Electrical and Humidity-Sensing Properties of EuCl2, Eu2O3 and EuCl2/Eu2O3 Blend Films
Abstract
:1. Introduction
2. Experimental Methods
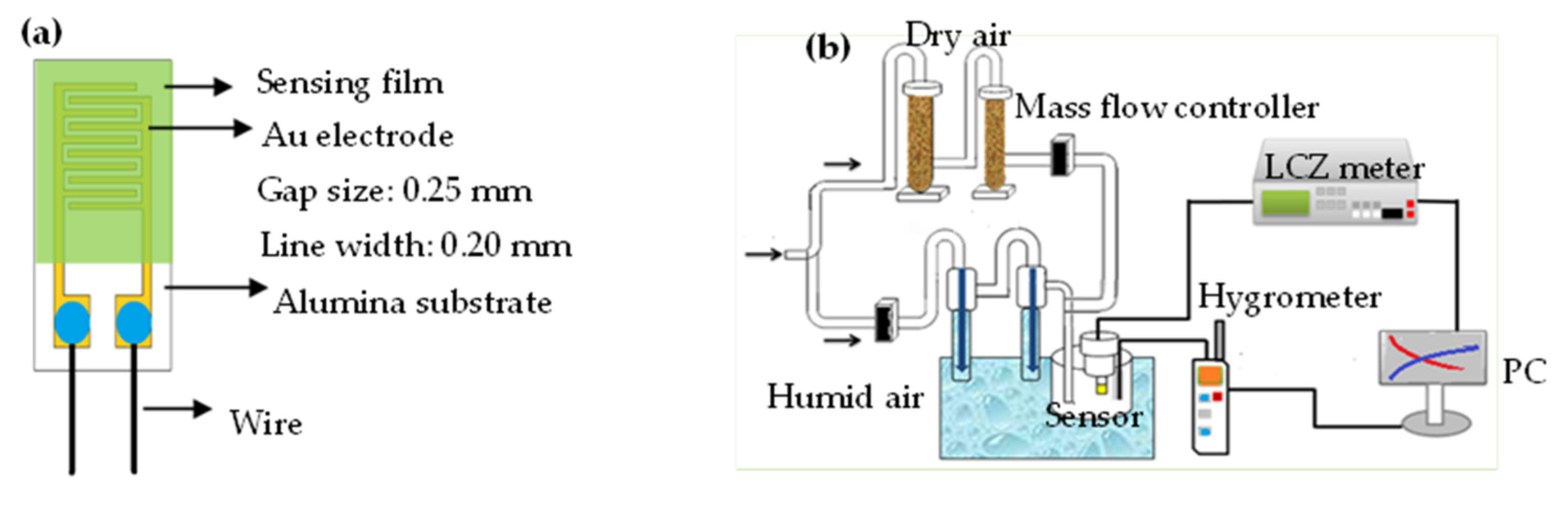
2.1. Materials and Humidity Sensors Preparation
2.2. Characterization of EuCl2, Eu2O3 and EuCl2/Eu2O3 Bend Films
2.3. Measurement of Electrical and Humidity-Sensing Properties
3. Results and Discussion
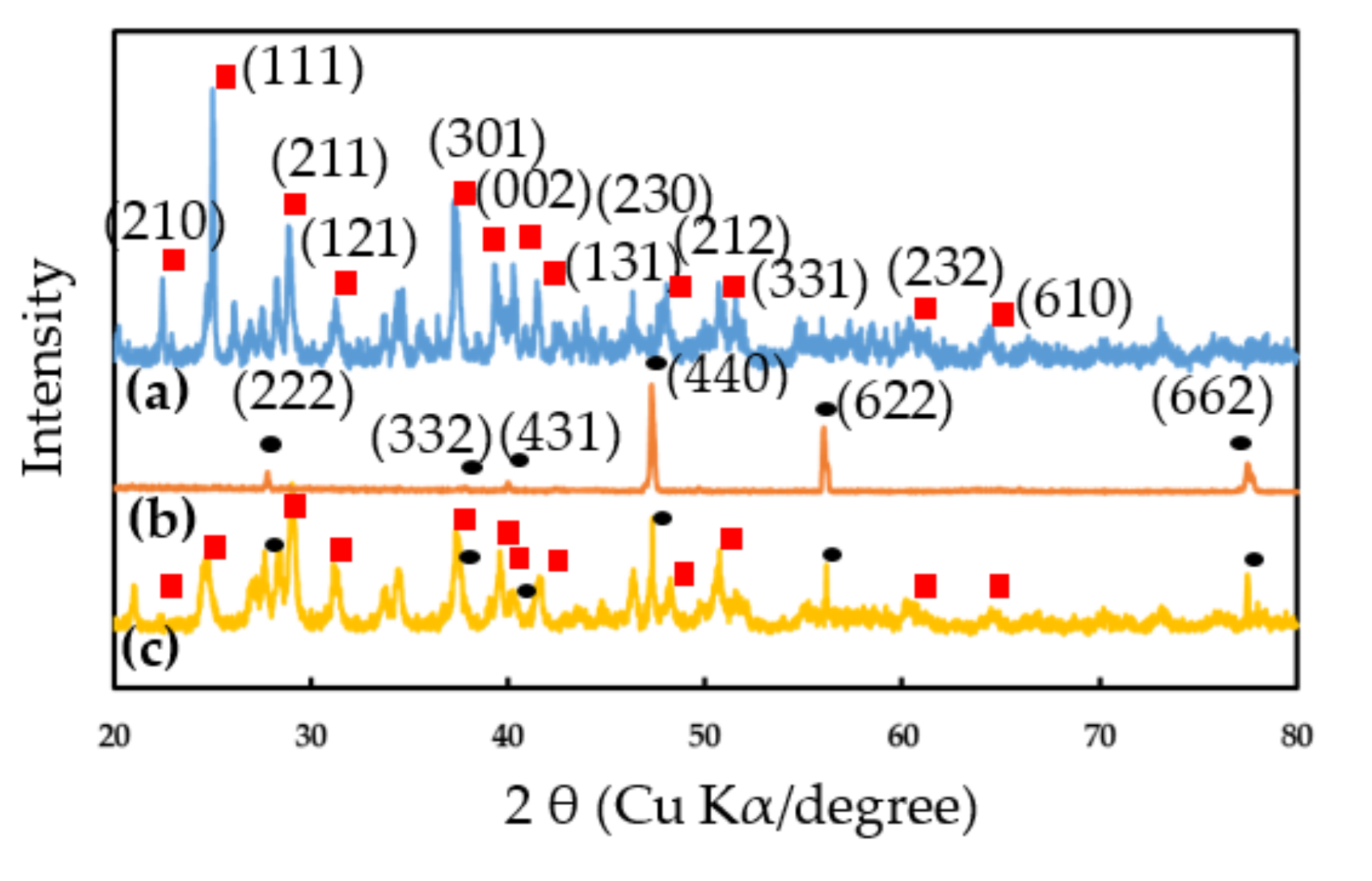
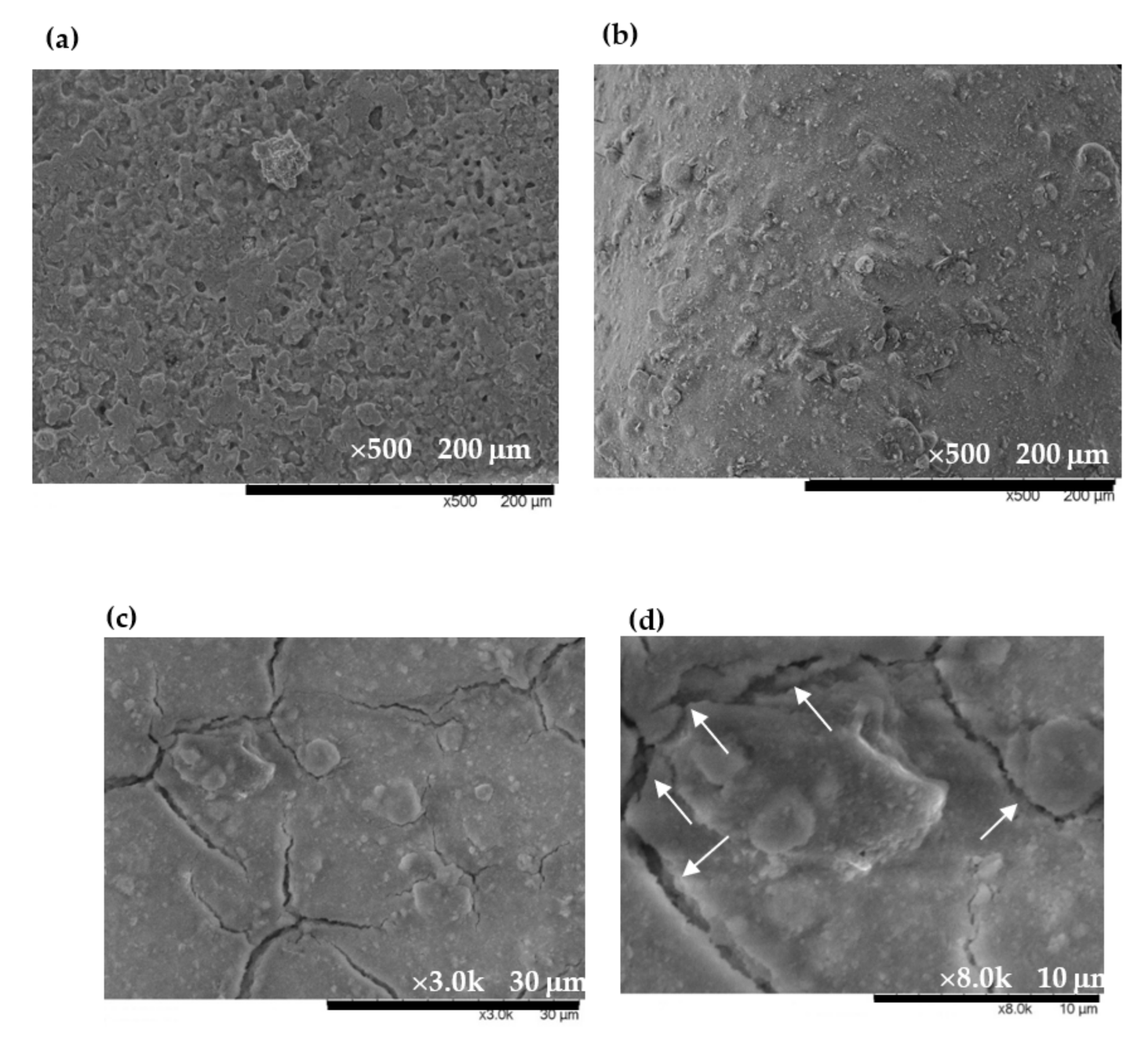
3.1. Characteristics of EuCl2, Eu2O3 and EuCl2/Eu2O3 Blend Films
XRD Characterization and Morphology Observations
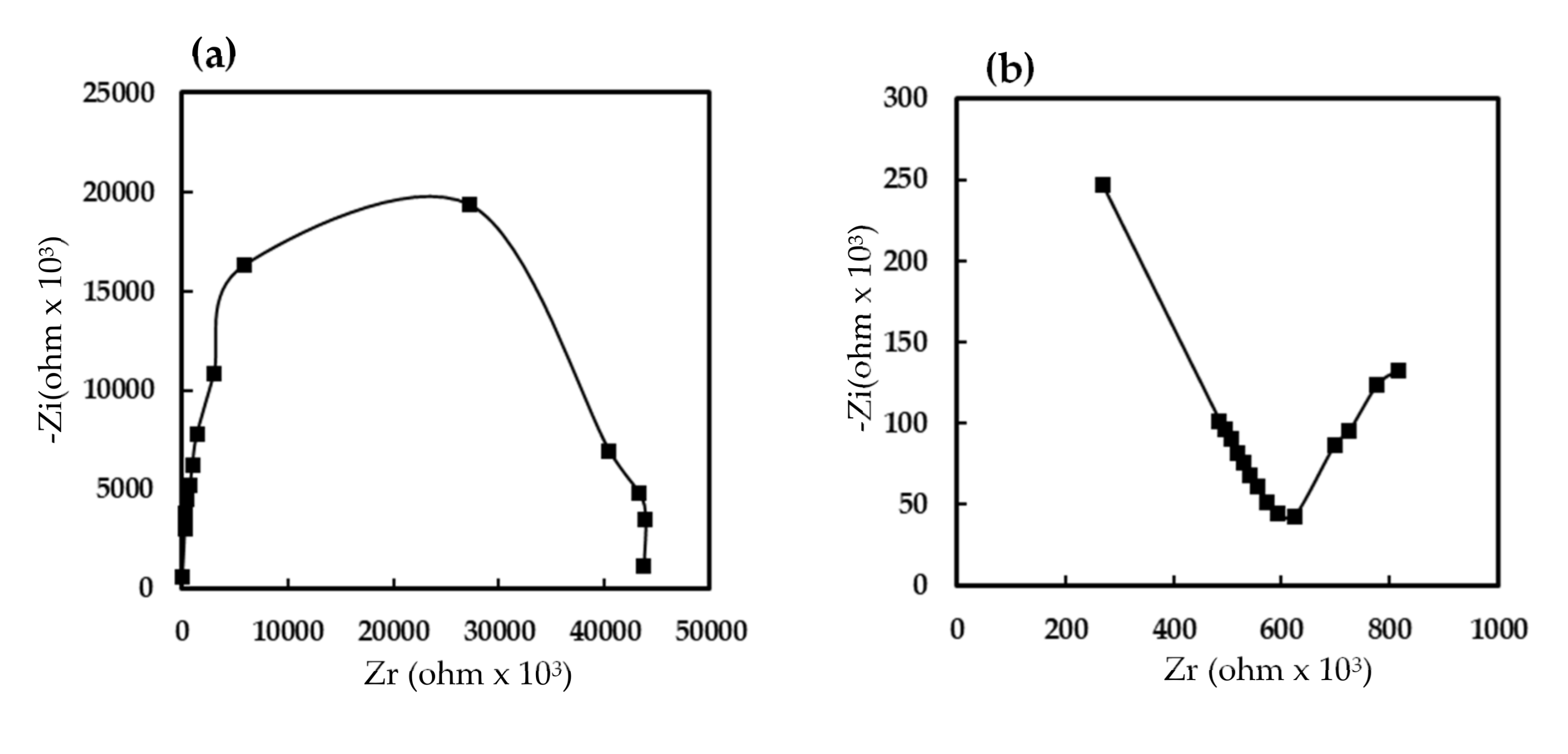
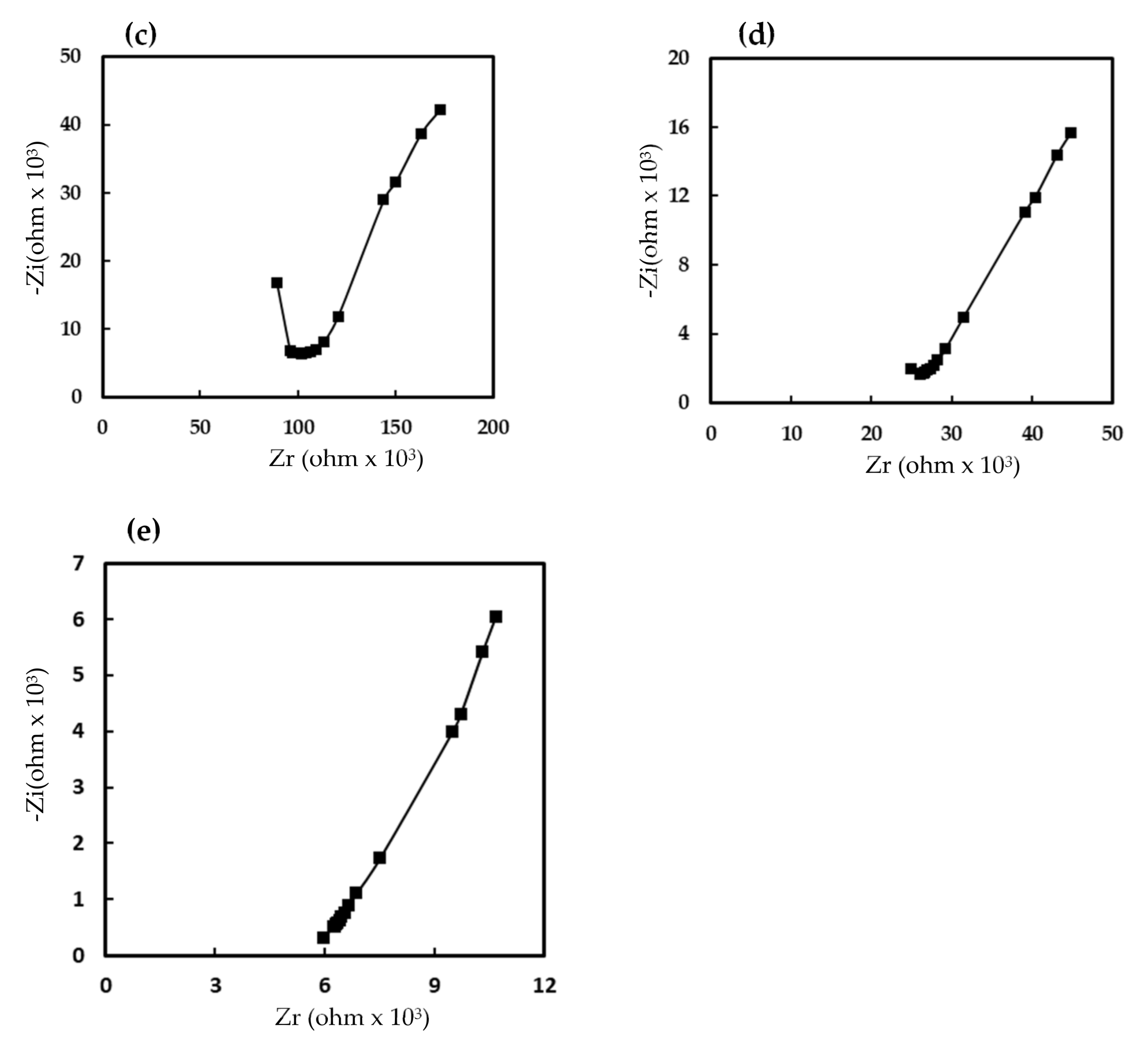
3.2. Electrical and Humidity-Sensing Properties of Humidity Sensors Based on EuCl2, Eu2O3 and EuCl2/Eu2O3 Blend Films
3.3. Humidity-Sensing Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sakai, Y.; Sadaoka, Y.; Matsuguchi, M. Humidity sensors based on polymer thin films. Sens. Actuators B 1996, 35–36, 85–90. [Google Scholar] [CrossRef]
- Luo, Y.; Kun, Y.; Shi, Y.; Shang, C. Research of radiosonde humidity sensor with temperature compensation function and experimental verification. Sens. Actuators A 2014, 218, 49–59. [Google Scholar] [CrossRef]
- Lee, S.W.; Choi, B.I.; Kim, J.C.; Woo, S.B.; Kim, Y.G.; Yoo, J.; Seo, Y.S. Reduction and compensation of humidity measurement errors at cold temperatures using dual QCM humidity sensors based on graphene oxides. Sens. Actuators B 2019, 284, 386–394. [Google Scholar] [CrossRef]
- Farahani, H.; Wagiran, R.; Hamidon, M.N. Humidity sensors principle, mechanism, and fabrication technologies: A comprehensive review. Sensors 2014, 14, 7881–7939. [Google Scholar] [CrossRef] [Green Version]
- Najeeb, M.A.; Ahmad, Z.; Shakoor, R.A. Organic thin-film capacitive and resistive humidity sensors: A focus review. Adv. Mater. Interfaces 2018, 5, 1–19. [Google Scholar] [CrossRef]
- Blank, T.A.; Eksperiandova, L.P.; Belikov, K.N. Recent trends of ceramic humidity sensors development: A review. Sens. Actuators B 2016, 228, 416–442. [Google Scholar] [CrossRef]
- Zhang, D.; Tong, J.; Xia, B.; Xue, Q. Ultrahigh performance humidity sensor based on layer-by-layer self-assembly of graphene oxide/polyelectrolyte nanocomposite film. Sens. Actuators B 2014, 203, 263–270. [Google Scholar] [CrossRef]
- Sakai, Y.; Matsuguchi, M.; Hurukawa, T. Humidity sensor using crosslinked poly(chloromethyl styrene). Sens. Actuators B 2000, 66, 135–138. [Google Scholar] [CrossRef]
- Zhang, D.; Sun, Y.; Li, P.; Zhang, Y. Facile fabrication of MoS2-modified SnO2 hybrid nanocomposite for ultrasensitive humidity sensing. ACS Appl. Mater. Interfaces 2016, 8, 14142–14149. [Google Scholar] [CrossRef]
- Taccola, S.; Greco, F.; Zucca, A.; Innocenti, C.; de Julián Fernández, C.; Campo, G.; Sangregorio, C.; Mazzolai, B.; Mattoli, V. Characterization of free-standing PEDOT: PSS/iron oxide nanoparticle composite thin films and application as conformable humidity sensors. ACS Appl. Mater. Interfaces 2013, 5, 6324–6332. [Google Scholar] [CrossRef]
- Zhang, D.; Tong, J.; Xia, B. Humidity-sensing properties of chemically reduced graphene oxide/polymer nanocomposite film sensor based on layer-by-layer nano self-assembly. Sens. Actuators B 2014, 197, 66–72. [Google Scholar] [CrossRef]
- Ziegler, D.; Boschetto, F.; Marin, E.; Palmero, P.; Pezzotti, G.; Tulliani, J.M. Rice husk ash as a new humidity sensing material and its aging behavior. Sens. Actuators B 2021, 328, 129049. [Google Scholar] [CrossRef]
- Chen, Q.; Yao, Y.; Huang, X.H.; Liu, D.; Mao, K.l. Simulation analysis and experimental verification for sensitivity of IDE-QCM humidity sensors. Sens. Actuators B 2021, 341, 129992. [Google Scholar] [CrossRef]
- Chen, Q.; Huang, X.H.; Yao, Y.; Luo, K.B.; Pan, H.Z.; Wang, Q. Ringed electrode configuration enhances the sensitivity of QCM humidity sensor based on lignin through fringing field effect. IEEE Sens. J. 2021, 3109446. [Google Scholar] [CrossRef]
- Faia, P.M.; Furtado, C.S.; Ferreira, A.J. Humidity sensing properties of a thick-film titania prepared by a slow spinning process. Sens. Actuators B 2004, 101, 183–190. [Google Scholar] [CrossRef]
- Bârsan, N.; Simion, C.; Heine, T.; Pokhrel, S.; Weimar, U. Modeling of sensing and transduction for p-type semiconducting metal oxide based gas sensors. J. Electroceramics 2010, 25, 11–18. [Google Scholar] [CrossRef]
- Niu, X.; Zhong, H.; Wang, X.; Jiang, K. Sensing properties of rare earth oxide doped In2O3 by a sol–gel method. Sens. Actuators B 2006, 115, 434–438. [Google Scholar] [CrossRef]
- Stănoiu, A.; Simiona, C.E.; Somăcescu, S. NO2 sensing mechanism of ZnO–Eu2O3 binary oxide under humid air conditions. Sens. Actuators B 2013, 186, 687–694. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhao, R.; Sun, B.; Nie, G.; Ji, H.; Lei, J.; Wang, C. Highly sensitive acetone sensor based on Eu-doped SnO2 electrospun nanofibers. Ceram. Int. 2016, 42, 15881–15888. [Google Scholar] [CrossRef]
- Ortega, P.P.; Rocha, L.S.R.; Cortés, J.A.; Ramirez, M.A.; Buono, C.; Ponce, M.A.; Simões, A.Z. Towards carbon monoxide sensors based on europium doped cerium dioxide. Appl. Surf. Sci. 2019, 464, 692–699. [Google Scholar] [CrossRef]
- Sarf, F.; Er, I.K.; Yakar, E.; Acar, S. The role of rare-earth metal (Y, Ru and Cs)-doped ZnO thin films in NH3 gas sensing performances at room temperature. J. Mater Sci. Mater Electron. 2020, 31, 10084–10095. [Google Scholar] [CrossRef]
- Zhang, W.; Li, G.; She, C.; Liu, A.; Cheng, J.; Li, H.; Liu, S.; Jing, C.; Cheng, Y.; Chu, J. High performance tube sensor based on PANI/Eu3+ nanofiber for low-volume NH3 detection. Anal. Chim. Acta 2020, 1093, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Fois, M.; Cox, T.; Ratcliffe, N.; de Lacy Costello, B. Rare earth doped metal oxide sensor for the multimodal detection of volatile organic compounds (VOCs). Sens. Actuators B 2021, 330, 129264. [Google Scholar] [CrossRef]
- Mokoena, T.P.; Swart, H.C.; Hillie, K.T.; Motaung, D.E. Engineering of rare-earth Eu3+ ions doping on p-type NiO for selective detection of toluene gas sensing and luminescence properties. Sens. Actuators B 2021, 347, 130530. [Google Scholar] [CrossRef]
- Zhao, S.; Shen, Y.; Li, A.; Chen, Y.; Gao, S.; Liu, W.; Wei, D. Effects of rare earth elements doping on gas sensing properties of ZnO nanowires. Ceram. Int. 2021, 47, 24218–24226. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, H.; Lin, C.; Bian, M. The enhancement of humidity sensing performance based on Eu-doped ZnO. Curr. Appl. Phys. 2019, 19, 82–88. [Google Scholar] [CrossRef]
- Fu, X.Q.; Wang, C.; Yu, H.C.; Wang, Y.G.; Wang, T.H. Fast humidity sensors based on CeO2 nanowires. Nanotechnology 2007, 18, 145503. [Google Scholar] [CrossRef]
- Lyle, S.J.; Westall, W.A. A study of the thermal decomposition of hydrated europium (III) chloride and europium (III) bromide. Thermochim. Acta 1983, 68, 51–58. [Google Scholar] [CrossRef]
- Jiang, W.; Bian, Z.; Hong, C.; Huang, C. A mild liquid reduction route toward uniform blue-emitting EuCl2 nanoprisms and nanorods. Inorg. Chem. 2011, 50, 6862–6864. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Zhang, G.; Zhou, H.; Sun, P.; Li, B.; Ding, D.; Xhen, T. Macroporous lanthanide-organic coordination polymer foams and their corresponding lanthanide oxides. Adv. Mater. 2008, 20, 984–988. [Google Scholar] [CrossRef]
- Wang, S.; Wang, W.; Qian, Y. Preparation and characterization of Eu2O3 nanometer thin films by pulse ultrasonic spray pyrolysis method. Mater. Res. Bull. 2000, 35, 2057–2062. [Google Scholar] [CrossRef]
- Buvailo, A.I.; Xing, Y.; Hines, J.; Dollahon, N.; Borguet, E. TiO2/LiCl-based nanostructured thin film for humidity sensor applications. ACS Appl. Mater. Interfaces 2011, 3, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, Z.; Liu, L.; Zhang, H.; Zheng, W.; Wang, Y.; Huang, H.; Wang, Z.; Wang, C. Humidity sensor based on LiCl-doped ZnO electrospun nanofibers. Sens. Actuators B 2009, 141, 404–409. [Google Scholar] [CrossRef]
- Song, X.; Qi, Q.; Zhang, T.; Wang, C. A humidity sensor based on KCl-doped SnO2 nanofibers. Sens. Actuators B 2009, 138, 368–373. [Google Scholar] [CrossRef]
- Feng, C.D.; Sun, S.L.; Wang, H.; Segre, C.U.; Stetter, J.R. Humidity sensing properties of Nafion and sol−gel derived SiO2/Nafion composite thin films, Sens. Actuators B 1997, 40, 217–222. [Google Scholar] [CrossRef]
- Wang, J.; Lin, Q.; Zhang, T.; Zhou, R.; Xu, B. Humidity sensor based on composite material of nano-BaTiO3 and polymer RMX. Sens. Actuators B 2002, 81, 248–253. [Google Scholar] [CrossRef]
- Wang, J.; Xu, B.K.; Ruan, S.P.; Wang, S.P. Preparation and electrical properties of humidity sensing films of BaTiO3/polystrene sulfonic sodium. Mater. Chem. Phys. 2003, 78, 746–750. [Google Scholar] [CrossRef]
- Casalbore-Miceli, G.; Yang, M.J.; Camaioni, N.; Mari, C.M.; Li, Y.; Sun, H.; Ling, M. Investigations on the ion transport mechanism in conduction polymer films. Solid State Ionics 2000, 131, 311–321. [Google Scholar] [CrossRef]


| Materials | Linear Fitting Curve | Sensitivity a (log Z/% RH) | Linearity b (R2) |
|---|---|---|---|
| EuCl2 | Y = −0461 X + 5.612 | −0.0461 | 0.7267 |
| Eu2O3 | Y = −0118 X + 8.072 | −0.0118 | 0.9230 |
| 2 wt% EuCl2/Eu2O3 | Y = −0245 X + 7.546 | −0.0245 | 0.8983 |
| 5 wt% EuCl2/Eu2O3 | Y = −0296 X + 5.464 | −0.0296 | 0.6983 |
| 6 wt% EuCl2/Eu2O3 | Y = −0346 X + 5.782 | −0.0346 | 0.7797 |
| 7 wt% EuCl2/Eu2O3 | Y = −0427 X + 6.015 | −0.0427 | 0.8601 |
| 8 wt% EuCl2/Eu2O3 | Y = −0411 X + 5.741 | −0.0411 | 0.7582 |
| Sensing Material | Working Range (% RH) | Sensitivity | Hysteresis (% RH) | Response Time (s) | Ref. |
|---|---|---|---|---|---|
| LiCl-doped TiO2 | 13–65 | ― | ― | 0.5 | [32] |
| LiCl-doped ZnO | 11–95 | ― | 2 | 3 | [33] |
| KCl-doped SnO2 | 11–95 | 4 order a | ― | 5 | [34] |
| EuCl2-blended Eu2O3 | 20–90 | 0.0427 b | <1.1 | 40 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, P.-G.; Choy, N.-H. Electrical and Humidity-Sensing Properties of EuCl2, Eu2O3 and EuCl2/Eu2O3 Blend Films. Chemosensors 2021, 9, 288. https://doi.org/10.3390/chemosensors9100288
Su P-G, Choy N-H. Electrical and Humidity-Sensing Properties of EuCl2, Eu2O3 and EuCl2/Eu2O3 Blend Films. Chemosensors. 2021; 9(10):288. https://doi.org/10.3390/chemosensors9100288
Chicago/Turabian StyleSu, Pi-Guey, and Nok-Him Choy. 2021. "Electrical and Humidity-Sensing Properties of EuCl2, Eu2O3 and EuCl2/Eu2O3 Blend Films" Chemosensors 9, no. 10: 288. https://doi.org/10.3390/chemosensors9100288





