Hydrogen Production from Biomass and Organic Waste Using Dark Fermentation: An Analysis of Literature Data on the Effect of Operating Parameters on Process Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Prospection
2.2. Regression Modelling and Statistical Analysis
- Operating mode: continuous (CN), batch (BA);
- Residence time (RT): RT1 ≤ 2 d, 2 d < RT2 ≤ 10 d, RT3 > 10 d;
- Substrate concentration as total chemical oxygen demand (tCOD);
- Substrate type: soluble substrates (SS) made up of single substrates or synthetic models of wastewaters, non-soluble substrates (SNS) made up of biomass, sludge, or organic/food waste;
- Pretreatment of the substrate: yes (SY), no (SN);
- Methanogens’ inhibition: yes (IY), no (IN);
- Temperature: mesophilic (Tm), thermophilic (Tt);
- pH: acidic (pHa < 6), neutral (6 ≤ pHn ≤ 8), alkaline (pHb > 8).
- τb < ±0.10, very weak;
- ±0.10 < τb < 0.19, weak;
- ±0.20 < τb < 0.29, moderate;
- ≥−0.30, strong.
- -
- Highly significant ‘***’: 0 ≤ p-value < 0.001;
- -
- Fairly significant ‘**’: 0.001 ≤ p-value < 0.01;
- -
- Significant ‘*’: 0.01 ≤ p-value < 0.05;
- -
- Non-significant ‘•’: 0.05 ≤ p-value < 0.1;
- -
- Highly non-significant ‘no symbol’: p-value ≥ 0.1.
3. Results and discussion
3.1. General Observation and Descriptive Statistics
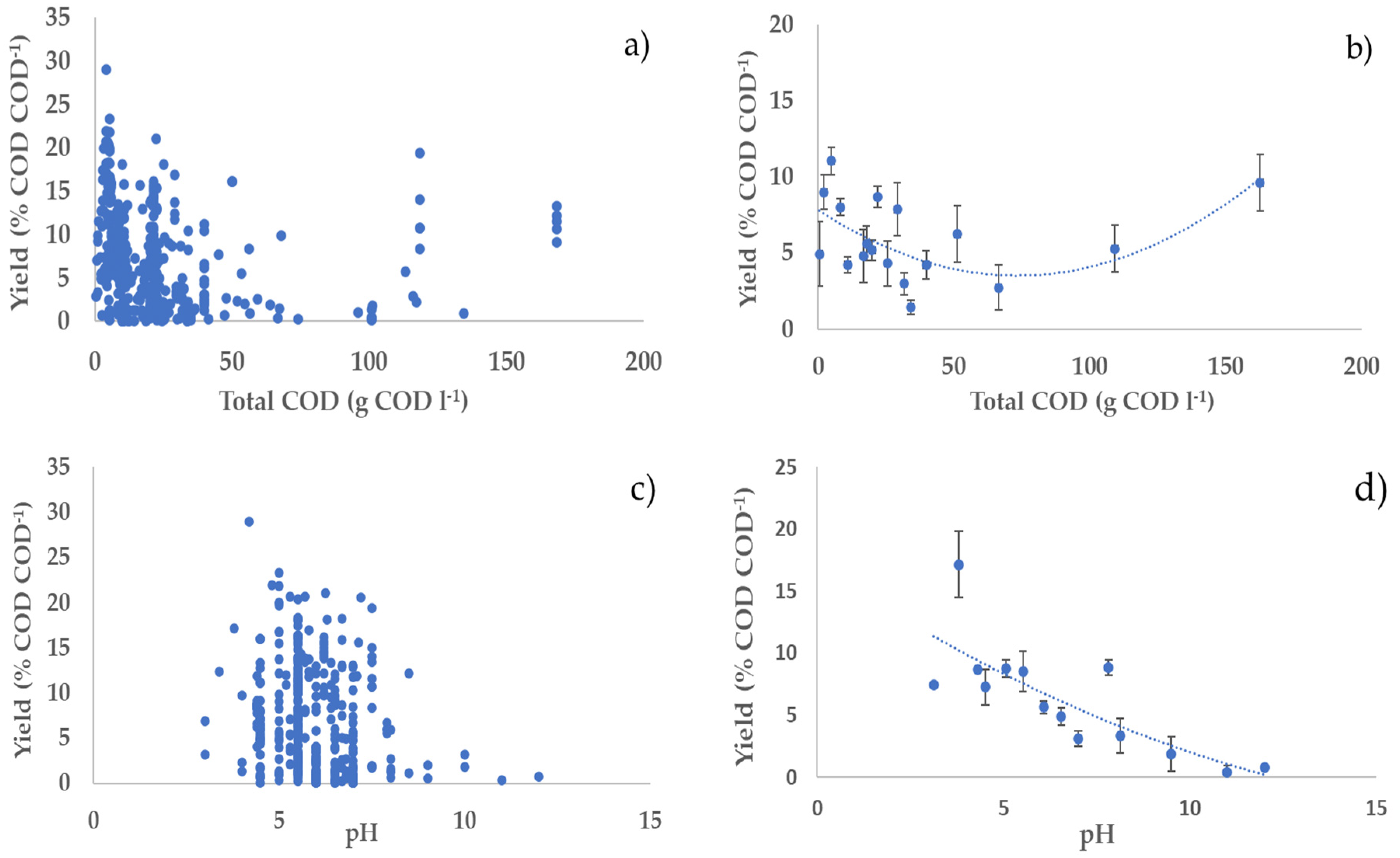
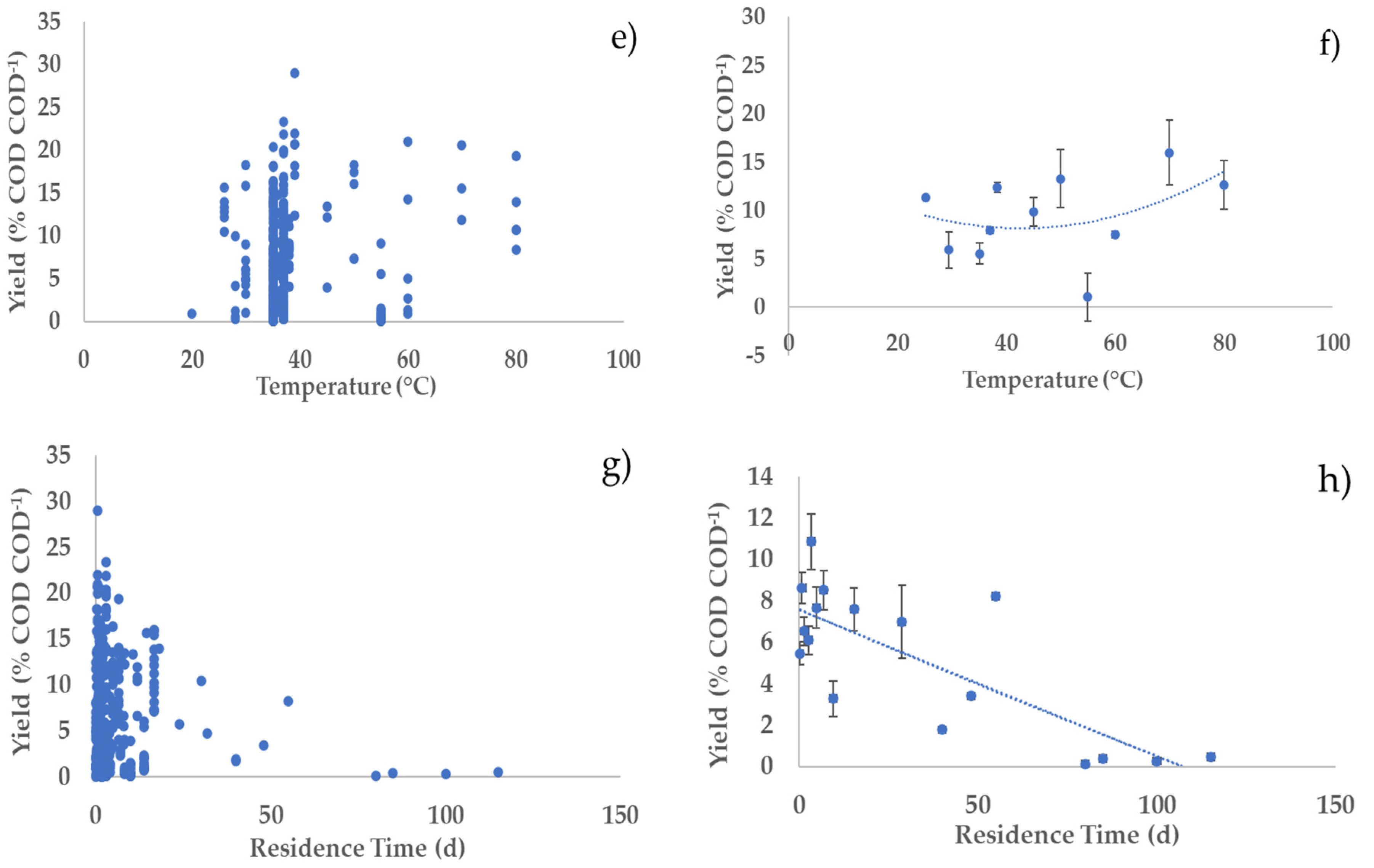
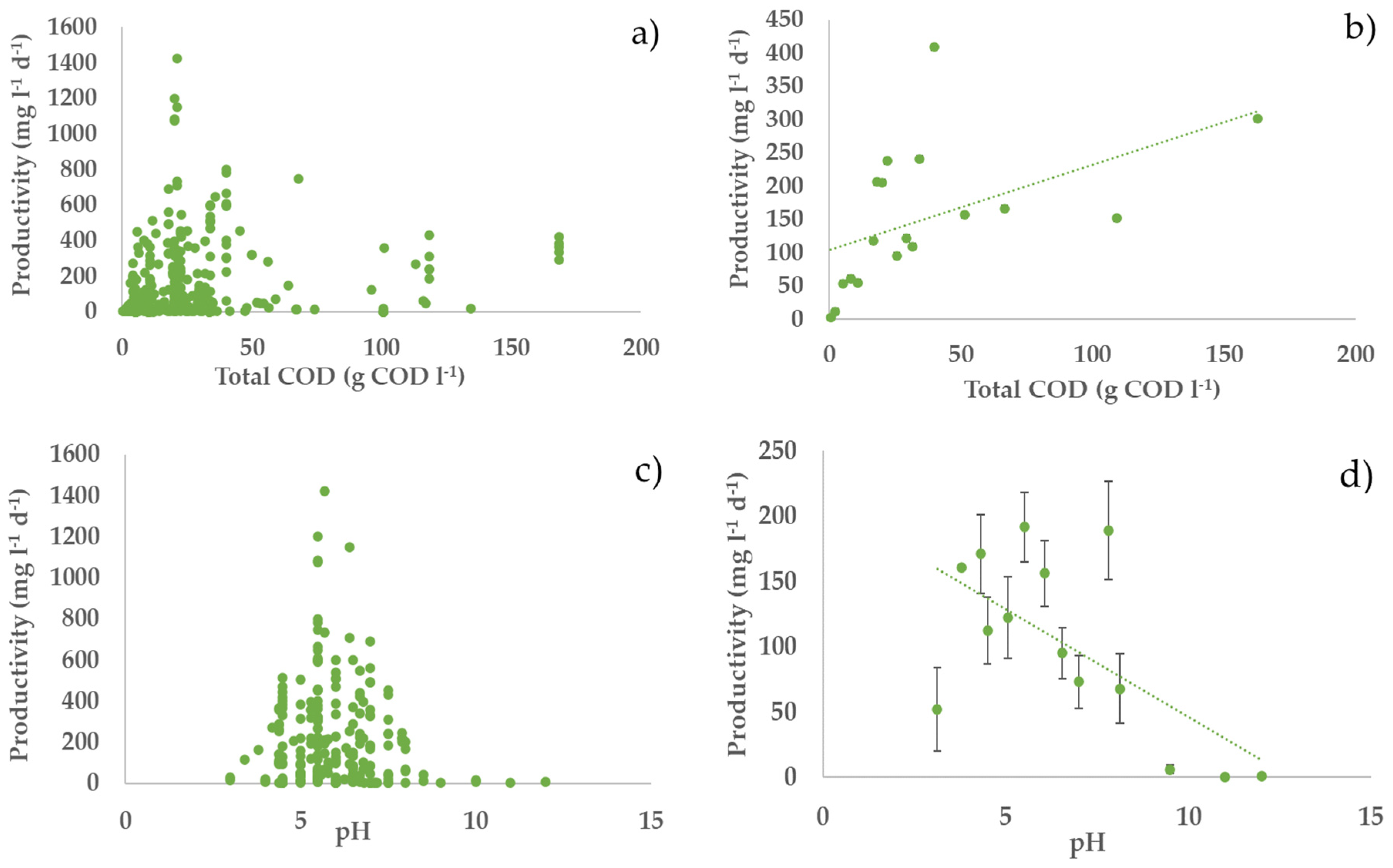
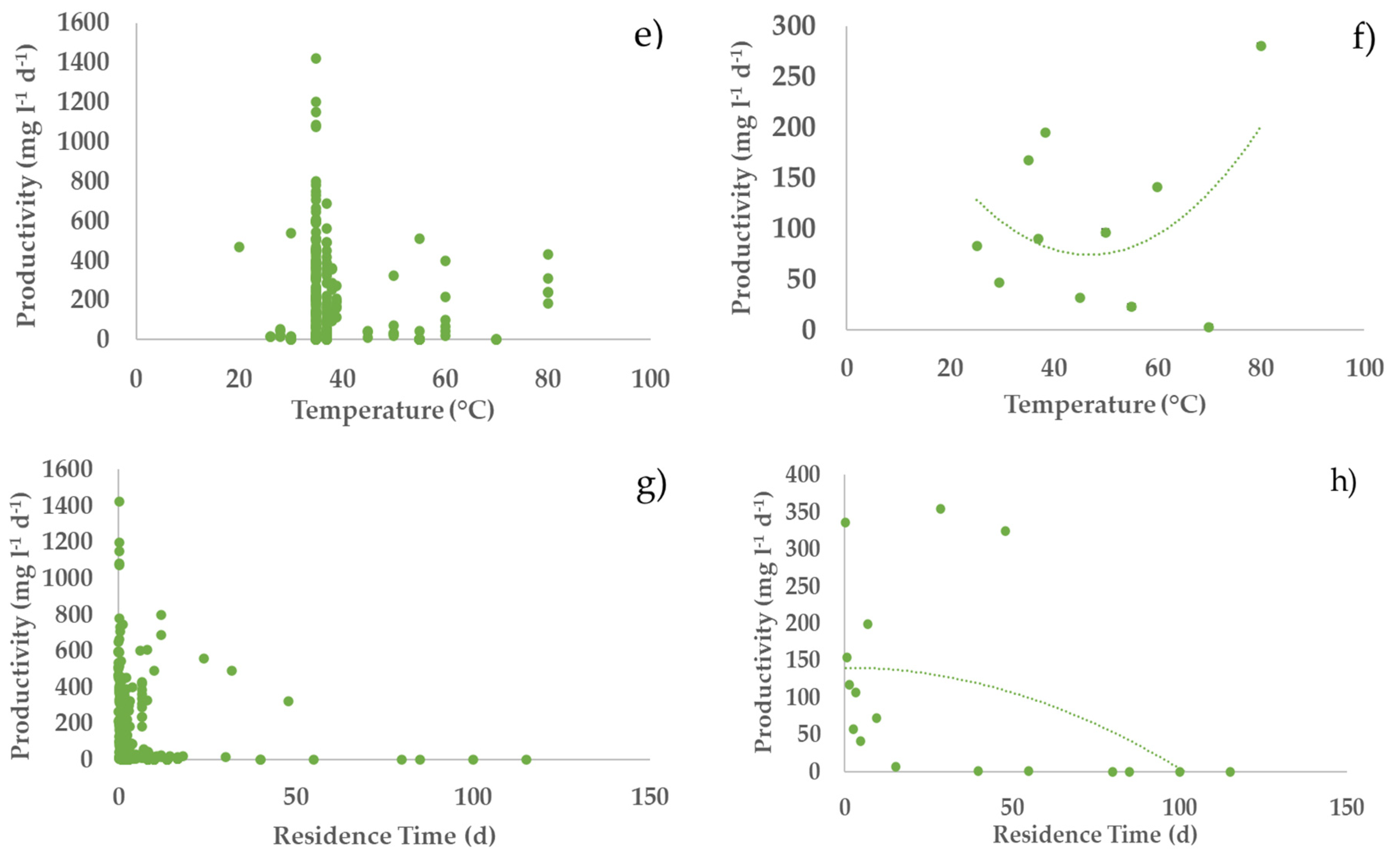
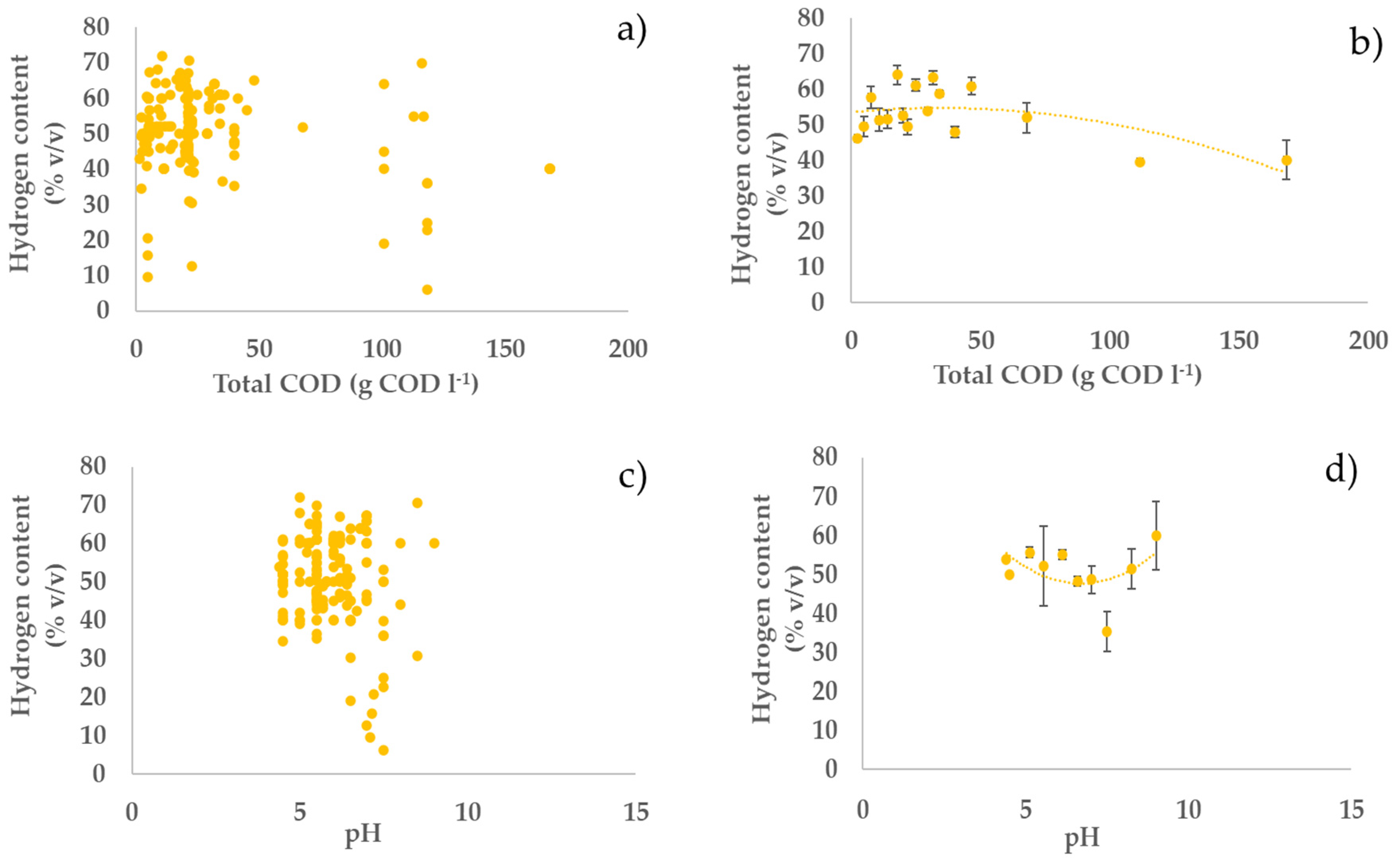
3.2. Profiles of the Performance Variables vs. Operating Parameters
3.3. Descriptive Statistics and Performances
3.4. Bivariate Analysis
3.5. Multi-Linear Regression Model
3.5.1. Hydrogen Yield
3.5.2. Hydrogen Productivity
3.5.3. H2 Content
4. General Discussion
4.1. Comparison with Other Studies in the Literature
4.2. Process Considerations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- De Bere, L. Anaerobic digestion of solid waste: State-of-the-art. Water Sci. Technol. 2000, 41, 283–290. [Google Scholar] [CrossRef]
- Mata-Ãlvarez, J. Biomethanization of the Organic Fraction of Municipal Solid Wastes; IWA publishing: London, UK, 2002; Volume 4. [Google Scholar]
- Zupančič, G.D.; Uranjek-Ževart, N.; Roš¡, M. Full-scale anaerobic co-digestion of organic waste and municipal sludge. Biomass Bioenergy 2008, 32, 162–167. [Google Scholar] [CrossRef]
- Ghyoot, W.; Verstraete, W. Anaerobic digestion of primary sludge from chemical pre-precipitation. Water Sci. Technol. 1997, 36, 357–365. [Google Scholar] [CrossRef]
- Kleerebezem, R.; Joosse, B.; Rozendal, R.; Van Loosdrecht, M.C.M. Anaerobic digestion without biogas? Rev. Environ. Sci. Bio/Technol. 2015, 14, 787–801. [Google Scholar] [CrossRef] [Green Version]
- Das, D. Advances in biohydrogen production processes: An approach towards commercialization. Int. J. Hydrogen Energy 2009, 34, 7349–7357. [Google Scholar] [CrossRef]
- Dionisi, D.; Silva, I.M.O. Production of ethanol, organic acids and hydrogen: An opportunity for mixed culture biotechnology? Rev. Environ. Sci. Bio/Technol. 2016, 15, 213–242. [Google Scholar] [CrossRef]
- Pinto, S.; Jamshidi Far, A.; Dionisi, D. Land and water requirements for the supply of renewable heating and transport energy using anaerobic digestion and water electrolysis. A case study for the UK. Sustain. Energy Technol. Assess. 2021, 48, 101636. [Google Scholar] [CrossRef]
- Dahiya, S.; Chatterjee, S.; Sarkar, O.; Mohan, S.V. Renewable hydrogen production by dark-fermentation: Current status, challenges and perspectives. Bioresour. Technol. 2021, 321, 124354. [Google Scholar] [CrossRef]
- Ueno, Y.; Otsuka, S.; Morimoto, M. Hydrogen production from industrial wastewater by anaerobic microflora in chemostat culture. J. Ferment. Bioeng. 1996, 82, 194–197. [Google Scholar] [CrossRef]
- Lay, C.; Wu, J.; Hsiao, C.; Chang, J.; Chen, C.; Lin, C. Biohydrogen production from soluble condensed molasses fermentation using anaerobic fermentation. Int. J. Hydrogen Energy 2010, 35, 13445–13451. [Google Scholar] [CrossRef]
- Ren, N.; Li, J.; Li, B.; Wang, Y.; Liu, S. Biohydrogen production from molasses by anaerobic fermentation with a pilot-scale bioreactor system. Int. J. Hydrogen Energy 2006, 31, 2147–2157. [Google Scholar] [CrossRef]
- Yu, H.; Zhu, Z.; Hu, W.; Zhang, H. Hydrogen production from rice winery wastewater in an upflow anaerobic reactor by using mixed anaerobic cultures. Int. J. Hydrog. Energy Biohydrog. 2002, 27, 1359–1365. [Google Scholar] [CrossRef]
- Lin, C.Y.; Lay, C.H. A nutrient formulation for fermentative hydrogen production using anaerobic sewage sludge microflora. Int. J. Hydrogen Energy 2005, 30, 285–292. [Google Scholar] [CrossRef]
- Roychowdhury, S.; Cox, D.; Levandowsky, M. Production of hydrogen by microbial fermentation. Int. J. Hydrogen Energy 1988, 13, 407–410. [Google Scholar] [CrossRef]
- Kataoka, N.; Miya, A.; Kiriyama, K. Studies on hydrogen production by continuous culture system of hydrogen-producing anaerobic bacteria. Water Sci. Technol. 1997, 36, 41–47. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, H.; Fang, H.H.P. Biohydrogen production from starch in wastewater under thermophilic condition. J. Environ. Manag. 2003, 69, 149–156. [Google Scholar] [CrossRef]
- Jung, K.; Kim, D.; Shin, H. Continuous fermentative hydrogen production from coffee drink manufacturing wastewater by applying UASB reactor. Int. J. Hydrogen Energy 2010, 35, 13370–13378. [Google Scholar] [CrossRef]
- Mu, Y.; Yu, H.; Wang, Y. The role of pH in the fermentative hydrogen production from an acidogenic granule-based reactor. Chemosphere 2006, 64, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; Yu, H. Biological hydrogen production in a UASB reactor with granules. I: Physicochemical characteristics of hydrogen-producing granules. Biotechnol. Bioeng. 2006, 94, 980–987. [Google Scholar] [CrossRef]
- Chang, F.; Lin, C. Biohydrogen production using an up-flow anaerobic sludge blanket reactor. Int. J. Hydrogen Energy 2004, 29, 33–39. [Google Scholar] [CrossRef]
- Yu, H.; Mu, Y. Biological hydrogen production in a UASB reactor with granules. II: Reactor performance in 3-year operation. Biotechnol. Bioeng. 2006, 94, 988–995. [Google Scholar] [CrossRef] [PubMed]
- Noike, T. Biological Hydrogen Production of Organic Wastes—Development of the Two-Phase Hydrogen Production Process; International Symposium on Hydrogen and Methane Fermentation of Organic Wastes: Tokyo, Japan, 2002; pp. 31–39. [Google Scholar]
- Kotsopoulos, T.A.; Zeng, R.J.; Angelidaki, I. Biohydrogen production in granular up-flow anaerobic sludge blanket (UASB) reactors with mixed cultures under hyper-thermophilic temperature (70 degrees C). Biotechnol. Bioeng. 2006, 94, 296–302. [Google Scholar] [CrossRef]
- Arriaga, S.; Rosas, I.; Alatriste-Mondragón, F.; Razo-Flores, E. Continuous production of hydrogen from oat straw hydrolysate in a biotrickling filter. Fuel Energy Abstr. 2011, 36, 3442–3449. [Google Scholar] [CrossRef]
- Li, J.; Li, B.; Zhu, G.; Ren, N.; Bo, L.; He, J. Hydrogen production from diluted molasses by anaerobic hydrogen producing bacteria in an anaerobic baffled reactor (ABR). Int. J. Hydrogen Energy 2005, 32, 3274–3283. [Google Scholar] [CrossRef]
- Chen, C.; Lin, C.; Lin, M. Acid-base enrichment enhances anaerobic hydrogen production process. Appl. Microbiol. Biotechnol. 2002, 58, 224–228. [Google Scholar] [PubMed]
- Guo, W.; Ren, N.; Chen, Z.; Liu, B.; Wang, X.; Xiang, W.; Ding, J. Simultaneous biohydrogen production and starch wastewater treatment in an acidogenic expanded granular sludge bed reactor by mixed culture for long-term operation. Int. J. Hydrogen Energy 2008, 33, 7397–7404. [Google Scholar] [CrossRef]
- Guo, L.; Lu, M.; Li, Q.; Zhang, J.; She, Z. A comparison of different pretreatments on hydrogen fermentation from waste sludge by fluorescence excitation-emission matrix with regional integration analysis. Int. J. Hydrogen Energy 2015, 40, 197–208. [Google Scholar] [CrossRef]
- Arooj, M.F.; Han, S.; Kim, S.; Kim, D.; Shin, H. Continuous biohydrogen production in a CSTR using starch as a substrate. Int. J. Hydrogen Energy 2008, 33, 3289–3294. [Google Scholar] [CrossRef]
- Chen, S.; Lee, K.; Lo, Y.; Chen, W.; Wu, J.; Lin, C.; Chang, J. Batch and continuous biohydrogen production from starch hydrolysate by Clostridium species. Int. J. Hydrogen Energy 2008, 33, 1803–1812. [Google Scholar] [CrossRef]
- Datar, R.; Huang, J.; Maness, P.; Mohagheghi, A.; Czernik, S.; Chornet, E. Hydrogen production from the fermentation of corn stover biomass pretreated with a steam-explosion process. Int. J. Hydrogen Energy 2007, 32, 932–939. [Google Scholar] [CrossRef]
- Pattra, S.; Sangyoka, S.; Boonmee, M.; Reungsang, A. Bio-hydrogen production from the fermentation of sugarcane bagasse hydrolysate by Clostridium butyricum. Int. J. Hydrogen Energy 2008, 33, 5256–5265. [Google Scholar] [CrossRef]
- Ntaikou, I.; Gavala, H.N.; Kornaros, M.; Lyberatos, G. Hydrogen production from sugars and sweet sorghum biomass using Ruminococcus albus. Int. J. Hydrogen Energy 2008, 33, 1153–1163. [Google Scholar] [CrossRef]
- Wang, Z.; Shao, S.; Zhang, C.; Lu, D.; Ma, H.; Ren, X. Pretreatment of vinegar residue and anaerobic sludge for enhanced hydrogen and methane production in the two-stage anaerobic system. Int. J. Hydrogen Energy 2015, 40, 4494–4501. [Google Scholar] [CrossRef]
- Sagnak, R.; Kargi, F.; Kapdan, I.K. Bio-hydrogen production from acid hydrolyzed waste ground wheat by dark fermentation. Int. J. Hydrogen Energy 2011, 36, 12803–12809. [Google Scholar] [CrossRef]
- Chu, C.; Tung, L.; Lin, C. Effect of substrate concentration and pH on biohydrogen production kinetics from food industry wastewater by mixed culture. Int. J. Hydrogen Energy 2013, 38, 15849–15855. [Google Scholar] [CrossRef]
- Zhu, H.; Beland, M. Evaluation of alternative methods of preparing hydrogen producing seeds from digested wastewater sludge. Int. J. Hydrogen Energy 2006, 31, 1980–1988. [Google Scholar] [CrossRef]
- Ziara, R.M.M.; Miller, D.N.; Subbiah, J.; Dvorak, B.I. Lactate wastewater dark fermentation: The effect of temperature and initial pH on biohydrogen production and microbial community. Int. J. Hydrogen Energy 2019, 44, 661–673. [Google Scholar] [CrossRef]
- Khanal, S.K.; Chen, W.; Li, L.; Sung, S. Biological hydrogen production: Effects of pH and intermediate products. Int. J. Hydrogen Energy 2004, 29, 1123–1131. [Google Scholar] [CrossRef]
- Yang, H.; Shao, P.; Lu, T.; Shen, J.; Wang, D.; Xu, Z.; Yuan, X. Continuous bio-hydrogen production from citric acid wastewater via facultative anaerobic bacteria. Int. J. Hydrogen Energy 2006, 31, 1306–1313. [Google Scholar] [CrossRef]
- Chang, J.; Wu, J.; Wen, F.; Hung, K.; Chen, Y.; Hsiao, C.; Lin, C.; Huang, C. Molecular monitoring of microbes in a continuous hydrogen-producing system with different hydraulic retention time. Int. J. Hydrogen Energy 2006, 33, 1579–1585. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, P.; Song, X.; Qu, Y. Hydrogen production from cellulose by co-culture of Clostridium thermocellum JN4 and Thermoanaerobacterium thermosaccharolyticum GD17. Int. J. Hydrogen Energy 2008, 33, 2927–2933. [Google Scholar] [CrossRef]
- Thong, S.; Prasertsan, P.; Karakashev, D.; Angelidaki, I. Thermophilic fermentative hydrogen production by the newly isolated Thermoanaerobacterium thermosaccharolyticum PSU-2. Int. J. Hydrogen Energy 2008, 33, 1204–1214. [Google Scholar] [CrossRef]
- Wang, X.; Jin, B.; Mulcahy, D. Impact of carbon and nitrogen sources on hydrogen production by a newly isolated Clostridium butyricum W5. Int. J. Hydrogen Energy 2008, 33, 4998–5005. [Google Scholar] [CrossRef]
- Vatsala, T.M.; Raj, S.M.; Manimaran, A. A pilot-scale study of biohydrogen production from distillery effluent using defined bacterial co-culture. Int. J. Hydrogen Energy 2008, 33, 5404–5415. [Google Scholar] [CrossRef]
- Fang, H.H.P.; Li, C.; Zhang, T. Acidophilic biohydrogen production from rice slurry. Int. J. Hydrogen Energy 2006, 31, 683–692. [Google Scholar] [CrossRef]
- Hussy, I.; Hawkes, F.R.; Dinsdale, R.; Hawkes, D.L. Continuous Fermentative Hydrogen Production from a Wheat Starch Co-Product by Mixed Microflora. Biotechnol. Bioeng. 2003, 84, 619–626. [Google Scholar] [CrossRef]
- Davila-Vazquez, G.; Alatriste-Mondragón, F.; de León-Rodríguez, A.; Razo-Flores, E. Fermentative hydrogen production in batch experiments using lactose, cheese whey and glucose: Influence of initial substrate concentration and pH. Int. J. Hydrogen Energy 2008, 33, 4989–4997. [Google Scholar] [CrossRef]
- Wang, C.C.; Chang, C.W.; Chu, C.P.; Lee, D.J.; Chang, B.V.; Liao, C.S. Producing hydrogen from wastewater sludge by Clostridium bifermentans. J. Biotechnol. 2003, 102, 83–92. [Google Scholar] [CrossRef]
- Liu, H.; Fang, H.H. Hydrogen production from wastewater by acidogenic granular sludge. Water Sci. Technol. J. Int. Assoc. Water Pollut. Res. 2002, 47, 153–158. [Google Scholar] [CrossRef]
- Han, H.; Wei, L.; Liu, B.; Yang, H.; Shen, J. Optimization of biohydrogen production from soybean straw using anaerobic mixed bacteria. Int. J. Hydrogen Energy 2012, 37, 13200–13208. [Google Scholar] [CrossRef]
- Wang, Y.; Ping, A.; Hu, C.; Zhang, Y. Effects of various pretreatment methods of anaerobic mixed microflora on biohydrogen production and the fermentation pathway of glucose. Int. J. Hydrogen Energy 2011, 36, 390–396. [Google Scholar] [CrossRef]
- Fangkum, A.; Reungsang, A. Biohydrogen production from sugarcane bagasse hydrolysate by elephant dung: Effects of initial pH and substrate concentration. Int. J. Hydrogen Energy 2011, 36, 8687–8696. [Google Scholar] [CrossRef]
- Cui, M.; Yuan, Z.; Zhi, X.; Wei, L.; Shen, J. Biohydrogen production from poplar leaves pretreated by different methods using anaerobic mixed bacteria. Int. J. Hydrogen Energy 2010, 35, 4041–4047. [Google Scholar] [CrossRef]
- Chen, C.; Chuang, Y.; Lin, C.; Lay, C.; Sen, B. Thermophilic dark fermentation of untreated rice straw using mixed cultures for hydrogen production. Int. J. Hydrogen Energy 2012, 37, 15540–15546. [Google Scholar] [CrossRef]
- Sreela-or, C.; Imai, T.; Plangklang, P.; Reungsang, A. Optimization of key factors affecting hydrogen production from food waste by anaerobic mixed cultures. Int. J. Hydrogen Energy 2011, 36, 14120–14133. [Google Scholar] [CrossRef]
- Kim, D.; Kim, S.; Kim, H.; Kim, M.; Shin, H. Sewage sludge addition to food waste synergistically enhances hydrogen fermentation performance. Bioresource Technol. 2011, 102, 8501–8506. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, M.; Miyahara, T.; Mizuno, O.; Noike, T. Biological hydrogen potential of materials characteristic of the organic fraction of municipal solid wastes. Water Sci. Technol. 2000, 41, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Yuan, Z.; Sun, Y.; Kong, X.; Zhang, Y. Hydrogen production characteristics of the organic fraction of municipal solid wastes by anaerobic mixed culture fermentation. Int. J. Hydrogen Energy 2009, 34, 812–820. [Google Scholar] [CrossRef]
- Kim, S.; Han, S.; Shin, H. Feasibility of biohydrogen production by anaerobic co-digestion of food waste and sewage sludge. Int. J. Hydrogen Energy 2004, 29, 1607–1616. [Google Scholar] [CrossRef]
- Chen, W.; Chen, S.; Kumar Khanal, S.; Sung, S. Kinetic study of biological hydrogen production by anaerobic fermentation. Int. J. Hydrogen Energy 2006, 31, 2170–2178. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Han, S.J.; Kim, J.P.; Kim, M.S.; Oh, Y.K.; Sim, S.J. Hydrogen production by the hyperthermophilic eubacterium, Thermotoga neapolitana, using cellulose pretreated by ionic liquid. Int. J. Hydrogen Energy 2008, 33, 5161–5168. [Google Scholar] [CrossRef]
- Kyazze, G.; Dinsdale, R.; Guwy, A.J.; Hawkes, F.R.; Premier, G.C.; Hawkes, D.L. Performance characteristics of a two-stage dark fermentative system producing hydrogen and methane continuously. Biotechnol. Bioeng. 2007, 97, 759–770. [Google Scholar] [CrossRef] [PubMed]
- Henze, M. (Ed.) Wastewater Treatment: Biological and Chemical Processes, 3rd ed.; Springer: Berlin, Germany; New York, NY, USA, 2002. [Google Scholar]
- Dionisi, D.; Bolaji, I.; Nabbanda, D.; Silva, I.M.O. Calculation of the potential production of methane and chemicals using anaerobic digestion. Biofuels Bioprod. Biorefining 2018, 12, 788–801. [Google Scholar] [CrossRef]
- Grady, L.; Daigger, G.; Love, N.; Filipe, C. (Eds.) Biological Wastewater Treatment, 3rd ed.; IWA Publishing: London, UK, 2011. [Google Scholar]
- Lin, C.; Chang, R. Hydrogen production during the anaerobic acidogenic conversion of glucose. J. Chem. Technol. Biotechnol. 1999, 74, 498–500. [Google Scholar] [CrossRef]
- Dabrock, B.; Bahl, H.; Gottschalk, G. Parameters Affecting Solvent Production by Clostridium pasteurianum. Appl. Environ. Microbiol. 1992, 58, 1233–1239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lay, J.J. Modeling and optimization of anaerobic digested sludge converting starch to hydrogen. Biotechnol. Bioeng. 2000, 68, 269–278. [Google Scholar] [CrossRef]
- Lay, J.; Li, Y.; Noike, T. Influences of pH and moisture content on the methane production in high-solids sludge digestion. Water Res. 1997, 31, 1518–1524. [Google Scholar] [CrossRef]
- Temudo, M.F.; Kleerebezem, R.; van Loosdrecht, F.A.U.; van Loosdrecht, M. Influence of the pH on (open) mixed culture fermentation of glucose: A chemostat study. Biotechnol. Bioeng. 2007, 98, 69–79. [Google Scholar] [CrossRef]
- Lee, K.; Lin, P.; Chang, J. Temperature effects on biohydrogen production in a granular sludge bed induced by activated carbon carriers. Int. J. Hydrogen Energy 2006, 31, 465–472. [Google Scholar] [CrossRef]
- Liu, D.; Zeng, R.J.; Angelidaki, I. Effects of pH and hydraulic retention time on hydrogen production versus methanogenesis during anaerobic fermentation of organic household solid waste under extreme-thermophilic temperature (70 °C). Biotechnol. Bioeng. 2008, 100, 1108–1114. [Google Scholar] [CrossRef]
- Amorim, N.C.S.; Alves, I.; Martins, J.S.; Amorim, E.L.C. Biohydrogen production from cassava wastewater in an anaerobic fluidized bed reactor. Braz. J. Chem. Eng. 2014, 31, 603–612. [Google Scholar] [CrossRef]

| Conversion Unit | Conversion Factor | Reference |
|---|---|---|
| gCOD gVS−1 carbohydrates | 1.13 | [65] |
| gCOD gVS−1 proteins | 1.6 | [65] |
| gCOD gVS−1 lipids | 2.03 | [65,66] |
| gCOD gDS−1 wastewaters | 1.2 | [67] |
| Substrate Category | Frequency | Percentage |
|---|---|---|
| Pure substrate (glucose, sucrose, starch) | 96 | 28.2 |
| Biomass (oat straw, corn, sugarcane, wheat, soybean, rice, cellulose) | 79 | 23.2 |
| Food waste (bean curd manufacturing, molasses, vinegar, mixed food waste) | 47 | 13.8 |
| Organic waste (waste ground wheat, waste potato starch, rice slurry, sugarcane bagasse, and poplar leaves | 42 | 12.3 |
| Carbohydrate-rich wastewater (sugar factory, condensed molasses, and sucrose-rich wastewater) | 22 | 6.5 |
| Industrial wastewater (rice winery, coffee drink manufacturing, brewery, citric acid, and distillery wastewaters) | 21 | 6.2 |
| Synthetic wastewater | 13 | 3.8 |
| Wastewater sludge | 10 | 2.9 |
| Municipal wastewater | 10 | 2.9 |
| Performance Parameter | Mean | 90th Percentile |
|---|---|---|
| Hydrogen yield (% COD COD−1) | 6.49 (0.27) | 15.55 |
| Hydrogen productivity (mg L−1 d−1) | 135.49 (9.96) | 305.16 |
| Hydrogen content in biogas (% v/v) | 51.00 (0.39) | 64.00 |
| All Experiments | Highest Hydrogen Yields (% COD COD−1) | Highest Hydrogen Productivities (mg L−1 d−1) | Highest Hydrogen Content (% v/v) | |
|---|---|---|---|---|
| Substrate concentration (g COD L−1) | 23.00 (1.32) | 14.23 (3.04) | 33.91 (4.22) | 28.47 (5.92) |
| Residence time (d) | 5.02 (0.53) | 3.57 (0.74) | 3.10 (1.00) | 6.23 (0.64) |
| Temperature (°C) | 37.98 (0.40) | 40.05 (1.66) | 37.98 (1.32) | 39.91 (2.47) |
| pH | 5.96 (0.05) | 5.62 (0.13) | 5.85 (0.12) | 4.39 (0.77) |
| Parameters | Estimate | ±SE | t-Value | p-Value |
|---|---|---|---|---|
| Intercept | 6.93 | 0.36 | 19.48 | <2 × 10−16 *** |
| pHa | 1.4 | 0.53 | 2.65 | 0.008 ** |
| pHn | −1.05 | 0.26 | −4.01 | 6.20 × 10−5 *** |
| pHb | −1.93 | 0.67 | −2.09 | 0.04 * |
| tCOD | −0.02 | 0.01 | −1.93 | 0.05 |
| SS | 1.2 | 0.51 | 2.34 | 0.02 * |
| SNS | −1.19 | 0.27 | −4.45 | 9.03 × 10−6 *** |
| Tm | −0.32 | 0.46 | −0.67 | 0.49 |
| Tt | −0.74 | 0.45 | −1.66 | 0.98 |
| CN | 0.12 | 0.6 | 0.2 | 0.85 |
| BA | −0.36 | 0.24 | −1.47 | 0.14 |
| RT1 | −0.16 | 0.53 | −0.3 | 0.76 |
| RT2 | −0.45 | 0.28 | −1.61 | 0.11 |
| RT3 | −0.007 | 0.3 | −0.025 | 0.98 |
| IY | −0.64 | 0.48 | −1.34 | 0.18 |
| IN | 0.05 | 0.32 | 0.16 | 0.87 |
| SY | −0.81 | 0.6 | −1.34 | 0.18 |
| SN | −0.25 | 0.26 | −0.95 | 0.34 |
| Parameters | Estimate | ±SE | t-Value | p-Value |
|---|---|---|---|---|
| Intercept | 96.72 | 12.6 | 7.67 | 2.24 × 10−14 *** |
| pHa | 64.13 | 18.7 | 3.43 | 6.12 × 10−4 *** |
| pHn | 9.38 | 9.28 | 1.01 | 0.31 |
| pHb | −28.76 | 23.67 | −1.22 | 0.25 |
| tCOD | 1.69 | 0.36 | 4.75 | 2.14 × 10−6 *** |
| SS | 107.34 | 18.16 | 5.91 | 3.73 × 10−9 *** |
| SNS | −21.09 | 9.49 | −2.22 | 0.03 * |
| Tm | 46.38 | 16.21 | 2.86 | 0.004 ** |
| Tt | −11.83 | 15.88 | −0.75 | 0.46 |
| CN | 174.52 | 21.08 | 8.28 | <2 × 10−16 *** |
| BA | −13.13 | 8.6 | −1.53 | 0.13 |
| RT1 | 111.81 | 18.75 | 5.96 | 2.72 × 10−9 *** |
| RT2 | −12.42 | 9.97 | −1.25 | 0.21 |
| RT3 | −13.26 | 9.76 | −1.36 | 0.17 |
| IY | 53.9 | 16.85 | 3.2 | 0.001 ** |
| IN | −2.15 | 11.34 | −0.19 | 0.85 |
| SY | −5.5 | 21.13 | −0.26 | 0.79 |
| SN | 29.87 | 8.59 | 3.48 | 5.14 × 10−4 *** |
| Parameters | Estimate | ±SE | t-Value | p-Value |
|---|---|---|---|---|
| Intercept | 53.96 | 1.16 | 46.23 | <2 × 10−16 *** |
| pHa | −1.63 | 1.67 | −0.97 | 0.33 |
| pHn | −1.11 | 0.91 | −1.23 | 0.22 |
| pHb | −4.34 | 1.18 | −3.67 | 0.0003 *** |
| tCOD | −0.09 | 0.03 | −3.48 | 0.0005 *** |
| SS | −2.93 | 1.65 | −1.78 | 0.07 • |
| SNS | −1.22 | 0.88 | −1.38 | 0.17 |
| Tm | −1.78 | 1.50 | −1.18 | 0.24 |
| Tt | −3.71 | 1.26 | −2.94 | 0.003 ** |
| CN | 3.00 | 2.92 | 1.03 | 0.30 |
| BA | −0.91 | 1.48 | −0.61 | 0.54 |
| RT1 | −1.71 | 1.66 | −1.03 | 0.31 |
| RT2 | −1.94 | 0.94 | −2.06 | 0.04 * |
| RT3 | −1.13 | 0.97 | −1.17 | 0.24 |
| IY | −3.09 | 1.50 | −2.05 | 0.04 * |
| IN | −0.17 | 1.24 | −0.14 | 0.89 |
| SY | −5.49 | 2.07 | −2.65 | 0.008 ** |
| SN | −0.12 | 0.80 | −0.15 | 0.88 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moussa, R.N.; Moussa, N.; Dionisi, D. Hydrogen Production from Biomass and Organic Waste Using Dark Fermentation: An Analysis of Literature Data on the Effect of Operating Parameters on Process Performance. Processes 2022, 10, 156. https://doi.org/10.3390/pr10010156
Moussa RN, Moussa N, Dionisi D. Hydrogen Production from Biomass and Organic Waste Using Dark Fermentation: An Analysis of Literature Data on the Effect of Operating Parameters on Process Performance. Processes. 2022; 10(1):156. https://doi.org/10.3390/pr10010156
Chicago/Turabian StyleMoussa, Rita Noelle, Najah Moussa, and Davide Dionisi. 2022. "Hydrogen Production from Biomass and Organic Waste Using Dark Fermentation: An Analysis of Literature Data on the Effect of Operating Parameters on Process Performance" Processes 10, no. 1: 156. https://doi.org/10.3390/pr10010156






