Macrophage-Targeted Sodium Chlorite (NP001) Slows Progression of Amyotrophic Lateral Sclerosis (ALS) through Regulation of Microbial Translocation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of ALS Phase 2A Trial and Participants
2.2. Analysis of Clinical Outcome Data
2.3. Source of Patient Specimens
2.4. Plasma Factors Evaluated
2.5. Statistical Analyses
3. Results
3.1. Demographics and Clinical Characteristics of ALS Patient Groups in the Study
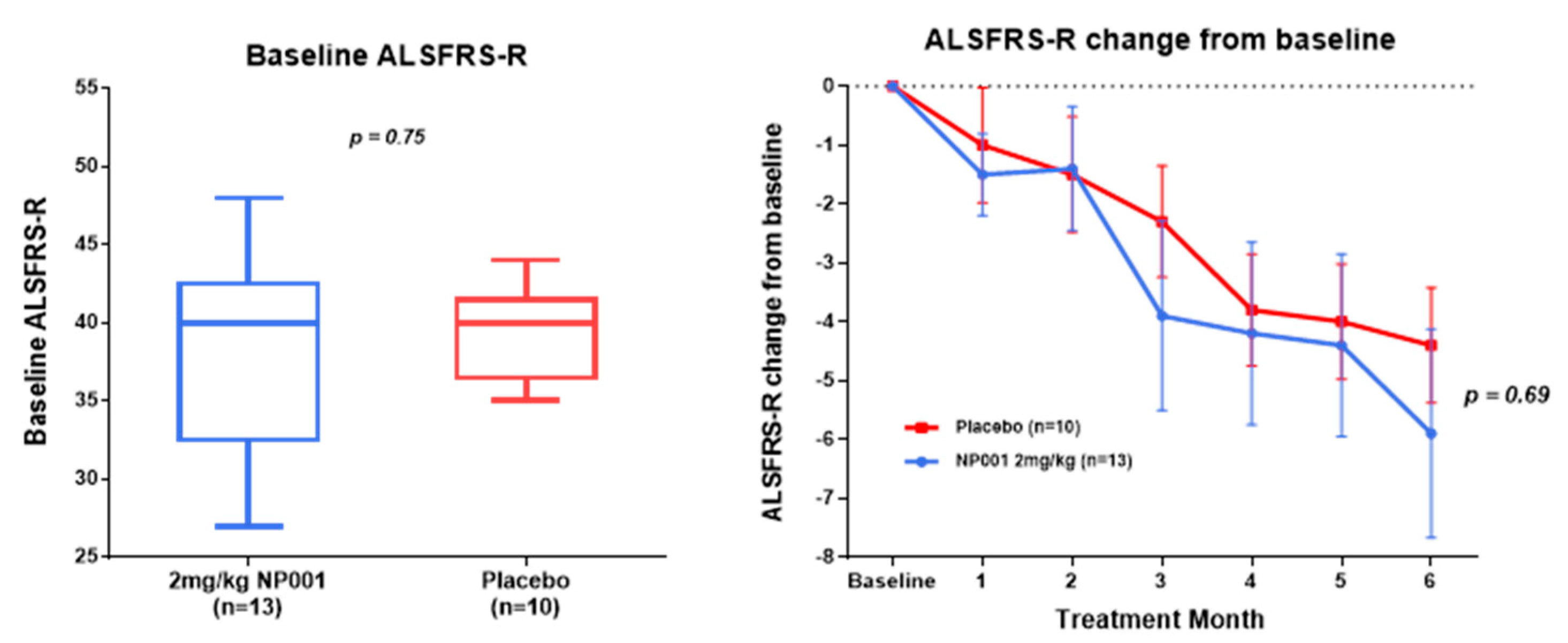
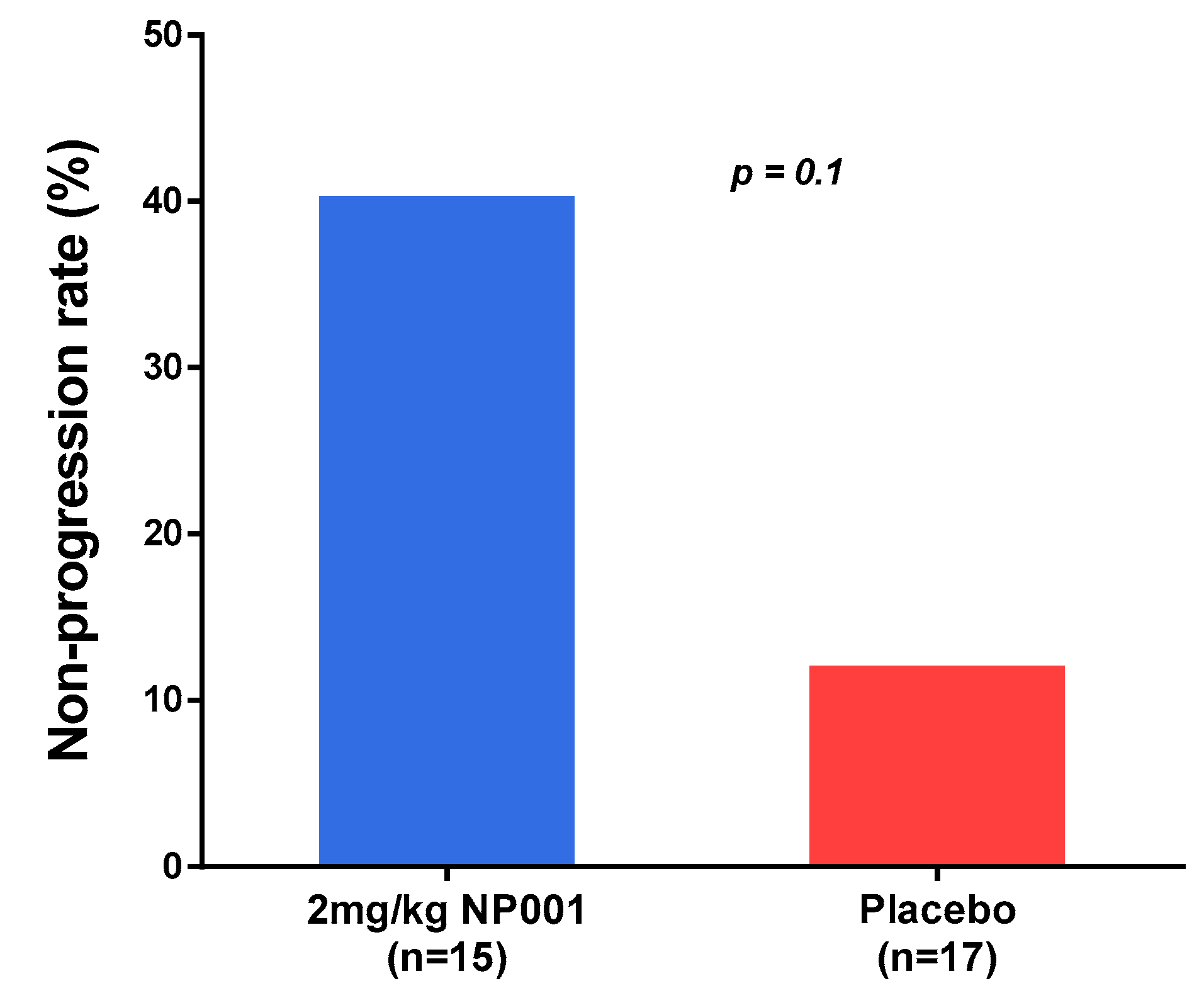
3.2. NP001 Significantly Slows Loss of ALSFRS-R in Treated as Compared to Placebo Patients over 6 Months
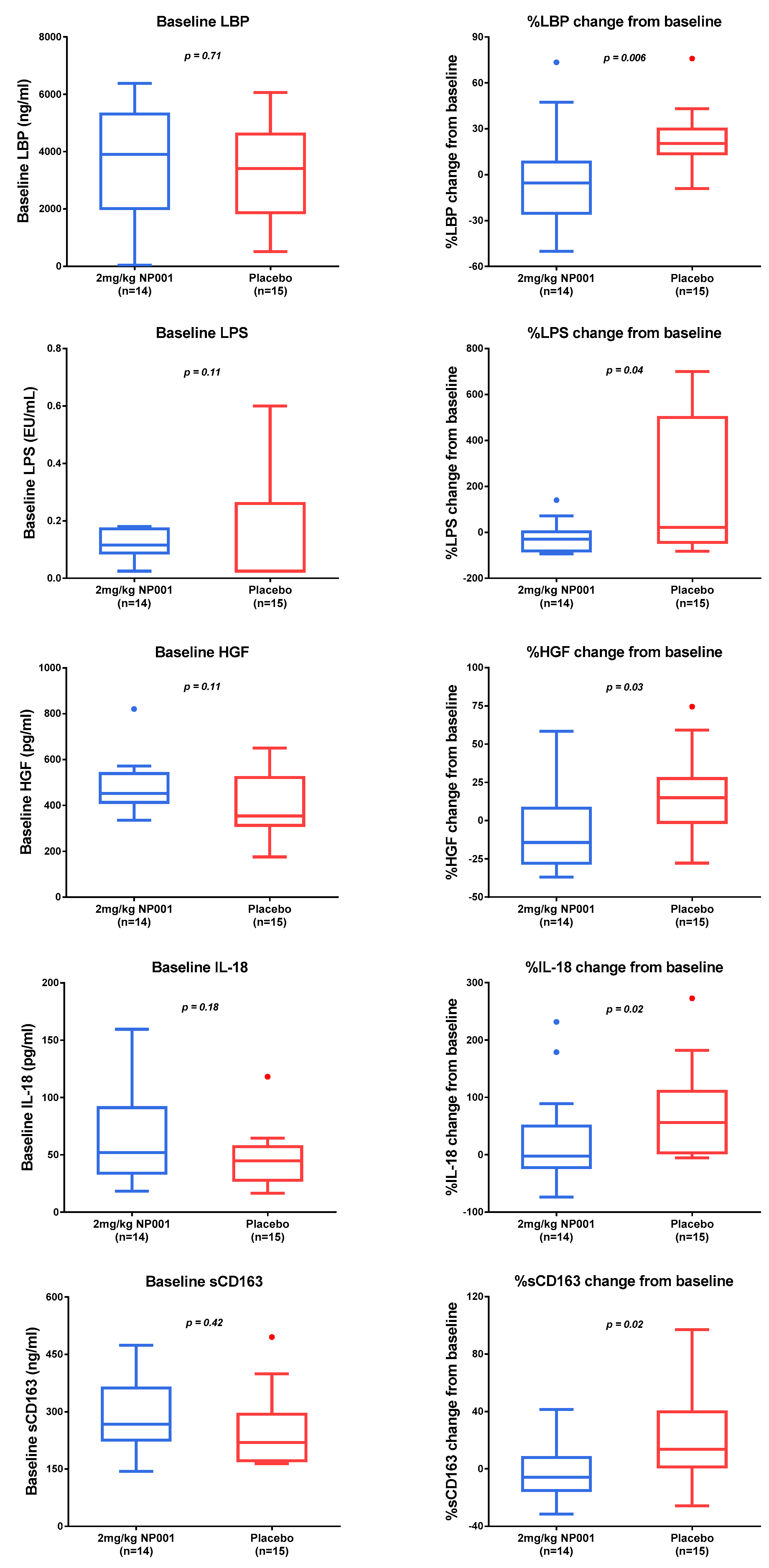
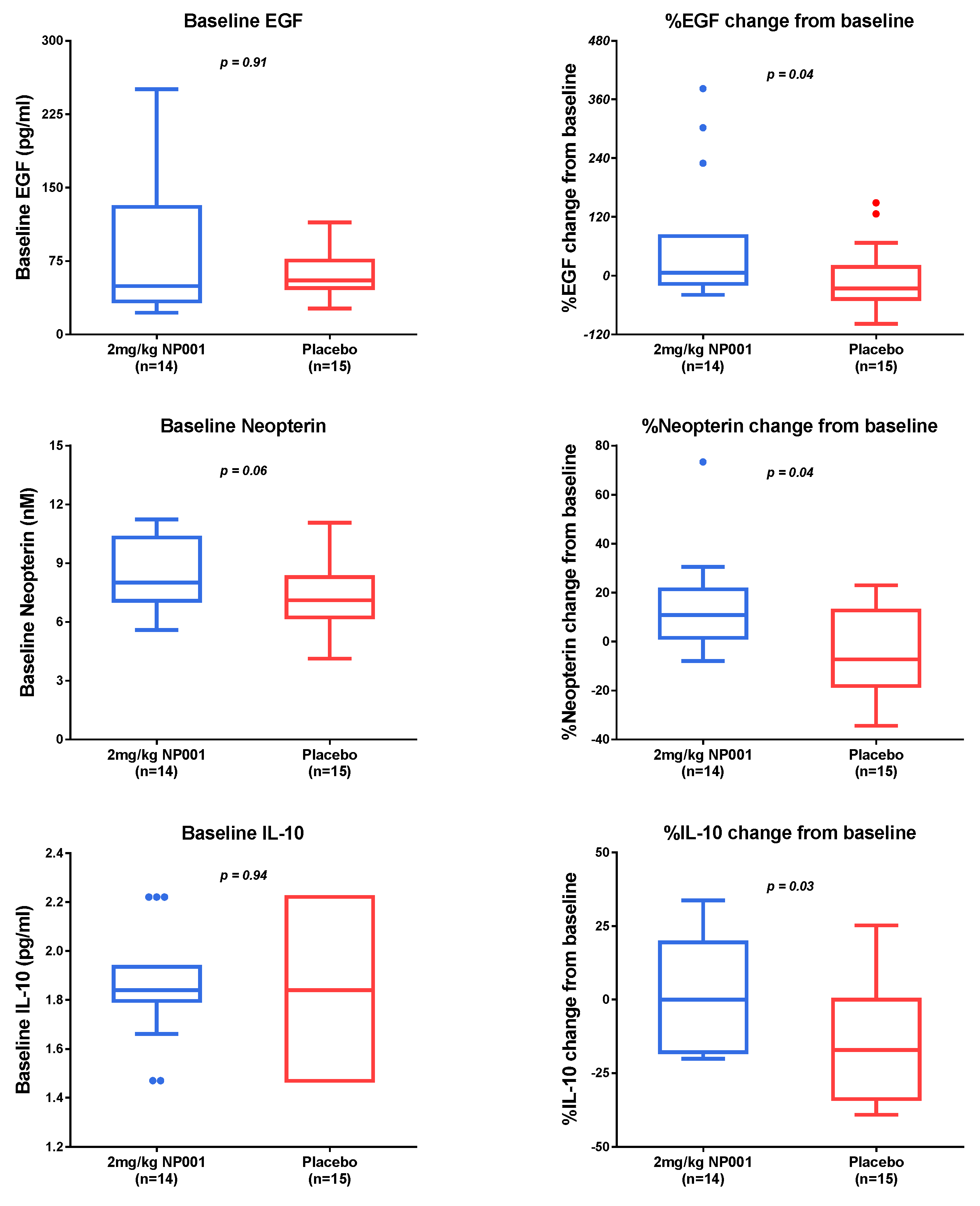
3.3. Plasma Biomarker Analysis
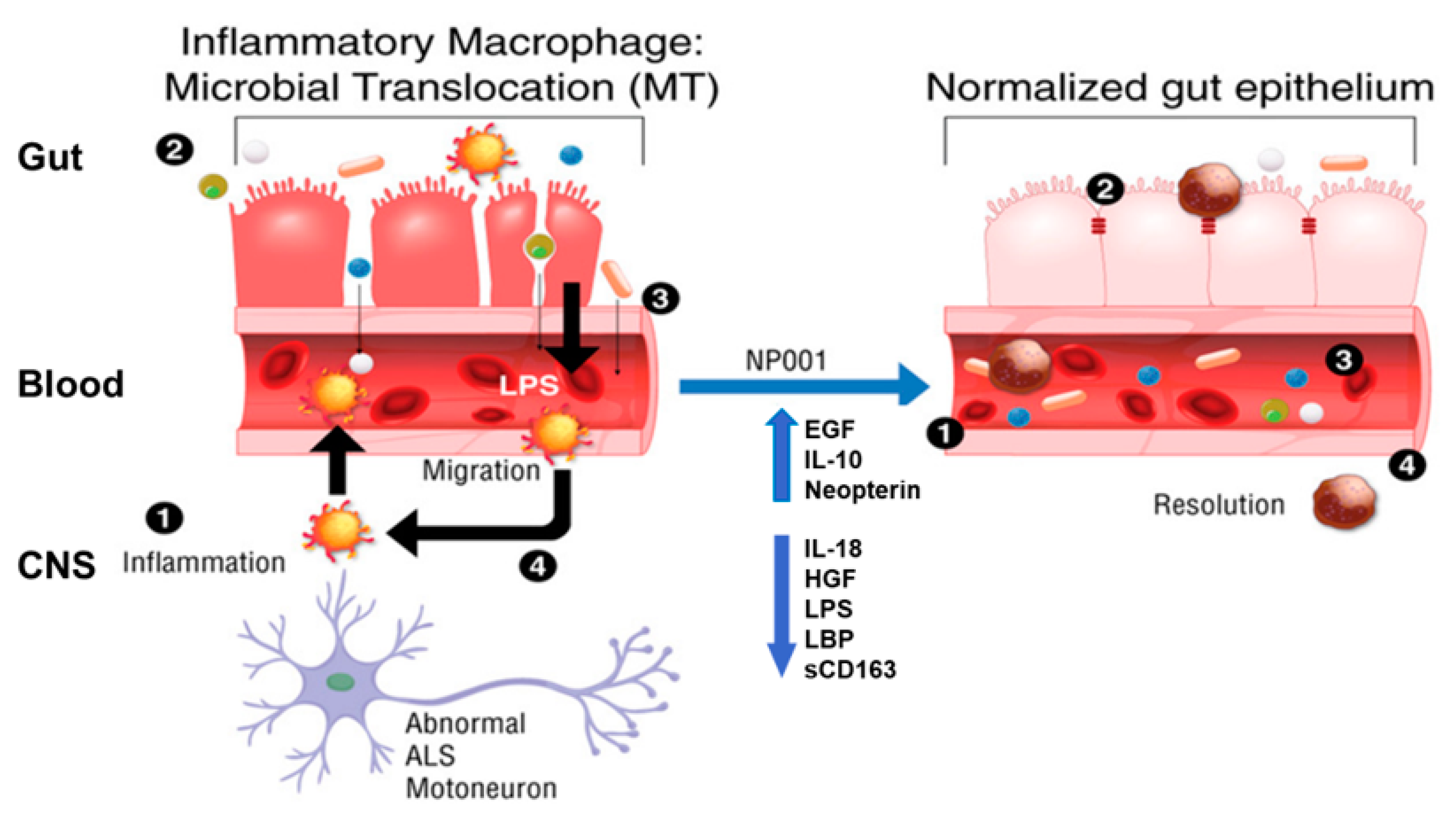
3.4. Microbial Translocation Is a Pathogenic Process in Part Reversed with NP001
- (1)
- ALS progression advances to the point wherein abnormal neurons stimulate local microglia to produce proinflammatory factors which activate blood monocytes to become proinflammatory [22].
- (2)
- Inflammatory macrophages do not phagocytose microorganisms or participate in wound healing. The epithelial cells of the colon turn over every 5 days, making the wound healing function critical for maintaining colonic epithelium integrity. The leakage of bacteria/bacterial products occurs in the blood [23,24].
- (3)
- Bacterial endotoxins (LPS) activate blood monocytes and tissue macrophages, leading to a systemic proinflammatory state [25].
- (4)
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feldman, E.L.; Goutman, S.A.; Petri, S.; Mazzini, L.; Savelieff, M.G.; Shaw, P.J.; Sobue, G. Amyotrophic lateral sclerosis. Lancet 2022, 400, 1363–1380. [Google Scholar] [CrossRef]
- Masrori, P.; Van Damme, P. Amyotrophic lateral sclerosis: A clinical review. Eur. J. Neurol. 2020, 27, 1918–1929. [Google Scholar] [CrossRef] [PubMed]
- Mejzini, R.; Flynn, L.L.; Pitout, I.L.; Fletcher, S.; Wilton, S.D.; Akkari, P.A. ALS Genetics, Mechanisms, and Therapeutics: Where Are We Now? Front. Neurosci. 2019, 13, 1310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- U.S. Department of Health and Human Services, Food and Drug Administration. Amyotrophic Lateral Sclerosis: Developing Drugs for Treatment Guidance for Industry; U.S. Department of Health and Human Services, Ed.; FDA: Silver Spring, MD, USA, 2019. Available online: www.fda.gov (accessed on 24 September 2019).
- Miller, T.M.; Cudkowicz, M.E.; Genge, A.; Shaw, P.J.; Sobue, G.; Bucelli, R.C.; Chiò, A.; Van Damme, P.; Ludolph, A.C.; Glass, J.D.; et al. Trial of Antisense Oligonucleotide Tofersen for SOD1 ALS. N. Engl. J. Med. 2022, 387, 1099–1110. [Google Scholar] [CrossRef]
- Abe, K.; Aoki, M.; Tsuji, S.; Itoyama, Y.; Sobue, G.; Togo, M.; Hamada, C.; Tanaka, M.; Akimoto, M.; Nakamura, K.; et al. Safety and efficacy of edaravone in well defined patients with amyotrophic lateral sclerosis: A randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2017, 16, 505–512. [Google Scholar] [CrossRef]
- Parakh, S.; Atkin, J.D. Protein folding alterations in amyotrophic lateral sclerosis. Brain Res. 2016, 1648 Pt B, 633–649. [Google Scholar] [CrossRef]
- Mehta, A.R.; Walters, R.; Waldron, F.; Pal, S.; Selvaraj, B.T.; MacLeod, M.R.; Hardingham, G.E.; Chandran, S.; Gregory, J.M. Targeting mitochondrial dysfunction in amyotrophic lateral sclerosis: A systematic review and meta-analysis. Brain Commun. 2019, 1, fcz009. [Google Scholar] [CrossRef] [Green Version]
- Gothelf, Y.; Kaspi, H.; Abramov, N.; Aricha, R. miRNA profiling of NurOwn(R): Mesenchymal stem cells secreting neurotrophic factors. Stem Cell Res. 2017, 8, 249. [Google Scholar] [CrossRef] [Green Version]
- Miller, R.G.; Zhang, R.; Bracci, P.M.; Azhir, A.; Barohn, R.; Bedlack, R.; Benatar, M.; Berry, J.D.; Cudkowicz, M.; Kasarskis, E.J.; et al. Phase 2B randomized controlled trial of NP001 in amyotrophic lateral sclerosis: Pre-specified and post hoc analyses. Muscle Nerve 2022, 66, 39–49. [Google Scholar] [CrossRef]
- Chiot, A.; Zaïdi, S.; Iltis, C.; Ribon, M.; Berriat, F.; Schiaffino, L.; Jolly, A.; de la Grange, P.; Mallat, M.; Bohl, D.; et al. Modifying macrophages at the periphery has the capacity to change microglial reactivity and to extend ALS survival. Nat. Neurosci. 2020, 23, 1339–1351. [Google Scholar] [CrossRef]
- Shiraishi, W.; Yamasaki, R.; Hashimoto, Y.; Ko, S.; Kobayakawa, Y.; Isobe, N.; Matsushita, T.; Kira, J.-I. Clearance of peripheral nerve misfolded mutant protein by infiltrated macrophages correlates with motor neuron disease progression. Sci. Rep. 2021, 11, 16438. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Cooper-Knock, J.; Weimer, A.K.; Shi, M.; Moll, T.; Marshall, J.N.; Harvey, C.; Nezhad, H.G.; Franklin, J.; Souza, C.D.S.; et al. Genome-wide identification of the genetic basis of amyotrophic lateral sclerosis. Neuron 2022, 110, 992–1008. [Google Scholar] [CrossRef]
- Giese, T.; McGrath, M.S.; Stumm, S.; Schempp, H.; Elstner, E.; Meuer, S.C. Differential effects on innate versus adaptive immune responses by WF10. Cell. Immunol. 2004, 229, 149–158. [Google Scholar] [CrossRef] [PubMed]
- McGrath, M.S.; Kahn, J.O.; Herndier, B.G. Development of WF10, a novel macrophage-regulating agent. Curr. Opin. Investig. Drugs 2002, 3, 365–373. [Google Scholar] [PubMed]
- Schempp, H.; Reim, M.; Dornisch, K.; Elstner, E.F. Chlorite-Hemoprotein Interaction as Key Role for the Pharmacological Activity of the Chlorite-based Drug WF10. Arzneimittelforschung 2001, 51, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.G.; Zhang, R.; Block, G.; Katz, J.; Barohn, R.; Kasarskis, E.; Forshew, D.; Gopalakrishnan, V.; McGrath, M.S. NP001 regulation of macrophage activation markers in ALS: A phase I clinical and biomarker study. Amyotroph. Lateral Scler. Front. Degener. 2014, 15, 601–609. [Google Scholar] [CrossRef] [Green Version]
- Barua, M.; Liu, Y.; Quinn, M.R. Taurine chloramine inhibits inducible nitric oxide synthase and TNF-alpha gene expression in activated alveolar macrophages: Decreased NF-kappaB activation and IkappaB kinase activity. J. Immunol. 2001, 167, 2275–2281. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.; Cha, Y.-N. Taurine chloramine produced from taurine under inflammation provides anti-inflammatory and cytoprotective effects. Amino Acids 2014, 46, 89–100. [Google Scholar] [CrossRef]
- Miller, R.G.; Block, G.; Katz, J.S.; Barohn, R.J.; Gopalakrishnan, V.; Cudkowicz, M.; Zhang, J.R.; McGrath, M.S.; Ludington, E.; Appel, S.H.; et al. Randomized phase 2 trial of NP001-a novel immune regulator: Safety and early efficacy in ALS. Neurol Neuroimmunol. Neuroinflamm. 2015, 2, e100. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Miller, R.G.; Gascon, R.; Champion, S.; Katz, J.; Lancero, M.; Narvaez, A.; Honrada, R.; Ruvalcaba, D.; McGrath, M.S. Circulating endotoxin and systemic immune activation in sporadic amyotrophic lateral sclerosis (sALS). J. Neuroimmunol. 2009, 206, 121–124. [Google Scholar] [CrossRef]
- Jin, X.; Liu, M.Y.; Zhang, D.F.; Zhong, X.; Du, K.; Qian, P.; Gao, H.; Wei, M.J. Natural products as a potential modulator of microglial polarization in neurodegenerative diseases. Pharmacol. Res. 2019, 145, 104253. [Google Scholar] [CrossRef] [PubMed]
- Natale, G.; Biagioni, F.; Busceti, C.L.; Gambardella, S.; Limanaqi, F.; Fornai, F. TREM Receptors Connecting Bowel Inflammation to Neurodegenerative Disorders. Cells 2019, 8, 1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sender, R.; Milo, R. The distribution of cellular turnover in the human body. Nat. Med. 2021, 27, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Hadlock, K.G.; Do, H.; Yu, S.; Honrada, R.; Champion, S.; Forshew, D.; Madison, C.; Katz, J.; Miller, R.G.; et al. Gene expression profiling in peripheral blood mononuclear cells from patients with sporadic amyotrophic lateral sclerosis (sALS). J. Neuroimmunol. 2011, 230, 114–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knoop, K.A.; Coughlin, P.E.; Floyd, A.N.; Ndao, I.M.; Hall-Moore, C.; Shaikh, N.; Gasparrini, A.J.; Rusconi, B.; Escobedo, M.; Good, M.; et al. Maternal activation of the EGFR prevents translocation of gut-residing pathogenic Escherichia coli in a model of late-onset neonatal sepsis. Proc. Natl. Acad. Sci. USA 2020, 117, 7941–7949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, N.; Wang, L.; Cao, H.; Liu, L.; Van Kaer, L.; Washington, M.K.; Rosen, M.J.; Dube, P.; Wilson, K.; Ren, X.; et al. Activation of the Epidermal Growth Factor Receptor in Macrophages Regulates Cytokine Production and Experimental Colitis. J. Immunol. 2014, 192, 1013–1023. [Google Scholar] [CrossRef] [Green Version]
- Kim, W.; Kim, S.H.; Jang, J.-H.; Kim, C.; Kim, K.; Suh, Y.-G.; Joe, Y.; Chung, H.T.; Cha, Y.-N.; Surh, Y.-J. Role of heme oxygenase-1 in potentiation of phagocytic activity of macrophages by taurine chloramine: Implications for the resolution of zymosan A-induced murine peritonitis. Cell. Immunol. 2018, 327, 36–46. [Google Scholar] [CrossRef]
- Burdo, T.H.; Soulas, C.; Orzechowski, K.; Button, J.; Krishnan, A.; Sugimoto, C.; Alvarez, X.; Kuroda, M.J.; Williams, K.C. Increased Monocyte Turnover from Bone Marrow Correlates with Severity of SIV Encephalitis and CD163 Levels in Plasma. PLoS Pathog. 2010, 6, e1000842. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Miller, R.G.; Madison, C.; Jin, X.; Honrada, R.; Harris, W.; Katz, J.; Forshew, D.A.; McGrath, M.S. Systemic immune system alterations in early stages of Alzheimer’s disease. J. Neuroimmunol. 2013, 256, 38–42. [Google Scholar] [CrossRef] [Green Version]
- Kelesidis, T.; Kendall, M.A.; Yang, O.O.; Hodis, H.N.; Currier, J.S. Biomarkers of Microbial Translocation and Macrophage Activation: Association With Progression of Subclinical Atherosclerosis in HIV-1 Infection. J. Infect. Dis. 2012, 206, 1558–1567. [Google Scholar] [CrossRef]
- Younas, M.; Psomas, C.; Reynes, C.; Cezar, R.; Kundura, L.; Portales, P.; Merle, C.; Atoui, N.; Fernandez, C.; Le Moing, V.; et al. Microbial Translocation Is Linked to a Specific Immune Activation Profile in HIV-1-Infected Adults With Suppressed Viremia. Front. Immunol. 2019, 10, 2185. [Google Scholar] [CrossRef] [PubMed]
- Bossù, P.; Cutuli, D.; Palladino, I.; Caporali, P.; Angelucci, F.; Laricchiuta, D.; Gelfo, F.; De Bartolo, P.; Caltagirone, C.; Petrosini, L. A single intraperitoneal injection of endotoxin in rats induces long-lasting modifications in behavior and brain protein levels of TNF-α and IL-18. J. Neuroinflammation 2012, 9, 101. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Lee, J.; Lee, J.; Lee, S.H.; Kim, S. Hepatocyte Growth Factor Regulates Macrophage Transition to the M2 Phenotype and Promotes Murine Skeletal Muscle Regeneration. Front. Physiol. 2019, 10, 914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Paula Martins, R.; Ghisoni, K.; Lim, C.K.; Aguiar, A.S.; Guillemin, G.J.; Latini, A. Neopterin preconditioning prevents inflammasome activation in mammalian astrocytes. Free Radic. Biol. Med. 2018, 115, 371–382. [Google Scholar] [CrossRef]
- Kim, H.S.; Son, J.; Lee, D.; Tsai, J.; Wang, D.; Chocron, E.S.; Jeong, S.; Kittrell, P.; Murchison, C.F.; Kennedy, R.E.; et al. Gut- and oral-dysbiosis differentially impact spinal- and bulbar-onset ALS, predicting ALS severity and potentially determining the location of disease onset. BMC Neurol. 2022, 22, 62. [Google Scholar] [CrossRef]
- Zingaropoli, M.A.; Nijhawan, P.; Carraro, A.; Pasculli, P.; Zuccalà, P.; Perri, V.; Marocco, R.; Kertusha, B.; Siccardi, G.; Del Borgo, C.; et al. Increased sCD163 and sCD14 Plasmatic Levels and Depletion of Peripheral Blood Pro-Inflammatory Monocytes, Myeloid and Plasmacytoid Dendritic Cells in Patients With Severe COVID-19 Pneumonia. Front. Immunol. 2021, 12, 627548. [Google Scholar] [CrossRef]
- Chen-Plotkin, A.S.; Hu, W.; Siderowf, A.; Weintraub, D.; Gross, R.G.; Hurtig, H.I.; Xie, S.X.; Arnold, S.E.; Grossman, M.; Clark, C.M.; et al. Plasma epidermal growth factor levels predict cognitive decline in Parkinson disease. Ann. Neurol. 2011, 69, 655–663. [Google Scholar] [CrossRef]
- Lim, N.S.; Swanson, C.R.; Cherng, H.R.; Unger, T.L.; Xie, S.X.; Weintraub, D.; Marek, K.; Stern, M.B.; Siderowf, A. Plasma EGF and cognitive decline in Parkinson’s disease and Alzheimer’s disease. Ann. Clin. Transl. Neurol. 2016, 3, 346–355. [Google Scholar] [CrossRef] [Green Version]
- Simons, J.P.; Loeffler, J.M.; Al-Shawi, R.; Ellmerich, S.; Hutchinson, W.L.; Tennent, G.A.; Petrie, A.; Raynes, J.G.; de Souza, J.B.; Lawrence, R.A.; et al. C-reactive protein is essential for innate resistance to pneumococcal infection. Immunology 2014, 142, 414–420. [Google Scholar] [CrossRef] [Green Version]
- Jones, N.R.; Pegues, M.A.; McCrory, M.A.; Kerr, S.W.; Jiang, H.; Sellati, R.; Berger, V.; Villalona, J.; Parikh, R.; McFarland, M.; et al. Collagen-induced arthritis is exacerbated in C-reactive protein-deficient mice. Arthritis Rheum. 2011, 63, 2641–2650. [Google Scholar] [CrossRef]
- Li, H.-Y.; Tang, Z.-M.; Wang, Z.; Lv, J.-M.; Liu, X.-L.; Liang, Y.-L.; Cheng, B.; Gao, N.; Ji, S.-R.; Wu, Y. C-Reactive Protein Protects Against Acetaminophen-Induced Liver Injury by Preventing Complement Overactivation. Cell. Mol. Gastroenterol. Hepatol. 2022, 13, 289–307. [Google Scholar] [CrossRef] [PubMed]
- Teupser, D.; Weber, O.; Rao, T.N.; Sass, K.; Thiery, J.; Fehling, H.J. No Reduction of Atherosclerosis in C-reactive Protein (CRP)-deficient Mice. J. Biol. Chem. 2011, 286, 6272–6279. [Google Scholar] [CrossRef] [PubMed]

| CRP > 1.13 mg/L | CRP < 1.13 mg/L | Overall | |
|---|---|---|---|
| Characteristics | (n = 29) | (n = 23) | (n = 52) |
| Sex, n (%) | |||
| Female | 9 (31.0%) | 9 (39.1%) | 18 (34.6%) |
| Male | 20 (69.0%) | 14 (60.9%) | 34 (65.4%) |
| Age at baseline, year | 52.7 ± 6.7 | 54.7 ± 7.3 | 53.5 ± 7.0 |
| Type of ALS, n (%) | |||
| Familial | 2 (6.9%) | 3 (13.0%) | 5 (9.6%) |
| Sporadic | 27 (93.1%) | 20 (87.0.0%) | 47 (90.4%) |
| Site of ALS onset, n (%) | |||
| Bulbar | 6 (20.7%) | 4 (17.4%) | 10 (19.2%) |
| Limb | 23 (79.3%) | 19 (82.6%) | 42 (80.8%) |
| El Escorial criteria for ALS, n (%) | |||
| Definite | 13 (44.8%) | 11 (47.8%) | 24 (46.2%) |
| Probable | 13 (44.8%) | 12 (52.2%) | 25 (48.1%) |
| Possible | 3 (10.3%) | 0 (0.0%) | 3 (5.8%) |
| Concurrent riluzole use, n (%) | |||
| Yes | 20 (69.0%) | 15 (65.2%) | 35 (67.3%) |
| No | 9 (31.0%) | 8 (34.8%) | 17 (32.7%) |
| ALSFRS-R score at baseline 1, mean ± SD | 38.1 ± 4.1 | 38.5 ± 5.1 | 38.3 ± 4.6 |
| Vital capacity at baseline, mean ± SD | 112.4 ± 15.6 | 108.8 ± 17.3 | 110.8 ± 16.3 |
| Months since ALS symptom onset 2, mean ± SD | 17.19 ± 7.80 | 15.73 ± 7.37 | 16.54 ± 7.57 |
| CRP at baseline (mg/L) 3, mean ± SD | 3.29 ± 2.42 | 0.71 ± 0.26 | 2.15 ± 2.22 |
| NP001 2 mg/kg | Placebo | Overall | |
|---|---|---|---|
| Characteristics | (n = 14) | (n = 15) | (n = 29) |
| Sex, n (%) | |||
| Female | 4 (28.6%) | 5 (33.3%) | 9 (31.0%) |
| Male | 10 (71.4%) | 10 (66.7%) | 20 (69.0%) |
| Age at baseline, year | 53.3 ± 8.1 | 52.1 ± 5.2 | 52.7 ± 6.7 |
| Type of ALS, n (%) | |||
| Familial | 0 (0.0%) | 2 (13.3%) | 2 (6.9%) |
| Sporadic | 14 (100.0%) | 13 (86.7%) | 27 (93.1%) |
| Site of ALS onset, n (%) | |||
| Bulbar | 3 (21.4%) | 3 (20.0%) | 6 (20.7%) |
| Limb | 11 (78.6%) | 12 (80.0%) | 23 (79.3%) |
| El Escorial criteria for ALS, n (%) | |||
| Definite | 6 (42.9%) | 7 (46.7%) | 13 (44.8%) |
| Probable | 7 (50.0%) | 6 (40.0%) | 13 (44.8%) |
| Possible | 1 (7.1%) | 2 (13.3%) | 3 (10.3%) |
| Concurrent riluzole use, n (%) | |||
| Yes | 11 (78.6%) | 9 (60.0%) | 20 (69.0%) |
| No | 3 (21.4%) | 6 (40.0%) | 9 (31.0%) |
| ALSFRS-R score at baseline 1, mean ± SD | 37.3 ± 4.2 | 38.8 ± 4.1 | 38.1 ± 4.1 |
| Vital capacity at baseline, mean ± SD | 116.0 ± 15.7 | 109.0 ± 15.3 | 112.4 ± 15.6 |
| Months since ALS symptom onset 2, mean ± SD | 20.11 ± 7.79 | 14.46 ± 6.98 | 17.19 ± 7.80 |
| CRP at baseline (mg/L) 3, mean ± SD | 3.80 ± 2.60 | 2.82 ± 2.23 | 3.29 ± 2.42 |
| NP001 2 mg/kg | Placebo | Overall | |
|---|---|---|---|
| Characteristics | (n = 13) | (n = 10) | (n = 23) |
| Sex, n (%) | |||
| Female | 5 (38.5%) | 4 (40.0%) | 9 (39.1%) |
| Male | 8 (61.5%) | 6 (60.0%) | 14 (60.9%) |
| Age at baseline, year | 52.5 ± 7.9 | 57.4 ± 5.6 | 54.7 ± 7.3 |
| Type of ALS, n (%) | |||
| Familial | 1 (7.7%) | 2 (20.0%) | 3 (13.0%) |
| Sporadic | 12 (92.3%) | 8 (80.0%) | 20 (87.0%) |
| Site of ALS onset, n (%) | |||
| Bulbar | 2 (15.4%) | 2 (20.0%) | 4 (17.4%) |
| Limb | 11 (84.6%) | 8 (80.0%) | 19 (82.6%) |
| El Escorial criteria for ALS, n (%) | |||
| Definite | 7 (53.8%) | 4 (40.0%) | 11 (47.8%) |
| Probable | 6 (46.2%) | 6 (60.0%) | 12 (52.2%) |
| Concurrent riluzole use, n (%) | |||
| Yes | 9 (69.2%) | 6 (60.0%) | 15 (65.2%) |
| No | 4 (30.8%) | 4 (40.0%) | 8 (34.8%) |
| ALSFRS-R score at baseline 1, mean ± SD | 37.8 ± 6.3 | 39.3 ± 3.1 | 38.5 ± 5.1 |
| Vital capacity at baseline, mean ± SD | 110.0 ± 16.3 | 107.3 ± 19.4 | 108.8 ± 17.3 |
| Months since ALS symptom onset 2, mean ± SD | 14.08 ± 7.24 | 17.88 ± 7.34 | 15.73 ± 7.37 |
| CRP at baseline (mg/L) 3, mean ± SD | 0.70 ± 0.25 | 0.71 ± 0.28 | 0.71 ± 0.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Bracci, P.M.; Azhir, A.; Forrest, B.D.; McGrath, M.S. Macrophage-Targeted Sodium Chlorite (NP001) Slows Progression of Amyotrophic Lateral Sclerosis (ALS) through Regulation of Microbial Translocation. Biomedicines 2022, 10, 2907. https://doi.org/10.3390/biomedicines10112907
Zhang R, Bracci PM, Azhir A, Forrest BD, McGrath MS. Macrophage-Targeted Sodium Chlorite (NP001) Slows Progression of Amyotrophic Lateral Sclerosis (ALS) through Regulation of Microbial Translocation. Biomedicines. 2022; 10(11):2907. https://doi.org/10.3390/biomedicines10112907
Chicago/Turabian StyleZhang, Rongzhen, Paige M. Bracci, Ari Azhir, Bruce D. Forrest, and Michael S. McGrath. 2022. "Macrophage-Targeted Sodium Chlorite (NP001) Slows Progression of Amyotrophic Lateral Sclerosis (ALS) through Regulation of Microbial Translocation" Biomedicines 10, no. 11: 2907. https://doi.org/10.3390/biomedicines10112907






