Kiwifruit Allergy in Children: Characterization of Main Allergens and Patterns of Recognition
Abstract
:1. Introduction
2. Methods
2.1. Patients
2.2. Ethical Considerations
2.3. Skin Tests
2.4. Specific IgE Determination
2.5. Preparation of Kiwifruit Protein Extract
2.6. Sodium Dodecyl Sulphate Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.7. IgE Immunoblotting
2.8. In Vitro Component Resolved Diagnosis
3. Results
3.1. Patients
| No. | Sex | Age (Years) | Clinical Manifestations | Other Food Allergies | Other Allergies | Other Allergens | Prick Kiwi (mm) | Prick-Prick Kiwi (mm) | Kiwifruit Specific IgE (kU/L) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Act d 1 | Act d 2 | Act d 5 | Act d 8 | Pru p 1 | Pru p 3 | Cor a 1.04 | Cor a 8 | Cor a 9 | ||||||||||
| 1 | M | 11 | Abdominal pain, vomiting | Rhinitis | Dust mites, timothy grass pollen | 4 × 4 | 4 × 3 | ND | 0 | 0 | 0 | 0 | 2.35 | 0 | 0 | 0 | 0 | |
| 2 | M | 5 | Itchy throat, lip swelling | Strawberry | Asthma, rhinitis, atopic dermatitis | Dust mites, timothy grass pollen, cat dander | 3 × 3 | 4 × 3 | ND | |||||||||
| 3 | F | 8 | Swelling of lips and eyelids, vomiting, abdominal pain | Egg and fish | Asthma, atopic dermatitis | Dust mites | 9 × 5 | 11 × 7 | 10.8 | 3.81 | 4.08 | 0 | 0 | 0 | 3.06 | 0 | 4 | 0 |
| 4 | M | 6 | Generalized hives and erythema | Atopic dermatitis | 8 × 2 | 3 × 4 | ND | 1.83 | 0 | 0 | 0 | 0 | 1.5 | 0 | 0 | 0 | ||
| 5 | F | 5 | Hives on the face, lip swelling | Asthma, atopic dermatitis | Dust mites | ND | 6 × 3 | <0.35 | ||||||||||
| 6 | F | 12 | Itchy mouth | Rhinitis, conjunctivitis | Dust mites | 10 × 9 | 5 × 4 | ND | 4.2 | 0 | 0 | 0 | 2.24 | 0 | 0 | 0 | 0 | |
| 7 | M | 12 | Hives, itchy throat | Asthma, rhinitis, atopic dermatitis | Dust mites, timothy grass pollen, herbaceous | 4 × 4 | 11 × 7 | ND | 2.28 | 0 | 0 | 0 | 2.44 | 0 | 0 | 0 | 0 | |
| 8 | M | 9 | Lips swelling | Seafood | Rhinitis, atopic dermatitis | Dust mites | 10 × 6 | 20 × 12 | <0.35 | 5.54 | 0 | 0 | 0 | 2.49 | 0 | 0 | 0 | 0 |
| 9 | F | 7 | Hives and erythema, wheezing, moderate dispnea | Egg | Asthma | Dust mites | 7 × 7 | ND | 9.22 | |||||||||
| 10 | M | 11 | Generalized hives and erythema | Rhinitis | Dust mites | 7 × 6 | 7 × 6 | <0.35 | 0 | 0 | 0 | 0 | 1.89 | 0 | 0 | 0 | 0 | |
| 11 | M | 8 | Vomiting, abdominal pain | Fish, egg and peanut | Asthma, rhinitis | Dust mites, cat dander | 12 × 7 | 17 × 10 | 17.8 | 9.81 | 0 | 0 | 0 | 2.31 | 0 | 0 | 0 | 0 |
| 12 | F | 10 | Facial swelling, contact urticaria | Tree nuts | Asthma, rhinitis | ND | 5 × 9 | 0.97 | 0 | 0 | 0 | 0 | 2.61 | 0 | 0 | 0 | 22 | |
| 13 | M | 3 | Perioral erythema and edema | Cow, milk, protein | Atopic dermatitis | 10 × 6 | 10 × 7 | <0.35 | 0 | 0 | 0 | 0 | 1.69 | 0 | 0 | 0 | 0 | |
| 14 | F | 6 | Itchy throat and mouth, lips swelling | Egg, peanut, hazelnut, walnut and peanut | 5 × 3 | 14 × 4 | 0.82 | 0 | 0 | 0 | 0 | 1.67 | 0 | 0 | 0 | 0 | ||
| 15 | F | 12 | Hives and lips swelling | Asthma, rhinitis | Dust mites, timothy grass pollen, birch pollen, herbaceous, cat dander | 13 × 8 | ND | 4.28 | 3.27 | 0 | 0 | 0 | 1.68 | 0 | 1.7 | 0 | 0 | |
| 16 | M | 9 | Generalized hives, vomiting and diarrhea | Egg and banana | Atopic dermatitis, latex allergy | Dust mites | 7 × 7 | 15 × 7 | 17.9 | 7.74 | 2.73 | 40.33 | 29.5 | 0 | 18.66 | 0 | 8.6 | 0 |
| 17 | F | 6 | Erythema and edema of lips and ear | Egg | Atopic dermatitis | 7 × 9 | ND | ND | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 18 | M | 8 | Eyelids swelling, red and watery eyes, nasal congestion and itching, sneezing | Asthma, rhinitis, atopic dermatitis | Dust mites, timothy grass pollen, latex | ND | 5 × 7 | 3.33 | ||||||||||
| 19 | M | 11 | Lips swelling, oral itching, repetitive coughing and wheezing, dyspnea | Banana, chickpea, prawn, pea, squid, apple, pear, peach, watermelon, plum, cherry | Asthma, rhinitis, atopic dermatitis | Dust mites, cat and dog dander | ND | 7 × 7 | ND | |||||||||
| 20 | M | 7 | Itchy throat and mouth, lips swelling, throat tightness | Egg | Rhinitis, conjunctivitis | Timothy grass pollen | 5 × 5 | 5 × 7 | <0.35 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 21 | F | 9 | Erythema, hives, lip swelling, oral itching | Asthma, rhinitis, atopic dermatitis | Dust mites, fungus, cat dander | 12 × 7 | 7 × 10 | ND | 0 | 0 | 0 | 7.02 | 0 | 0 | 0 | 0 | 0 | |
| 22 | M | 8 | Lips swelling, itchy throat, red or watery eyes | Seafood | Rhinitis, conjunctivitis | Dust mites, timothy grass pollen | 3 × 3 | 3 × 3 | <0.35 | 0 | 0 | 0 | 0 | 1.84 | 0 | 1.4 | 0 | 0 |
| 23 | M | 3 | Erythema and edema of lips and ear, oral itching | Milk, egg. | Asthma, rhinitis, atopic dermatitis | Dust mites, timothy grass pollen | ND | 19 × 15 | ND | |||||||||
| 24 | M | 12 | Abdominal pain, vomiting, diarrhea, | Egg, fish | Asthma, rhinitis, atopic dermatitis | Dust mites, timothy grass pollen | 5 × 6 | 11 × 7 | 9.47 |
3.2. Skin Testing and Serum Specific IgE
3.3. Detection of IgE-Binding Proteins in the Kiwifruit Extract
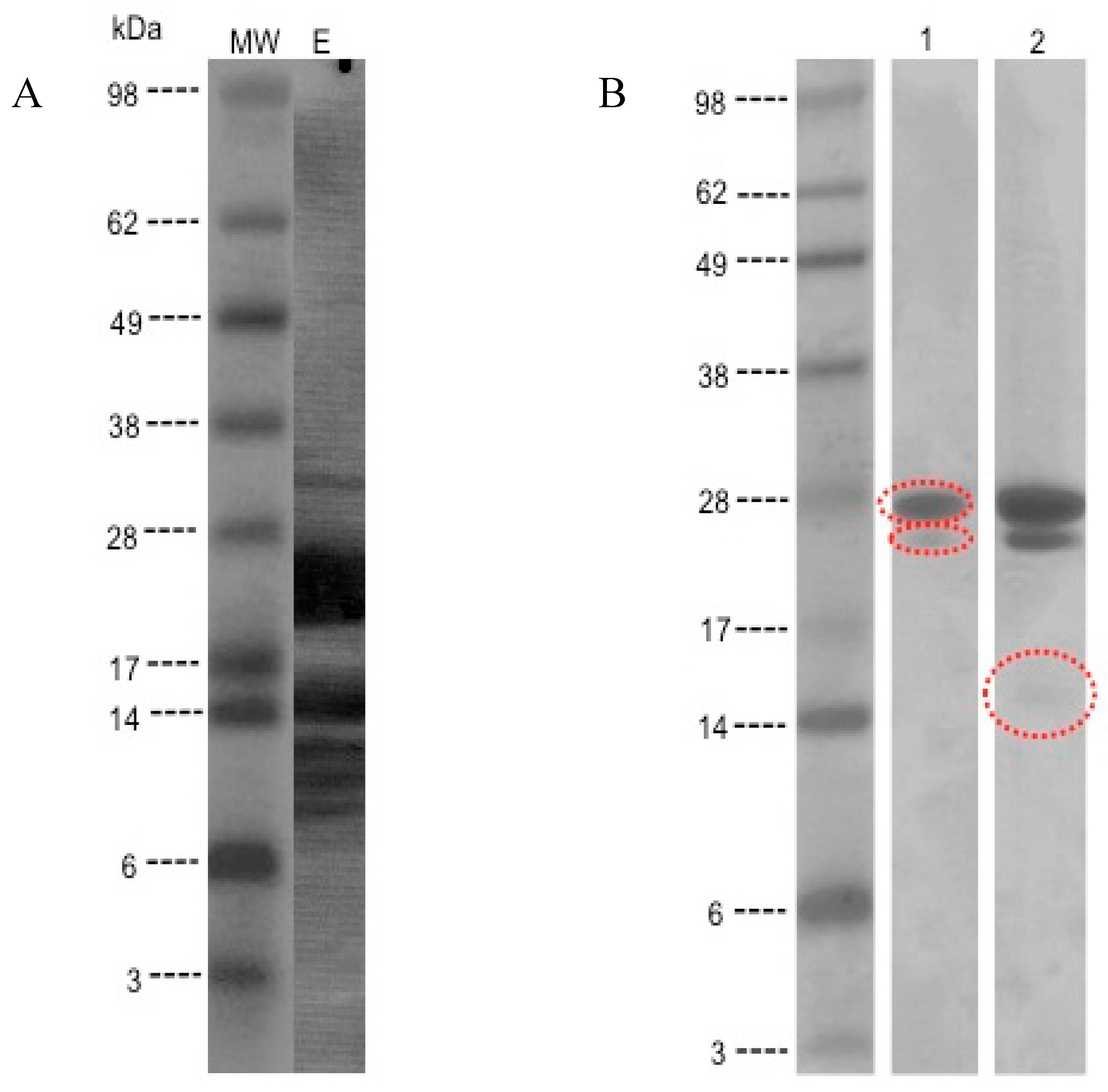

3.4. MS/MS Peptide Sequence Analysis

| Peptide | Position |
|---|---|
| SAGAVVDIK | 135-143 |
| SQECGGCWAFSAIATVEGINK | 144-165 |
| IVTGVLISLSEQELIDCGR | 166-184 |
| YVTIDTYENVPYNNEWALQTAVTYQPVSVALDAAGDAF | 233-271 |
| QYSSGIFTG PCGTAVDHAV TIVGYGTEGG IDYWIVK | 272-307 |
| NSW DTTWGEEGYMR | 308-321 |
| NVGGAGTCGIATMPSYPVK | 325-343 |

| Peptide | Position |
|---|---|
| GATFNIINNCPFTVWAA AVPGGGK | 24-47 |
| GQNWIINPGAGTK | 52-64 |
| GQNWIINPGAGTKGAR | 52-67 |
| APGGCNNPCTVFK | 160-172 |
| TDQYCCNSGNCGLTNFSK | 173-190 |
| DDQTSTFTCPAGTNYK | 205-220 |

| Peptide | Position |
|---|---|
| ISSCNGPCR | 1-9 |
| D LNDCDGQLICIK | 10-22 |
| CNDDPQVGTHICR | 25-37 |
| PSGTLTCR | 50-57 |
| S YPTYDCSPPVTSSTPAK | 60-77 |
| IVALSTGWYNGGSR | 106-119 |
| VVDECDSR | 138-145 |
| EHAGQPPCRN | 151-159 |
| NIVDGSNAVWSALGLDK | 160-177 |
| NVGVVDITWSMA | 178-189 |
3.5. Evaluation of the ISAC
4. Discussion
Acknowledgments
Author Contributions
Conflict of Interest
References
- Nishiyama, I. Fruits of the Actinidia genus. Adv. Food Nutr. Res. 2007, 52, 293–324. [Google Scholar] [PubMed]
- Fine, A.J. Hypersensitivity reaction to kiwi fruit (Chinese gooseberry, Actinidia chinensis). J. Allergy Clin. Immunol. 1981, 68, 235–237. [Google Scholar] [CrossRef]
- Mills, E.N.; Mackie, A.R.; Burney, P.; Beyer, K.; Frewer, L.; Madsen, C.; Botjes, E.; Crevel, R.W.; van Ree, R. The prevalence, cost and basis of food allergy across Europe. Allergy 2007, 62, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Lucas, J.S.; Grimshaw, K.E.; Collins, K.; Warner, J.O.; Hourihane, J.O. Kiwi fruit is a significant allergen and is associated with differing patterns of reactivity in children and adults. Clin. Exp. Allergy 2004, 34, 1115–1121. [Google Scholar] [CrossRef] [PubMed]
- Pastorello, E.; Pravettoni, V.; Ispano, M.; Farioli, L.; Ansaloni, R.; Rotondo, F.; Incorvaia, C.; Asman, I.; Bengtsson, A.; Ortolani, C. Identification of the allergenic components of kiwi fruit and evaluation of their cross- reactivity with timothy and birch pollens. J. Allergy Clin. Immunol. 1996, 98, 601–610. [Google Scholar] [CrossRef]
- Pastorello, E.A.; Conti, A.; Pravettoni, V.; Farioli, L.; Rivolta, F.; Ansaloni, R.; Ispano, M.; Incorvaia, C.; Giuffrida, M.G.; Ortolani, C. Identification of actinidin as the major allergen of kiwi fruit. J. Allergy Clin. Immunol. 1998, 101 4 Pt 1, 531–537. [Google Scholar] [CrossRef]
- Gavrovic-Jankulovic, M.; CIrkovic, T.; Vuckovic, O.; Atanaskovic-Markovic, M.; Petersen, A.; Gojgic, G.; Burazer, L.; Jankov, R.M. Isolation and biochemical characterization of a thaumatin-like kiwi allergen. J. Allergy Clin. Immunol. 2002, 110, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Aleman, A.; Sastre, J.; Quirce, S.; de las Heras, M.; Carnés, J.; Fernández-Caldas, E.; Pastor, C.; Blázquez, A.B.; Vivanco, F.; Cuesta-Herranz, J. Allergy to kiwi: A double-blind, placebo-controlled food challenge study in patients from a birch-free area. J. Allergy Clin. Immunol. 2004, 113, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Palacin, A.; Rodríguez, J.; Blanco, C.; López-Torrejón, G.; Sánchez-Monge, R.; Varela, J.; Jiménez, M.A.; Cumplido, J.; Carrillo, T.; Crespo, J.F.; et al. Immunoglobulin E recognition patterns to purified kiwifruit (Actinidia deliciosa) allergens in patients sensitized to kiwi with different clinical symptoms. Clin. Exp. Allergy 2008, 38, 1220–1228. [Google Scholar] [CrossRef] [PubMed]
- Bublin, M.; Pfister, M.; Radauer, C.; Oberhuber, C.; Bulley, S.; DeWitt, A.M.; Lidholm, J.; Reese, G.; Vieths, S.; Breiteneder, H.; et al. Component-resolved diagnosis of kiwifruit allergy with purified natural and recombinant kiwifruit allergens. J. Allergy Clin. Immunol. 2010, 125, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Tamburrini, M.; Cerasuolo, I.; Carratore, V.; Stanziola, A.A.; Zofra, S.; Romano, L.; Camardella, L.; Ciardiello, M.A. Kiwellin, a novel protein from kiwi fruit. Purification, biochemical characterization and identification as an allergen. Protein J. 2005, 24, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Bublin, M.; Dennstedt, S.; Buchegger, M.; Antonietta Ciardiello, M.; Bernardi, M.L.; Tuppo, L.; Harwanegg, C.; Hafner, C.; Ebner, C.; Ballmer-Weber, B.K.; et al. The performance of a component-based allergen microarray for the diagnosis of kiwifruit allergy. Clin. Exp. Allergy 2011, 41, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Oberhuber, C.; Bulley, S.M.; Ballmer-Weber, B.K.; Bublin, M.; Gaier, S.; DeWitt, A.M.; Briza, P.; Hofstetter, G.; Lidholm, J.; Vieths, S.; et al. Characterization of Bet v1-related allergens from kiwifruit relevant for patients with combined kiwifruit and birch pollen allergy. Mol. Nutr. Food Res. 2008, 52 (Suppl. 2), S230–S240. [Google Scholar] [PubMed]
- D’Avino, R.; Bernardi, M.L.; Wallner, M.; Palazzo, P.; Camardella, L.; Tuppo, L.; Alessandri, C.; Breiteneder, H.; Ferreira, F.; Ciardiello, M.A.; et al. Kiwifruit Act d 11 is the first member of the ripening-related protein family identified as an allergen. Allergy 2011, 66, 870–877. [Google Scholar] [CrossRef] [PubMed]
- Sirvent, S.; Cantó, B.; Gómez, F.; Blanca, N.; Cuesta-Herranz, J.; Canto, G.; Blanca, M.; Rodríguez, R.; Villalva, M.; Palomares, O. Detailed characterization of Act d 12 and Act d 13 from kiwi seeds: Implication in IgE cross-reactivity with peanut and tree nuts. Allergy 2014, 69, 1481–1488. [Google Scholar] [CrossRef] [PubMed]
- Tuano, K.; Davis, C. Utility of Component-Resolved Diagnostics in Food Allergy. Curr. Allergy Asthma Rep. 2015, 15, 32. [Google Scholar] [CrossRef] [PubMed]
- Rance, F.; Grandmottet, X.; Grandjean, H. Prevalence and main characteristics of school children diagnosed with food allergies in France. Clin. Exp. Allergy 2005, 35, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Vieira, T.; Cunha, L.; Neves, E.; Falcao, H. Diagnostic usefulness of component-resolved diagnosis by skin prick tests and specific IgE to single allergen components in children with allergy to fruits and vegetables. Allergol. Immunopathol. (Madr) 2014, 42, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Lucas, J.S.A.; Nieuwenhuizen, N.J.; Atkinson, R.G.; Macrae, E.A.; Cochrane, S.A.; Warner, J.O.; Hourihane, J.O. Kiwifruit allergy: Actinidin is not a major allergen in the United Kingdom. Clin. Exp. Allergy. 2007, 37, 1340–1348. [Google Scholar] [CrossRef] [PubMed]
- Le, T.M.; Bublin, M.; Breiteneder, H.; Fernandez-Rivas, M.; Asero, R.; Ballmer-Weber, B.; Barreales, L.; Bures, P.; Belohlavkova, S.; de Blay, F.; et al. Kiwifruit allergy across Europe: Clinical manifestation and IgE recognition patterns to kiwifruit allergens. J. Allergy Clin. Immunol. 2013, 131, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Tuppo, L.; Giangrieco, I.; Palazzo, P.; Bernardi, M.L.; Scala, E.; Carratore, V.; Tamburrini, M.; Mari, A.; Ciardiello, M.A. Kiwellin, a modular protein from green and gold kiwi fruits: Evidence of in vivo and in vitro processing and IgE binding. J. Agric. Food Chem. 2008, 56, 3812–3817. [Google Scholar] [CrossRef] [PubMed]
- Bublin, M.; Raudauer, C.; Knulst, A.; Wagner, S.; Scheneider, O.; Mackie, A.R.; Mills, E.N.; Breiteneder, H. Effects of gastrointestinal digestion and heating on the allergenicity of the kiwi allergens Act d 1, actinidin, and Act d 2, a thaumatin-like protein. Mol. Nutr. Food Res. 2008, 52, 1130–1139. [Google Scholar] [CrossRef]
- Ciardiello, M.A.; Giangrieco, I.; Tuppo, L.; Tamburrini, M.; Buccheri, M.; Palazzo, P.; Bernardi, M.L.; Ferrara, R.; Mari, A. Influence of the natural ripening stage, cold storage, and ethylene treatment on the protein and IgE-binding profiles of green and gold kiwi fruit extracts. J. Agric. Food Chem. 2009, 57, 1565–1571. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, M.L.; Picone, D.; Tuppo, L.; Giangrieco, I.; Petrella, G.; Palazzo P Ferrara, R.; Tamburrini, M.; Mari, A.; Ciardiello, M.A. Physico-chemical features of the environment affect the protein conformation and the Immunoglobulin E reactivity of kiwellin (Act d 5). Clin. Exp. Allergy 2010, 40, 1819–1826. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno Álvarez, A.; Sexto, L.V.; Bardina, L.; Grishina, G.; Sampson, H.A. Kiwifruit Allergy in Children: Characterization of Main Allergens and Patterns of Recognition. Children 2015, 2, 424-438. https://doi.org/10.3390/children2040424
Moreno Álvarez A, Sexto LV, Bardina L, Grishina G, Sampson HA. Kiwifruit Allergy in Children: Characterization of Main Allergens and Patterns of Recognition. Children. 2015; 2(4):424-438. https://doi.org/10.3390/children2040424
Chicago/Turabian StyleMoreno Álvarez, Ana, Leticia Vila Sexto, Luda Bardina, Galina Grishina, and Hugh. A. Sampson. 2015. "Kiwifruit Allergy in Children: Characterization of Main Allergens and Patterns of Recognition" Children 2, no. 4: 424-438. https://doi.org/10.3390/children2040424






