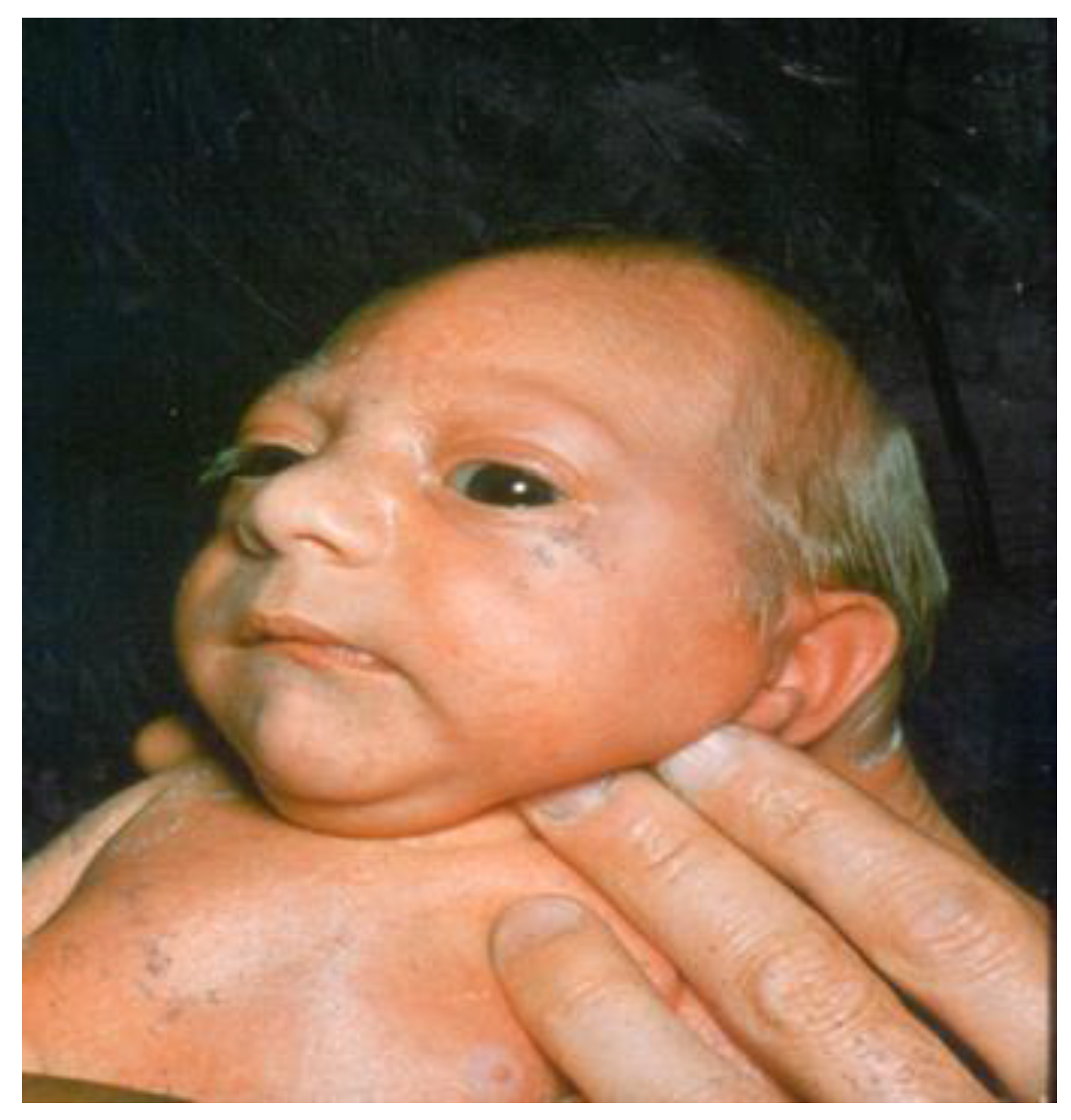
Microcephaly
Abstract
:1. Definition
2. Etiology
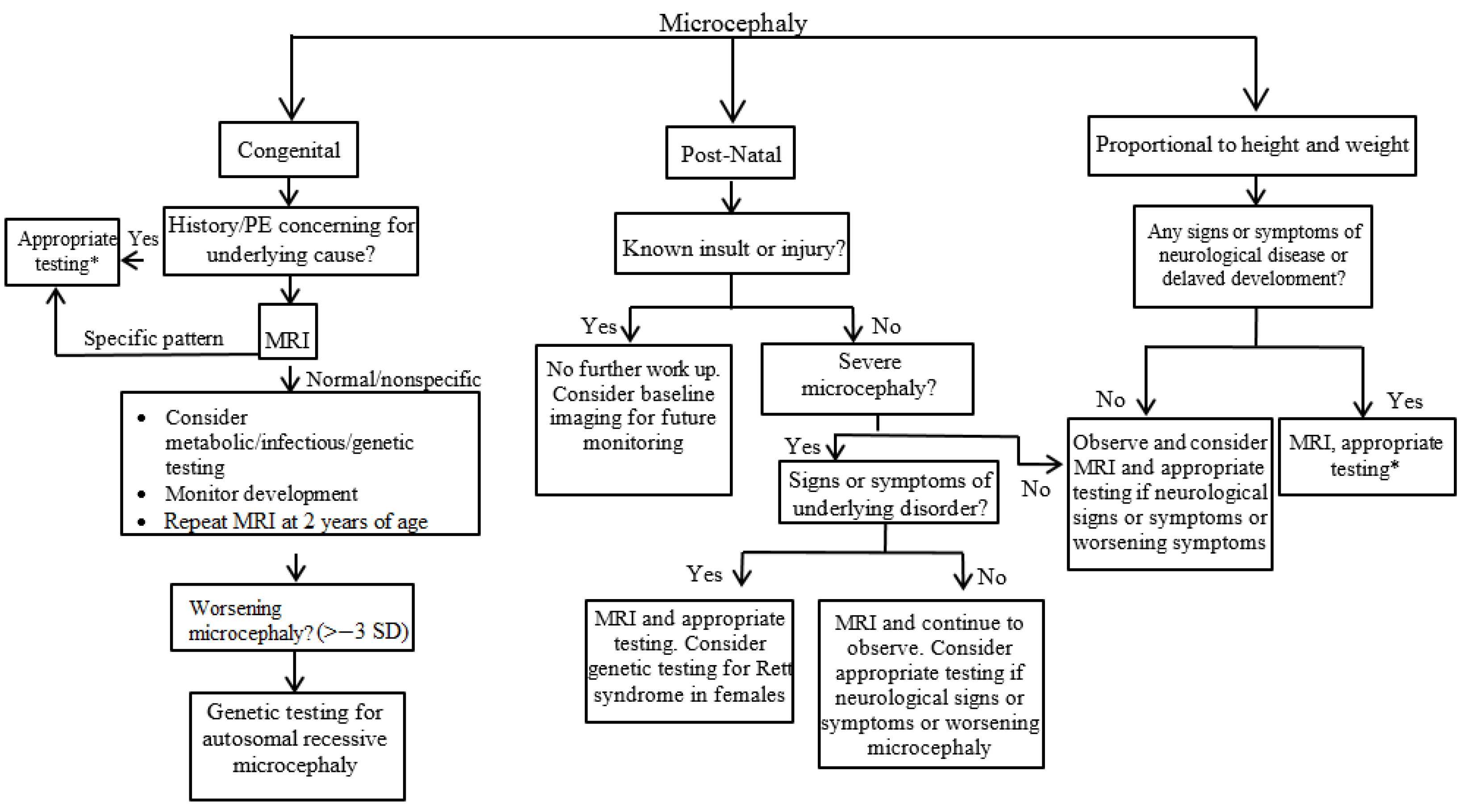
3. Evaluation
4. Prognosis
5. Specific Focus on Congenital Infections and Microcephaly: the Emergence of Zika Virus
6. Conclusions
Conflicts of Interest
References
- Abuelo, D. Microcephaly Syndromes. Semin. Pediatr. Neurol. 2007, 14, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Alamo-Junquera, D.; Sunyer, J.; Iniguez, C.; Ballester, F.; Garcia-Esteban, R.; Forns, J.; Turner, M.C.; Lertxundi, A.; Lertxundi, N.; Fernandez-Somoano, A.; et al. Prenatal head growth and child neuropsychological development at age 14 months. Am. J. Obstet. Gynecol. 2015, 212, e1–e11. [Google Scholar] [CrossRef] [PubMed]
- Ashwal, S.; Michelson, D.; Plauner, L.; Dobyns, W.B. Practice Parameter: Evaluation of the Child with Microcephaly (An Evidence-Based Review); American Academy of Neurology: Minneapolis, MN, USA, 2009. [Google Scholar]
- Stoler-Poria, S.; Lev, D.; Schweigher, A.; Lerman-Sagie, T.; Malinger, G. Developmental outcome of isolated fetal microcephaly. Ultrasound Obstet. Gynecol. 2010, 36, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Von der Hagen, M.; Pivarcsi, M.; Liebe, J.; von Bernuth, H.; Didonato, N.; Hennermann, J.B.; Buhrer, C.; Wieczorek, D.; Kaindl, A.M. Diagnostic approach to microcephaly in childhood: A two-center study and review of the literature. Dev. Med. Child Neurol. 2014, 56, 732–741. [Google Scholar] [CrossRef] [PubMed]
- Baxter, P.S.; Rigby, A.S.; Rotsaert, M.H.; Wright, I. Acquired Microcephaly: Causes, Patterns, Motor and IQ Effects, and Associated Growth Changes. Pediatrics 2009, 124, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Alcantara, D.; O’Driscoll, M. Congenital Microcephaly. Am. J. Med. Genet. Part C 2014, 166, 124–139. [Google Scholar] [CrossRef] [PubMed]
- Krauss, M.J.; Morrissey, A.E.; Winn, H.N.; Amon, E.; Leet, T.L. Microcephaly: An epidemiolgic analysis. Am. J. Obstet. Gynecol. 2002, 188, 1484–1490. [Google Scholar] [CrossRef]
- Rump, P.; Jazayeri, O.; Dijk-Bos, K.K.; Johansson, L.F.; van Essen, A.J.; Verheij, J.B.G.M.; Veenstra-Knol, H.E.; Redeker, E.J.W.; Mannens, M.M.A.M.; Swertz, M.A.; et al. Whole-exome sequencing is a powerful approach for establishing the etiological diagnosis in patients with intellectual disability and microcephaly. BMC Med. Genom. 2016, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Woods, C.G. Human microcephaly. Curr. Opin. Neurol. 2004, 14, 112–117. [Google Scholar] [CrossRef] [PubMed]
- National Birth Defects Prevention Network. Major birth defects data from population-based birth defects surveillance programs in the United States, 2006–2010. Birth Defects Res. Part A 2013, 97, S1–S172. [Google Scholar]
- Gilmore, E.C.; Walsh, C.A. Genetic causes of microcephaly and lessons for neuronal development. Wiley Interdisip. Rev. Dev. Biol. 2013, 2, 461–478. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, J.A.; Al-Harbi, K.M.; Ramzan, K.; Albalawi, A.M.; Mehmood, A.; Samman, M.I.; Basit, S. A novel splice-site mutation in the ASPM gene underlies autosomal recessive microcephaly. Ann. Saudi Med. 2016, 36, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Thornton, G.K.; Woods, C.G. Primary microcephaly: Do all roads lead to Rome? Trends Genet. 2009, 25, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Doobin, D.J.; Kemal, S.; Dantas, T.J.; Vallee, R.B. Severe NDE1-mediated microcephaly results from neural progenitor cell cycle arrests at multiple specific stages. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Cho, M.T.; Retterer, K.; Alexander, N.; Ben-Omran, T.; Al-Mureikhi, M.; Cristian, I.; Wheeler, P.G.; Crain, C.; Zand, D.; et al. De novo POGZ mutations are associated with neurodevelopmental disorders and microcephaly. Cold Spring Harb. Mol. Case Stud. 2015, 1, a000455. [Google Scholar] [CrossRef] [PubMed]
- Kadir, R.; Harel, T.; Markus, B.; Perez, Y.; Bakhrat, A.; Cohen, I.; Volodarsky, M.; Feintsein-Linial, M.; Chervinski, E.; Zlotogora, J.; et al. ALFY-Controlled DVL3 Autophagy Regulates Wnt Signaling, Determining Human Brain Size. PLoS ONE 2016, 12, e1005919. [Google Scholar] [CrossRef] [PubMed]
- Faheem, M.; Naseer, M.I.; Rasool, M.; Chaudhary, A.G.; Kumosani, T.A.; Ilyas, A.M.; Pushparaj, M.R.; Ahmed, F.; Algahtani, H.A.; Al-Qahtani, M.H.; et al. Molecular genetics of human primary microcephaly: An overview. BMC Med. Genom. 2015, 1. [Google Scholar] [CrossRef] [PubMed]
- Basit, S.; Al-Harbi, K.M.; Alhijji, S.A.M.; Albalawi, A.M.; Alharby, S.; Eldardear, A.; Samman, M.I. CIT, a gene involved in neurogenic cytokinesis, is mutated in human primary microcephaly. Hum. Genet. 2016, 135, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Leroy, J.G.; Frias, J.L. Nonsyndromic microcephaly: An overview. Adv. Pediatr. 2005, 52, 261–293. [Google Scholar] [CrossRef] [PubMed]
- Custer, D.A.; Vezina, L.G.; Vaught, D.R.; Brasseux, C.; Samango-Sprouse, C.A.; Cohen, M.S.; Rosenbaum, K.N. Neurodevelopmental and neuroimaging correlates in nonsyndromal microcephalic children. J. Dev. Behav. Pediatr. 2000, 21, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, T.; Yasuhara, A.; Nishida, N.; Murakami, K.; Woo, M.; Kobayashi, Y. MRI of the head in the evaluation of microcephaly. Neuropediatrics 1993, 24, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Michelson, D.; Ashwal, S.; Donley, D.; Plawner, L. Practice parameter: Evaluation of the child with global developmental delay: Report of the Quality Standards Subcommittee of the American Academy of Neurology and The Practice Committee of the Child Neurology Society. Neurology 2003, 60, 367–380. [Google Scholar]
- Den Hollander, N.S.; Wessels, M.W.; Los, F.J.; Ursem, N.T.; Niermeijer, M.F.; Wladimiroff, J.W. Congenital microcephaly detected by prenatal ultrasound: Genetic aspects and clinical significance. Ultrasound Obstet. Gynecol. 2000, 15, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Lalaguna-Mallada, P.; Alonso-del Val, B.; Abio-Albero, S.; Pena-Segura, J.L.; Rebage, V.; Lopez-Pison, J. Microcephalus as the reason for visiting a regional referral neuropaediatric service. Rev. Neurol. 2004, 38, 106–110. [Google Scholar] [PubMed]
- Naing, Z.W.; Scott, G.M.; Shand, A.; Hamilton, S.T.; van Zuylen, W.J.; Basha, J.; Hall, B.; Craig, M.E.; Rawlinson, W.D. Congenital cytomegalovirus infection in pregnancy: A review of prevalence, clinical features, diagnosis, and prevention. Aust. N. Z. J. Obstet. Gynecol. 2016, 56, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Noyola, D.E.; Demmler, G.J.; Nelson, C.T.; Griesser, C.; Williamson, W.D.; Atkins, J.T.; Rozelle, J.; Turcich, M.; Llorente, A.M.; Sellers-Vinson, S.; et al. Early predictors of neurodevelopmental outcome in symptomatic congenital cytomegalovirus infection. J. Pediatr. 2001, 138, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Russell, K.; Oliver, S.E.; Lewis, L.; Barfield, W.D.; Cragan, J.; Meaney-Delman, D.; Staples, J.E.; Fischer, M.; Peacock, G.; Oduyebo, T.; et al. Update: Interim Guidance for the Evaluation and Management of Infants with Possible Congenital Zika Virus Infection - United States, August 2016. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 870–878. [Google Scholar] [CrossRef] [PubMed]
- Souza, B.S.F.; Sampaio, G.L.A.; Pereira, C.S.; Campos, G.S.; Sardi, S.I.; Freitas, L.A.R.; Figueira, C.P.; Paredes, B.D.; Nonaka, C.K.V.; Azevedo, C.M.; et al. Zika virus infection induces mitosis abnormalities and apoptotic cell death of human neural progenitor cells. Sci. Rep. 2016. [Google Scholar] [CrossRef] [PubMed]
- Wolf, B.; Diop, F.; Ferraris, P.; Wichit, S.; Busso, C.; Misse, D.; Gonczy, P. Zika virus causes supernumerary foci with centriolar proteins and impaired spindle positioning. Open Biol. 2017, 7, 160231. [Google Scholar] [CrossRef] [PubMed]
- Shimada, M.; Matsuzaki, F.; Kato, A.; Kobayashi, J.; Matsumoto, T.; Komatsu, K. Induction of Excess Centrosomes in Neural Progenitor Cells during the Development of Radiation-Induced Microcephaly. PLoS ONE 2016, 11, e0158236. [Google Scholar] [CrossRef] [PubMed]
- Shao, Q.; Herrlinger, S.; Yang, S.L.; Lai, F.; Moore, J.M.; Brindley, M.A.; Chen, J.F. Zika virus infection disrupts neurovascular development and results in postnatal microcephaly with brain damage. Development 2016, 143, 4127–4136. [Google Scholar] [CrossRef] [PubMed]
- Soares de Araújo, J.S.; Regis, C.T.; Gomes, R.G.S.; Tavares, T.R.; Rocha dos Santos, C.; Assuncao, P.M.; Nóbrega, R.V.; Alves Pinto, D.d.F.; Bezerra, B.V.D.; da Silva Mattos, S. Microcephaly in north-east Brazil: A retrospective study on neonates born between 2012 and 2015. Bull. World Health Organ. 2016. [Google Scholar] [CrossRef]
- Rasmussen, S.A.; Jamieson, D.J.; Honein, M.A.; Peterson, L.R. Zika virus and birth defects—Reviewing the evidence for causality. 2016, 374, 1981–1987. [Google Scholar] [CrossRef] [PubMed]
- Pardi, N.; Hogan, M.J.; Pelc, R.S.; Muramatsu, H.; Andersen, H.; DeMaso, C.R.; Dowd, K.A.; Sutherland, L.L.; Scearce, R.M.; Parks, R.; et al. Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature 2017, 543, 248–251. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanzlik, E.; Gigante, J. Microcephaly. Children 2017, 4, 47. https://doi.org/10.3390/children4060047
Hanzlik E, Gigante J. Microcephaly. Children. 2017; 4(6):47. https://doi.org/10.3390/children4060047
Chicago/Turabian StyleHanzlik, Emily, and Joseph Gigante. 2017. "Microcephaly" Children 4, no. 6: 47. https://doi.org/10.3390/children4060047
APA StyleHanzlik, E., & Gigante, J. (2017). Microcephaly. Children, 4(6), 47. https://doi.org/10.3390/children4060047




