Highly Selective Production of Valuable Aromatic Hydrocarbons/Phenols from Forestry and Agricultural Residues Using Ni/ZSM-5 Catalyst
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Catalysts Synthesis
2.3. Experimental Setup
2.4. Catalyst Characterization
3. Results and Discussion
3.1. Biomass Characterization
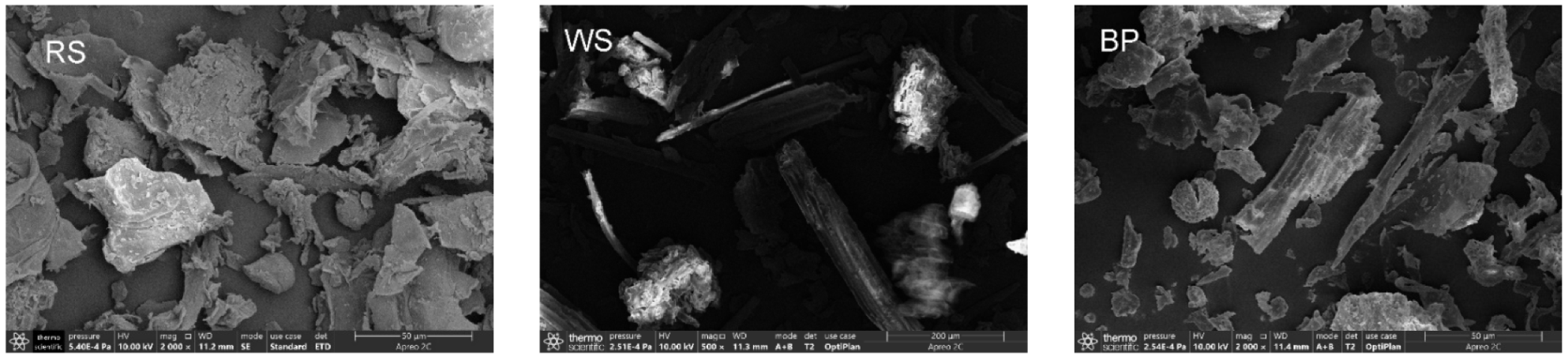
3.1.1. SEM Characterization
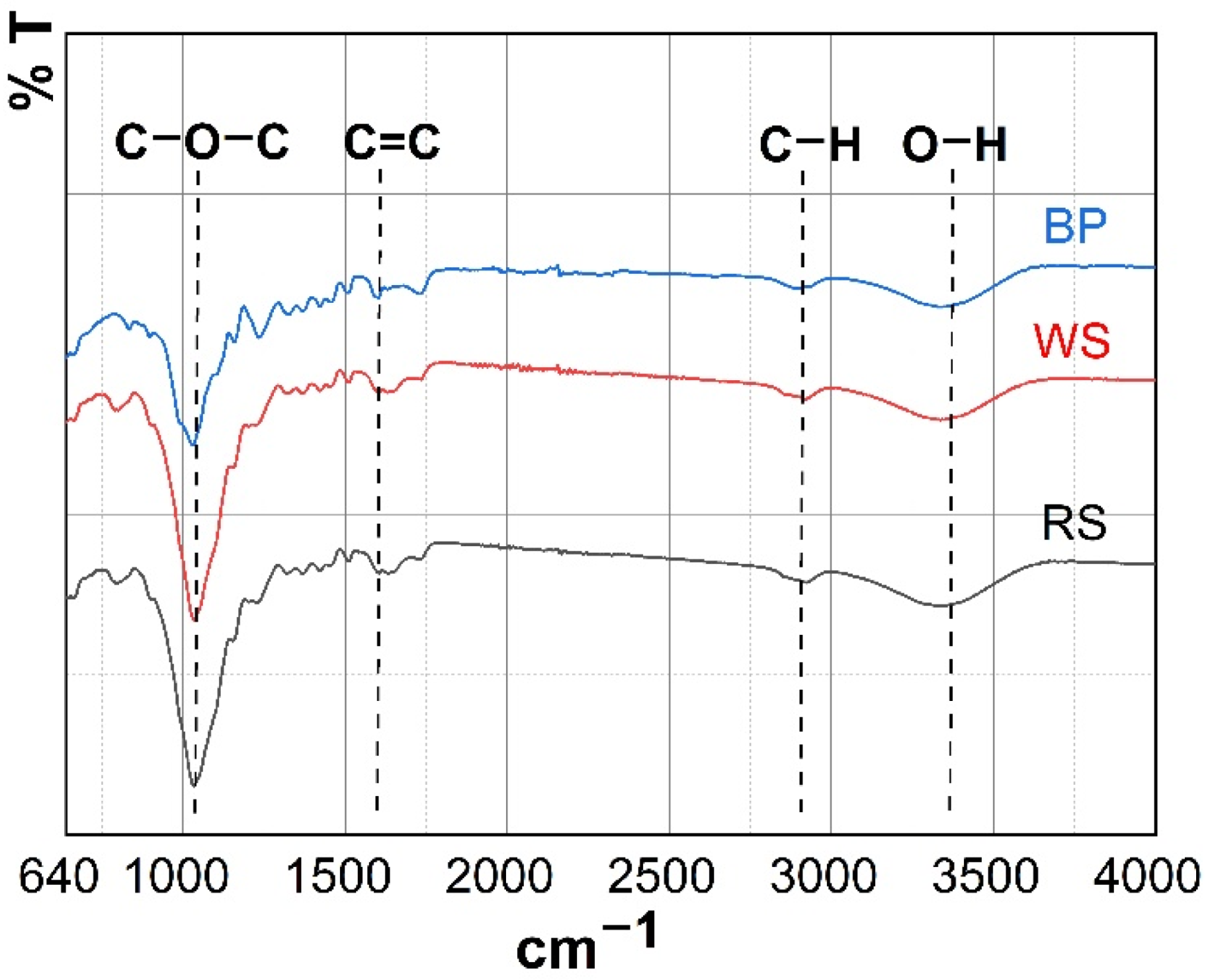
3.1.2. FTIR Characterization
3.1.3. TGA Characterization
3.2. Catalyst Characterization
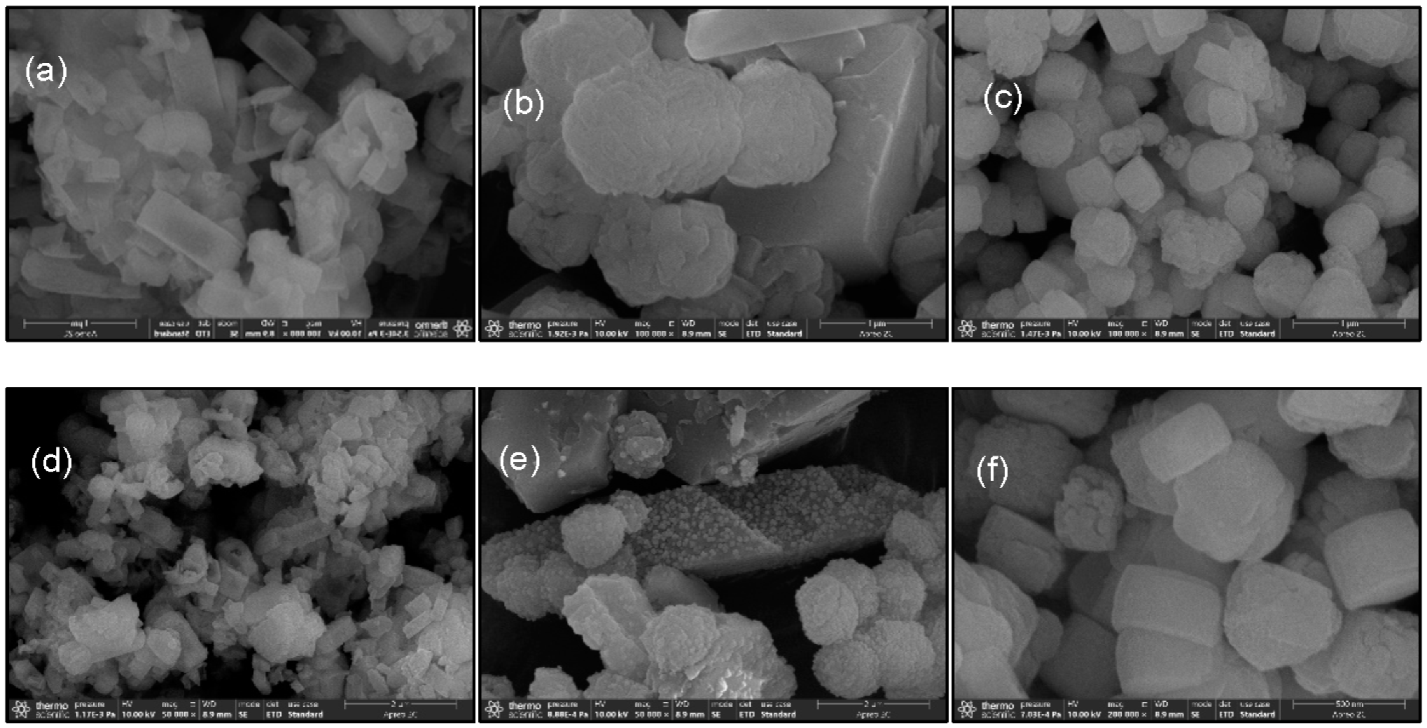
3.2.1. SEM Characterization
3.2.2. XRD Characterization
3.2.3. BET Characterization
3.3. Non-Catalytic Pyrolysis of Three Types of Waste Biomass
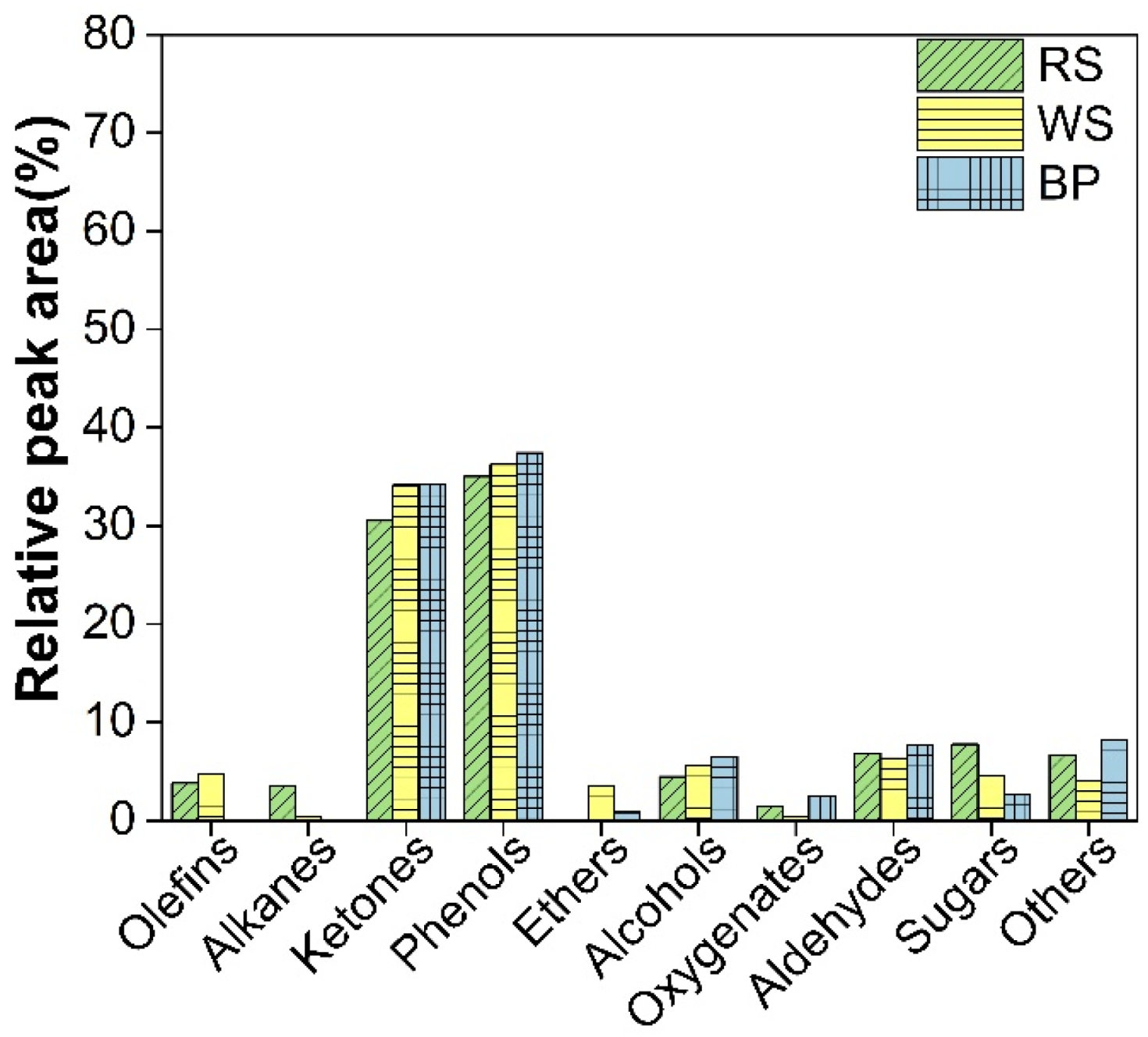
3.4. Catalytic Pyrolysis of Three Types of Waste Biomass with 65 wt.% Ni/SiO2-Al2O3
3.5. Catalytic Pyrolysis of Three Types of Waste Biomass with ZSM-5
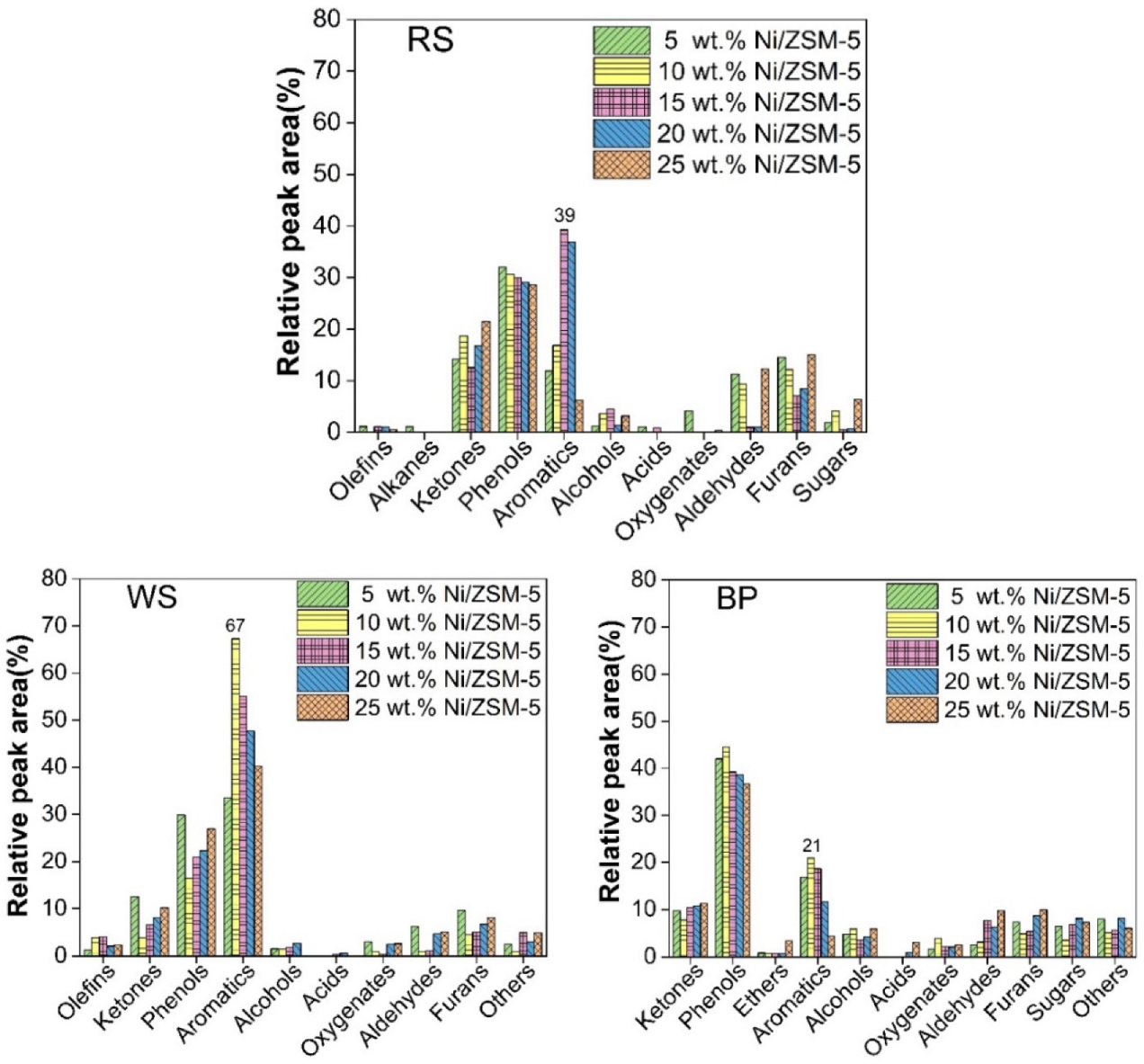
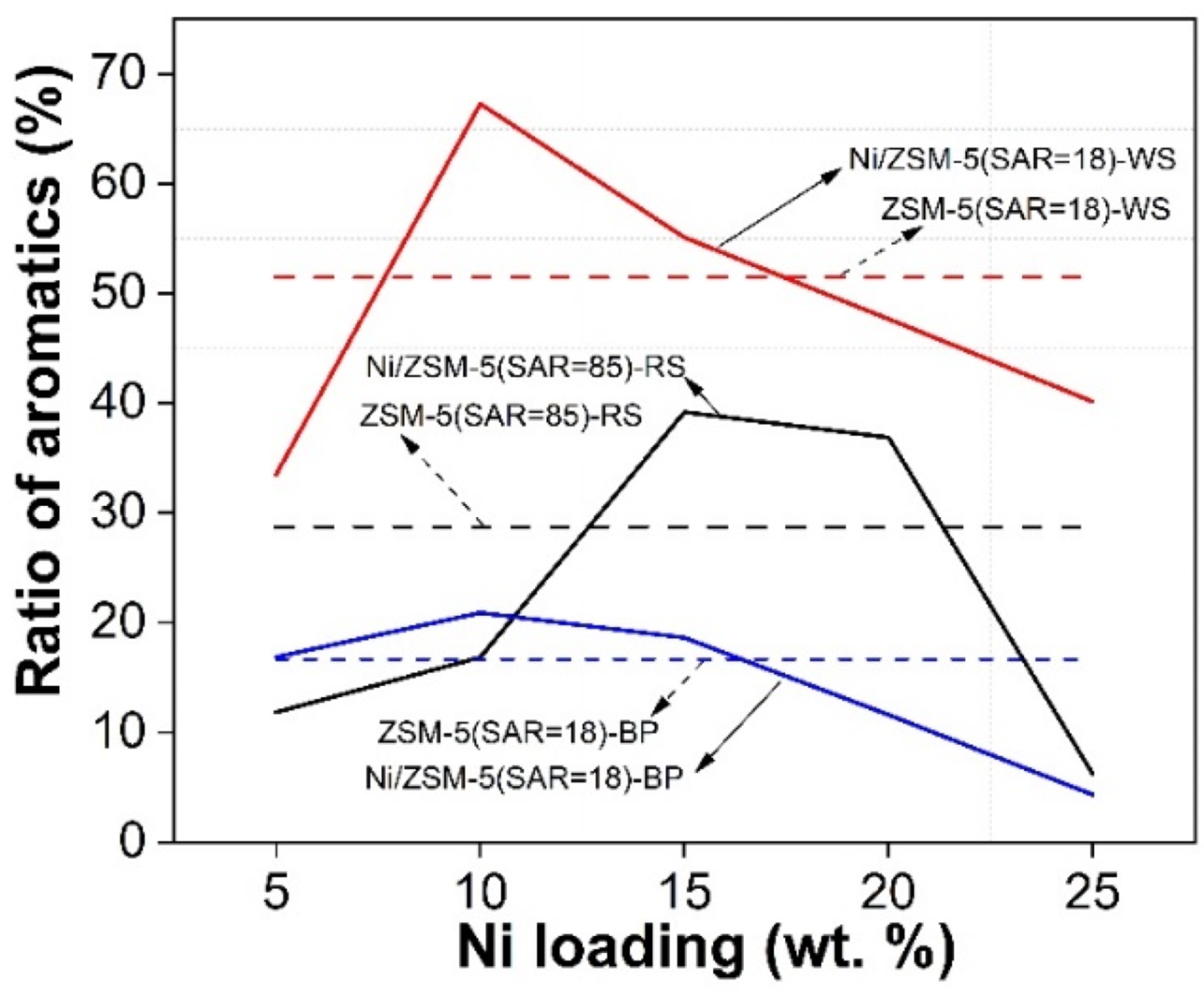
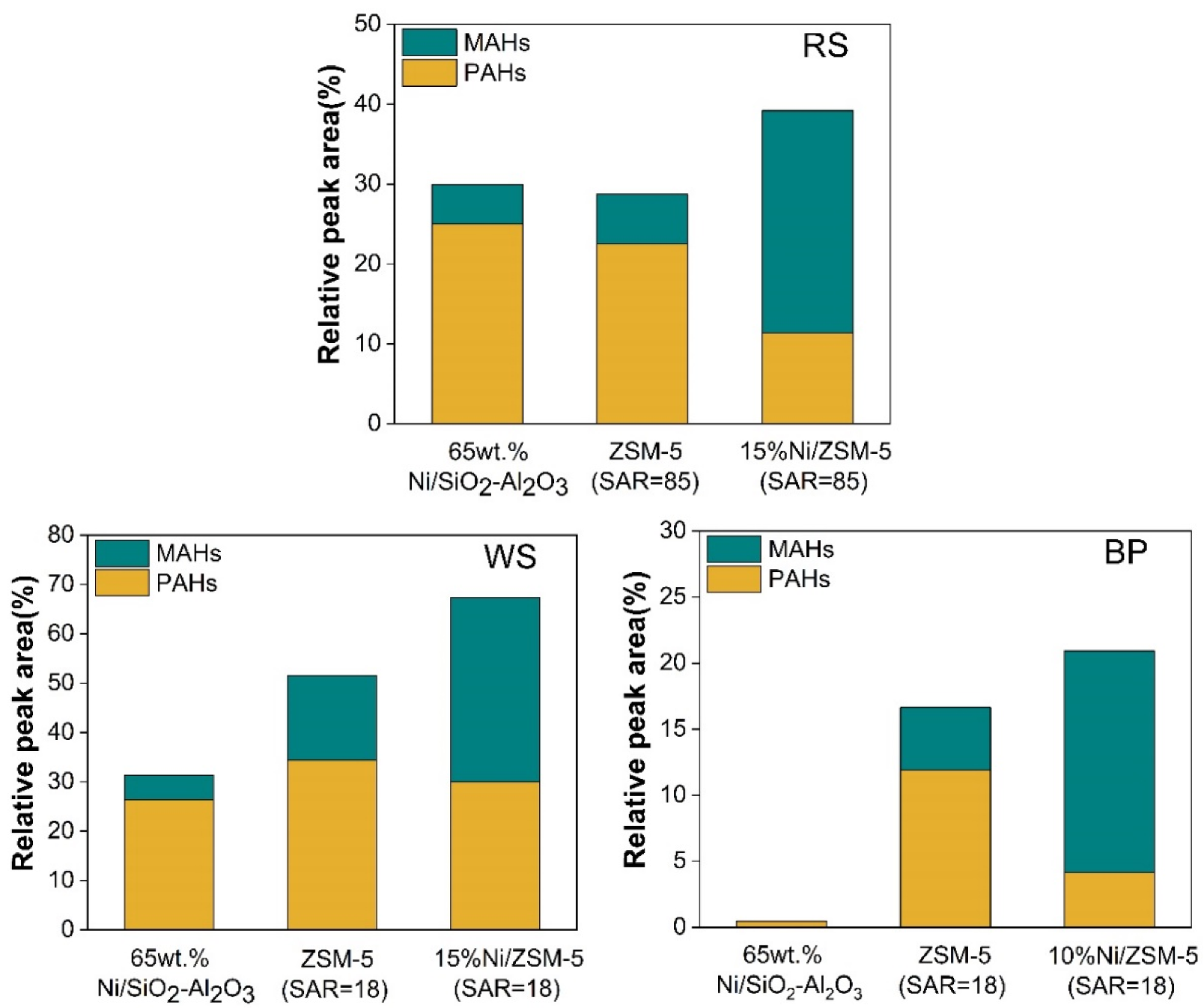
3.6. Catalytic Pyrolysis of Three Types of Waste Biomass with Ni/ZSM-5
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Laird, D.A.; Brown, R.C.; Amonette, J.E.; Lehmann, J. Review of the pyrolysis platform for coproducing bio-oil and biochar. Biofuels Bioprod. Biorefining 2009, 3, 547–562. [Google Scholar] [CrossRef]
- Donnis, B.; Egeberg, R.G.; Blom, P.; Knudsen, K.G. Hydroprocessing of bio-oils and oxygenates to hydrocarbons. Understanding the reaction routes. Top. Catal. 2009, 52, 229–240. [Google Scholar] [CrossRef]
- Ma, Y.; Li, H.; Yang, H.; Zhu, Y.; Zhao, L.; Li, M. Effects of solid acid and base catalysts on pyrolysis of rice straw and wheat straw biomass for hydrocarbon production. J. Energy Inst. 2022, 101, 140–148. [Google Scholar] [CrossRef]
- Yu, N.; Cai, Y.; Li, X.; Fan, Y.; Yin, H.; Zhang, R. Catalytic pyrolysis of rape straw for upgraded bio-oil production using HZSM-5 zeolite. Trans. Chin. Soc. Agric. Eng. 2014, 30, 264–271. [Google Scholar]
- Kim, Y.-M.; Park, S.; Kang, B.S.; Jae, J.; Rhee, G.H.; Jung, S.-C.; Park, Y.K. Suppressed char agglomeration by rotary kiln reactor with alumina ball during the pyrolysis of Kraft lignin. J. Ind. Eng. Chem. 2018, 66, 72–77. [Google Scholar] [CrossRef]
- Karnjanakom, S.; Suriya-umporn, T.; Bayu, A.; Kongparakul, S.; Samart, C.; Fushimi, C.; Abudula, A.; Guan, G. High selectivity and stability of Mg-doped Al-MCM-41 for in-situ catalytic upgrading fast pyrolysis bio-oil. Energy Convers. Manag. 2017, 142, 272–285. [Google Scholar] [CrossRef]
- Yildiz, G.; Pronk, M.; Djokic, M.; Van Geem, K.M.; Ronsse, F.; Van Duren, R.; Prins, W. Validation of a new set-up for continuous catalytic fast pyrolysis of biomass coupled with vapour phase upgrading. J. Anal. Appl. Pyrolysis 2013, 103, 343–351. [Google Scholar] [CrossRef]
- Liu, R.; Sarker, M.; Rahman, M.M.; Li, C.; Chai, M.; Cotillon, R.; Scott, N.R. Multiscale complexities of solid acid catalysts in the catalytic fast pyrolysis of biomass for bio-oil production—A review. Prog. Energy Combust. Sci. 2020, 80, 100852. [Google Scholar] [CrossRef]
- Fan, L.; Zhang, Y.; Liu, S.; Zhou, N.; Chen, P.; Liu, Y.; Wang, Y.; Peng, P.; Cheng, Y.; Addy, M.; et al. Ex-situ catalytic upgrading of vapors from microwave-assisted pyrolysis of low-density polyethylene with MgO. Energy Convers. Manag. 2017, 149, 432–441. [Google Scholar] [CrossRef]
- Ruddy, D.A.; Schaidle, J.A.; Ferrell, J.R., III; Wang, J.; Moens, L.; Hensley, J.E. Recent advances in heterogeneous catalysts for bio-oil upgrading via “ex situ catalytic fast pyrolysis”: Catalyst development through the study of model compounds. Green Chem. 2014, 16, 454–490. [Google Scholar] [CrossRef]
- Stefanidis, S.D.; Kalogiannis, K.G.; Iliopoulou, E.F.; Michailof, C.M.; Pilavachi, P.A.; Lappas, A.A. A study of lignocellulosic biomass pyrolysis via the pyrolysis of cellulose, hemicellulose and lignin. J. Anal. Appl. Pyrolysis 2014, 105, 143–150. [Google Scholar] [CrossRef]
- Ryu, H.W.; Lee, H.W.; Jae, J.; Park, Y.-K. Catalytic pyrolysis of lignin for the production of aromatic hydrocarbons: Effect of magnesium oxide catalyst. Energy 2019, 179, 669–675. [Google Scholar] [CrossRef]
- Zhao, W.; Huang, J.; Ni, K.; Zhang, X.; Lai, Z.; Cai, Y.; Li, X. Research on Non-Thermal Plasma assisted HZSM-5 online catalytic upgrading bio-oil. J. Energy Inst. 2018, 91, 595–604. [Google Scholar] [CrossRef]
- Kokotailo, G.; Lawton, S.; Olson, D.; Meier, W. Structure of synthetic zeolite ZSM-5. Nature 1978, 272, 437–438. [Google Scholar] [CrossRef]
- Dai, L.; Wang, Y.; Liu, Y.; Ruan, R.; Duan, D.; Zhao, Y.; Yu, Z.; Jiang, L. Catalytic fast pyrolysis of torrefied corn cob to aromatic hydrocarbons over Ni-modified hierarchical ZSM-5 catalyst. Bioresour. Technol. 2019, 272, 407–414. [Google Scholar] [CrossRef]
- Li, C.; Chai, M.; Rahman, M.M.; Li, Y.; Sarker, M.; Liu, R. Performance of alkali and Ni-modified ZSM-5 during catalytic pyrolysis of extracted hemicellulose from rice straw for the production of aromatic hydrocarbons. Renew. Energy 2021, 175, 936–951. [Google Scholar]
- Ding, Y.L.; Wang, H.-Q.; Xiang, M.; Yu, P.; Li, R.-Q.; Ke, Q.-P. The effect of Ni-ZSM-5 catalysts on catalytic pyrolysis and hydro-pyrolysis of biomass. Front. Chem. 2020, 8, 790. [Google Scholar] [CrossRef]
- Al-asadi, M.; Norbert, M. High temperature pyrolysis of municipal plastic waste using Me/Ni/ZSM-5 catalysts: The effect of metal/nickel ratio. Energies 2020, 13, 1284. [Google Scholar] [CrossRef]
- Jie, L.; Shan, G.C.; Sun, Y.F. Catalytic fast pyrolysis of lignocellulosic biomass: Critical role of zeolite catalysts. Renew. Sustain. Energy Rev. 2021, 139, 110707. [Google Scholar]
- French, R.; Czernik, S. Catalytic pyrolysis of biomass for biofuels production. Fuel Process. Technol. 2010, 91, 25–32. [Google Scholar] [CrossRef]
- Purwanto, W.W.; Supramono, D.; Muthia, R.; Firdaus, M.F. Effect of biomass types on bio-oil characteristics in a catalytic fast pyrolysis process with a Ni/ZSM-5 catalyst. Int. J. Technol. 2015, 6, 1069–1075. [Google Scholar] [CrossRef]
- Yung, M.M.; Starace, A.K.; Mukarakate, C.; Crow, A.M.; Leshnov, M.A.; Magrini, K.A. Biomass catalytic pyrolysis on Ni/ZSM-5: Effects of nickel pretreatment and loading. Energy Fuels 2016, 30, 5259–5268. [Google Scholar] [CrossRef]
- Stanton, A.R.; Iisa, K.; Yung, M.M.; Magrini, K.A. Catalytic fast pyrolysis with metal-modified ZSM-5 catalysts in inert and hydrogen atmospheres. J. Anal. Appl. Pyrolysis 2018, 135, 199–208. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, F.; Yang, X.; Huang, Y.; Liu, C.; Zheng, Z.; Gu, J. Study on aromatics production via the catalytic pyrolysis vapor upgrading of biomass using metal-loaded modified H-ZSM-5. J. Anal. Appl. Pyrolysis 2017, 126, 169–179. [Google Scholar] [CrossRef]
- Ghalibaf, M.; Lehto, J.; Alén, R. Fast pyrolysis of hot-water-extracted and delignified silver birch (Betula pendula) sawdust by Py-GC/MS. J. Anal. Appl. Pyrolysis 2017, 127, 17–22. [Google Scholar] [CrossRef]
- Zhang, B.; Zhong, Z.; Min, M.; Ding, K.; Xie, Q.; Ruan, R. Catalytic fast co-pyrolysis of biomass and food waste to produce aromatics: Analytical Py–GC/MS study. Bioresour. Technol. 2015, 189, 30–35. [Google Scholar] [CrossRef]
- Ding, K.; Zhong, Z.; Wang, J.; Zhang, B.; Addy, M.; Ruan, R. Effects of alkali-treated hierarchical HZSM-5 zeolites on the production of aromatic hydrocarbons from catalytic fast pyrolysis of waste cardboard. J. Anal. Appl. Pyrolysis 2017, 125, 153–161. [Google Scholar] [CrossRef]
- Kruk, M.; Jaroniec, M.; Joo, S.H.; Ryoo, R. Characterization of regular and plugged SBA-15 silicas by using adsorption and inverse carbon replication and explanation of the plug formation mechanism. J. Phys. Chem. B 2003, 107, 2205–2213. [Google Scholar] [CrossRef]
- Sun, R.; Tomkinson, J.; Ma, P.; Liang, S.F. Comparative study of hemicelluloses from rice straw by alkali and hydrogen per-oxide treatments. Carbohyd. Polym. 2000, 42, 111–122. [Google Scholar] [CrossRef]
- Liu, R.; Rahman, M.; Sarker, M.; Chai, M.; Li, C.; Cai, J. A review on the catalytic pyrolysis of biomass for the bio-oil production with ZSM-5: Focus on structure. Fuel Process. Technol. 2020, 199, 106301. [Google Scholar]
- Kong, X.; Zheng, R.; Zhu, Y.; Ding, G.; Zhu, Y.; Li, Y.-W. Rational design of Ni-based catalysts derived from hydrotalcite for selective hydrogenation of 5-hydroxymethylfurfural. Green Chem. 2015, 17, 2504–2514. [Google Scholar] [CrossRef]
- Bridgwater, A.V. Review of fast pyrolysis of biomass and product upgrading. Biomass Bioenergy 2012, 38, 68–94. [Google Scholar] [CrossRef]
- Foster, A.J.; Jae, J.; Cheng, Y.-T.; Huber, G.W.; Lobo, R.F. Optimizing the aromatic yield and distribution from catalytic fast pyrolysis of biomass over ZSM-5. Appl. Catal. A Gen. 2012, 423, 154–161. [Google Scholar] [CrossRef]
- Shang, J.; Fu, G.; Cai, Z.; Feng, X.; Tuo, Y.; Zhou, X.; Yan, H.; Peng, C.; Jin, X.; Liu, Y.; et al. Regulating light olefins or aromatics production in ex-situ catalytic pyrolysis of biomass by engineering the structure of tin modified ZSM-5 catalyst. Bioresour. Technol. 2021, 330, 124975. [Google Scholar] [CrossRef] [PubMed]
- Olson, D.H.; Kokotailo, G.T.; Lawton, S.L.; Meier, W.M. Crystal structure and structure-related properties of ZSM-5. J. Phys. Chem. 1981, 85, 238–2243. [Google Scholar] [CrossRef]
- Popescu, M.C.; Froidevaux, J.; Navi, P.; Popescu, C.M. Structural modifications of Tilia cordata wood during heat treatment investigated by FT-IR and 2D IR correlation spectroscopy. J. Mol. Struct. 2013, 1033, 176–186. [Google Scholar] [CrossRef]
- Peng, X.; Nie, S.; Li, X.; Huang, X.; Li, Q. Characteristics of the Water- and Alkali-Soluble Hemicelluloses Fractionated by Sequential Acidification and Graded-Ethanol from Sweet Maize Stems. Molecules 2019, 24, 212. [Google Scholar] [CrossRef]
- Ren, S.J.; Lei, H.; Wang, L.; Bu, Q.; Chen, S.; Wu, J. Thermal behaviour and kinetic study for woody biomass torrefaction and torrefied biomass pyrolysis by TGA. Biosyst. Eng. 2013, 116, 420–426. [Google Scholar] [CrossRef]
- Sudhakar, K.; Manickam, P. Characterization of micro algal biomass through FTIR/TGA/CHN analysis: Application to Scenedesmus sp. Energy Sources Part A Recovery Util. Environ. Eff. 2015, 37, 2330–2337. [Google Scholar] [CrossRef]
- Zhong, M.; Zhai, J.; Xu, Y.; Jin, L.; Ye, Y.; Hu, H.; Ma, F.; Fan, X. Catalytic cracking of coal-tar model compounds over ZrO2/Al2O3 and Ni-Ce/Al2O3 catalysts under steam atmosphere. Fuel 2020, 263, 116763. [Google Scholar] [CrossRef]
- Li, Y.; Fu, Q.; Flytzani-Stephanopoulos, M. Low-temperature water-gas shift reaction over Cu-and Ni-loaded cerium oxide catalysts. Appl. Catal. B Environ. 2000, 27, 179–191. [Google Scholar] [CrossRef] [Green Version]


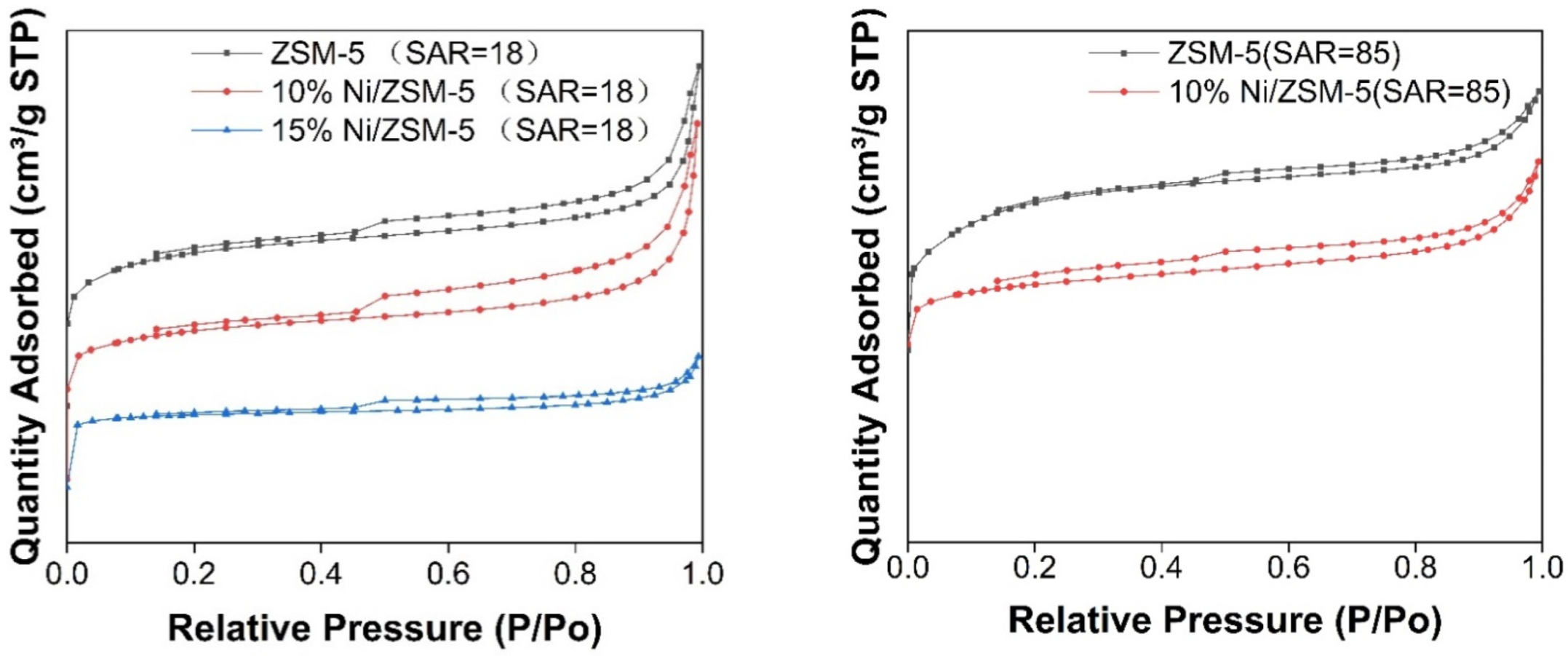




| Catalyst | Si/Al | a Pore Size, nm | Pore Volume, cm³/g | BET Surface, m²/g |
|---|---|---|---|---|
| ZSM-5 | 5:18 | 2.95 | 0.16 | 251.19 |
| ZSM-5 | 5:85 | 2.43 | 0.21 | 337.03 |
| 10 wt.% Ni/ZSM-5 | 5:18 | 3.49 | 0.17 | 192.00 |
| 15 wt.% Ni/ZSM-5 | 5:18 | 2.61 | 0.08 | 126.39 |
| 10 wt.% Ni/ZSM-5 | 5:85 | 2.74 | 0.18 | 257.65 |
| Biomass Type (Dry Base) | Cellulose, % | Hemicellulose, % | Lignin, % |
|---|---|---|---|
| RS (65.64) | 41.35 | 11.26 | 13.03 |
| WS (61.61) | 45.36 | 9.72 | 6.53 |
| BP (82.27) | 31.24 | 18.68 | 32.35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Pan, H.; Xie, S.; Li, G.; Du, Z.; Wang, X.; Luo, Y. Highly Selective Production of Valuable Aromatic Hydrocarbons/Phenols from Forestry and Agricultural Residues Using Ni/ZSM-5 Catalyst. Processes 2022, 10, 1970. https://doi.org/10.3390/pr10101970
Zhou X, Pan H, Xie S, Li G, Du Z, Wang X, Luo Y. Highly Selective Production of Valuable Aromatic Hydrocarbons/Phenols from Forestry and Agricultural Residues Using Ni/ZSM-5 Catalyst. Processes. 2022; 10(10):1970. https://doi.org/10.3390/pr10101970
Chicago/Turabian StyleZhou, Xuan, Hongling Pan, Shuixiang Xie, Guotao Li, Zhicai Du, Xiang Wang, and Yan Luo. 2022. "Highly Selective Production of Valuable Aromatic Hydrocarbons/Phenols from Forestry and Agricultural Residues Using Ni/ZSM-5 Catalyst" Processes 10, no. 10: 1970. https://doi.org/10.3390/pr10101970





