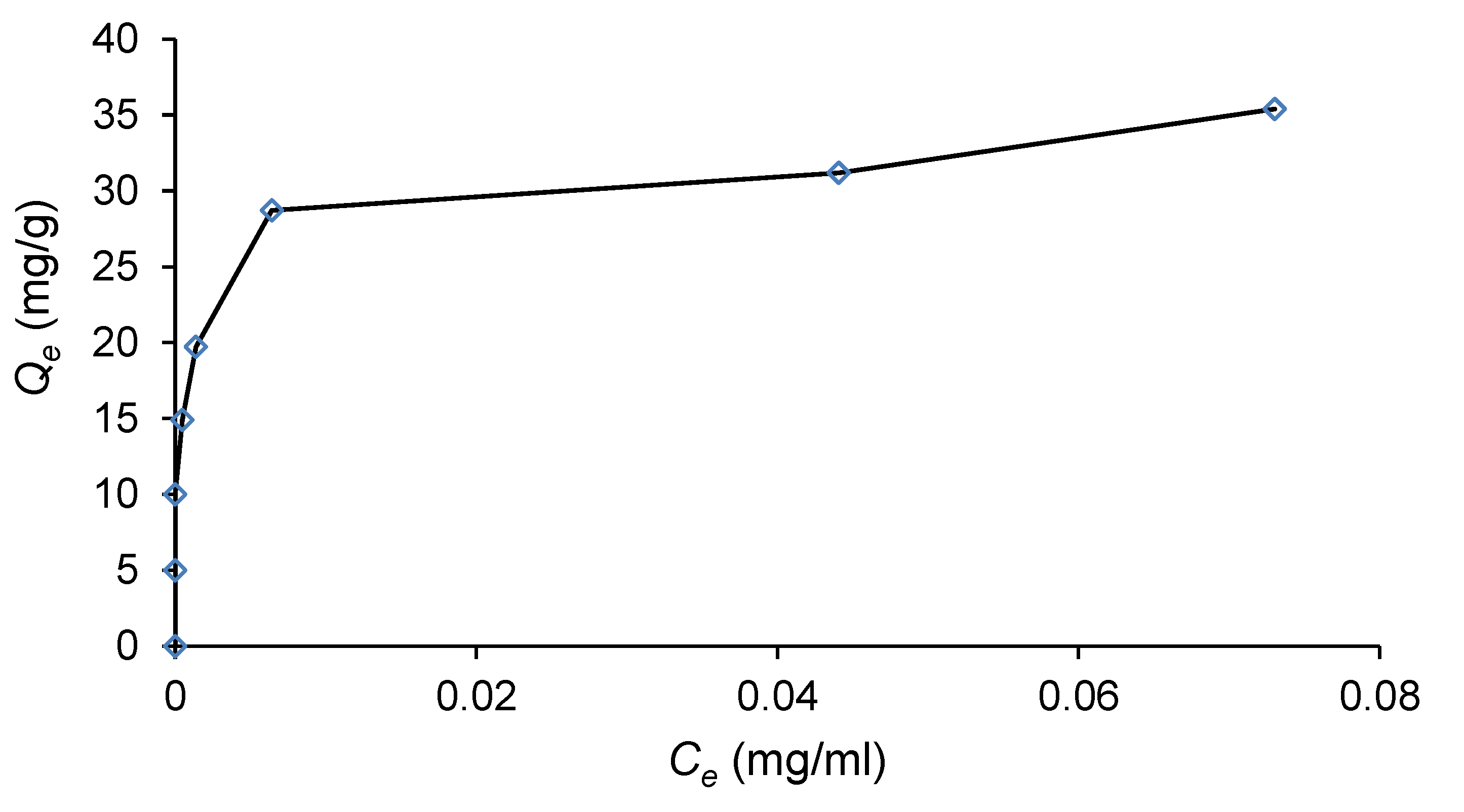

| Biomass Samples | Yield (%) |
|---|---|
| Coffee | 78 |
| Date pits | 74 |
| Almond shells | 73 |
| Peanut hulls | 80 |
| Grape vine sticks | 88 |
| Pistachio shells | 65 |
| Jute sticks | 85 |
| Sunflower shells | 80 |
| Biochar | Activated Carbon | |||
|---|---|---|---|---|
| Type of Adsorbent | Removal Efficiency (%) | Rate of Adsorption (L/min) | Removal Efficiency (%) | Rate of Adsorption (L/min) |
| Coffee | 90 | 0.0865 | 80 | 0.572 |
| Date pits | 77.2 | 0.084 | 100 | 0.951 |
| Almond shells | 89 | 0.0836 | 100 | 0.8503 |
| Peanut hulls | 99.6 | 0.0944 | 100 | 0.9911 |
| Grape vine sticks | 99.6 | 0.0988 | 100 | 0.9994 |
| Pistachio shells | 98.9 | 0.0978 | 100 | 0.9956 |
| Jute sticks | 99.94 | 0.099 | 100 | 0.9945 |
| Sunflower shells | 99.64 | 0.094 | 100 | 0.996 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sawalha, H.; Bader, A.; Sarsour, J.; Al-Jabari, M.; Rene, E.R. Removal of Dye (Methylene Blue) from Wastewater Using Bio-Char Derived from Agricultural Residues in Palestine: Performance and Isotherm Analysis. Processes 2022, 10, 2039. https://doi.org/10.3390/pr10102039
Sawalha H, Bader A, Sarsour J, Al-Jabari M, Rene ER. Removal of Dye (Methylene Blue) from Wastewater Using Bio-Char Derived from Agricultural Residues in Palestine: Performance and Isotherm Analysis. Processes. 2022; 10(10):2039. https://doi.org/10.3390/pr10102039
Chicago/Turabian StyleSawalha, Hassan, Aseel Bader, Jinan Sarsour, Maher Al-Jabari, and Eldon R. Rene. 2022. "Removal of Dye (Methylene Blue) from Wastewater Using Bio-Char Derived from Agricultural Residues in Palestine: Performance and Isotherm Analysis" Processes 10, no. 10: 2039. https://doi.org/10.3390/pr10102039





