High Sensitivity Detection of Copper Ions in Oysters Based on the Fluorescence Property of Cadmium Selenide Quantum Dots
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Synthesis of CdSe QDs
2.3. Instrumentation
2.4. Characterization of QDs
2.5. Detection of Different Concentrations of Copper Ion Standard Solution
2.6. Effects of Different Metal Ions on the Fluorescence Intensity of QDs
2.7. Determination of Cu2+ in Oysters
3. Results and Discussion
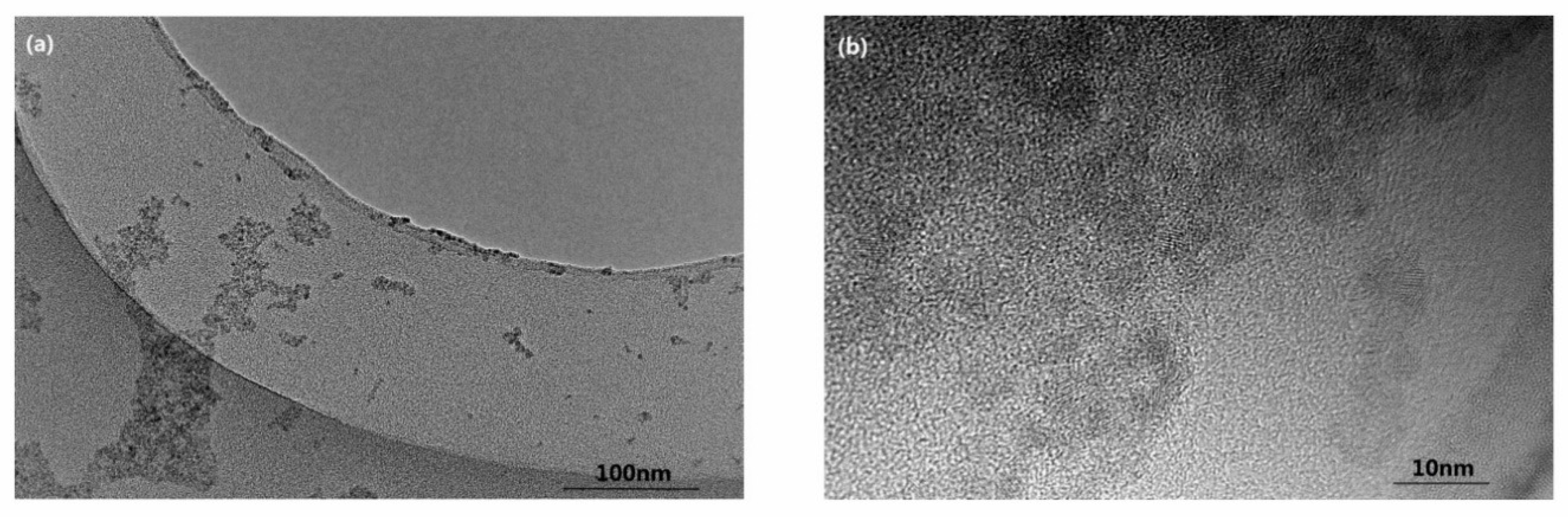
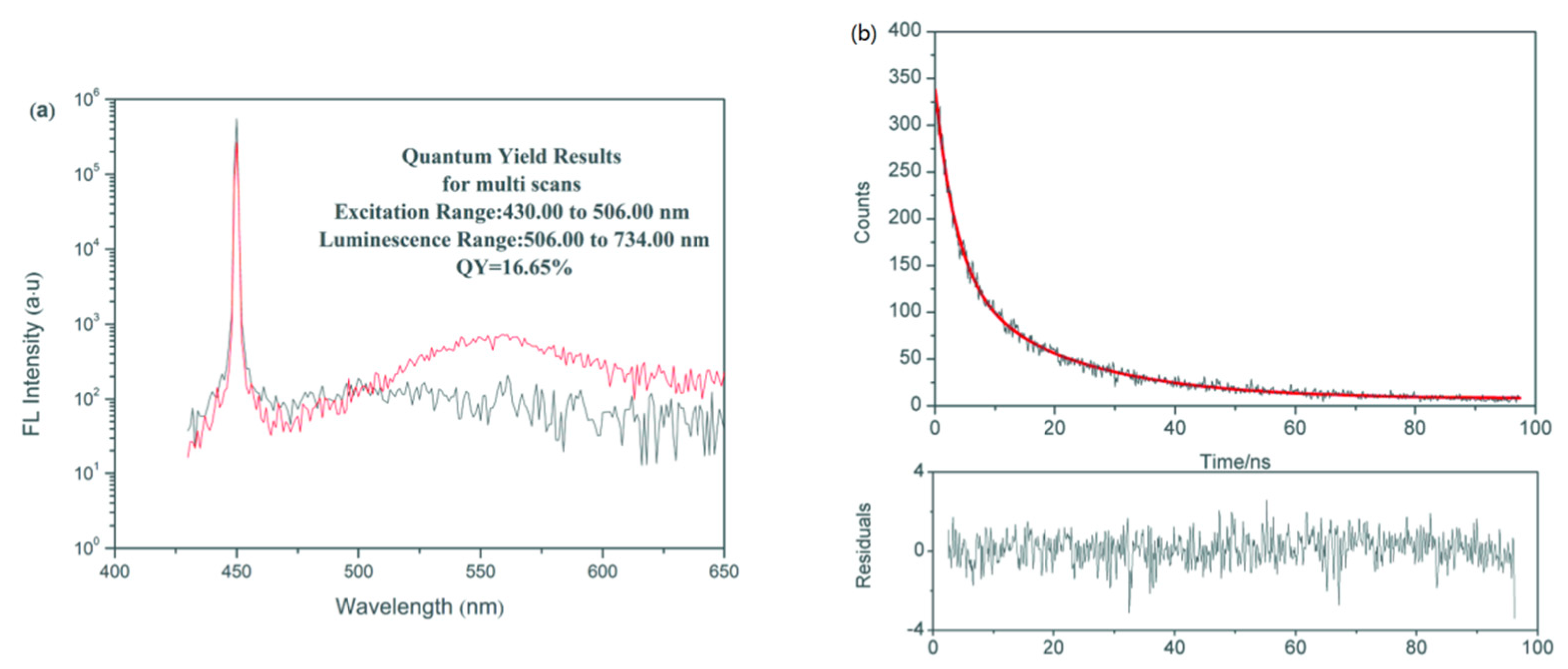
3.1. Characterization of CdSe QDs
3.2. Detection Performance of CdSe QDs for Cu2+
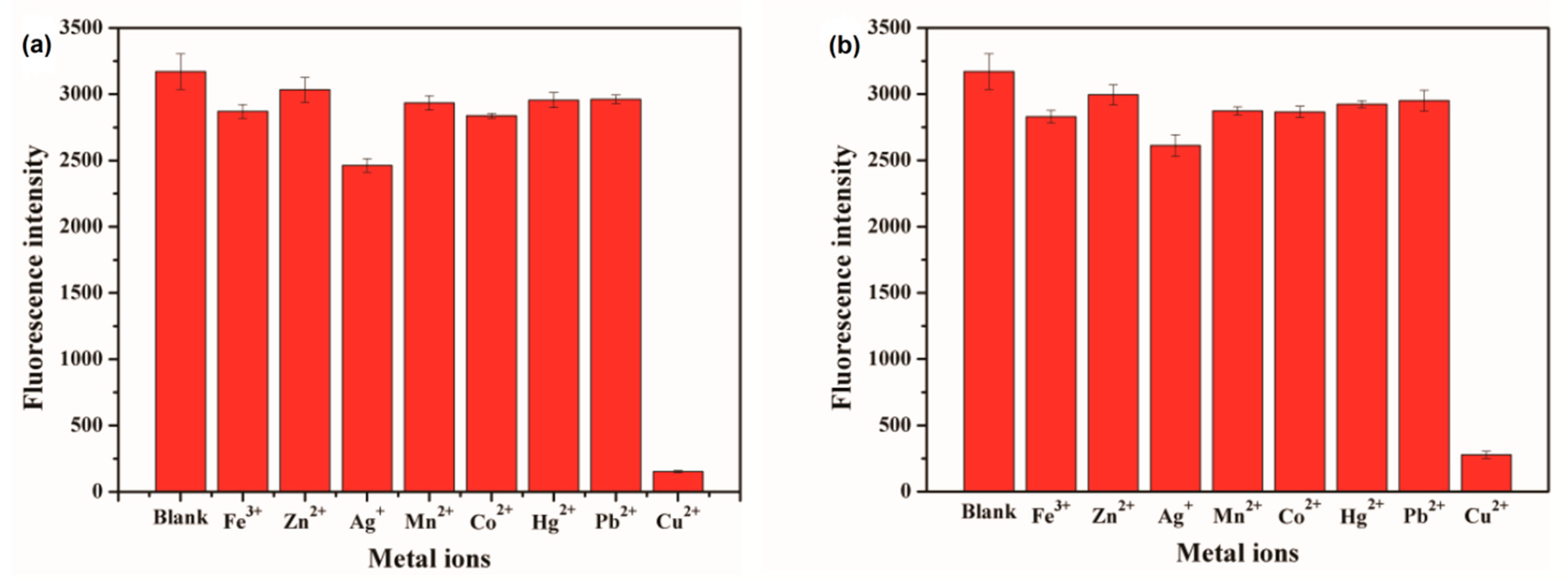
3.3. Selective Detection of CdSe QDs
3.4. Detection of Cu2+ in Oysters
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Buccolieri, A.; Buccolieri, G.; Cardellicchio, N.; Dell’Atti, A.; Di Leo, A.; Maci, A. Heavy metals in marine sediments of taranto gulf (Ionian Sea, Southern Italy). Mar. Chem. 2006, 99, 227–235. [Google Scholar] [CrossRef]
- Bosch, A.C.; O’Neill, B.; Sigge, G.O.; Kerwath, S.E.; Hoffman, L.C. Heavy metals in marine fish meat and consumer health: A review: Heavy metals in marine fish meat. J. Sci. Food Agric. 2016, 96, 32–48. [Google Scholar] [CrossRef] [PubMed]
- Mahurpawar, M. Effects of heavy metals on human health. Int. J. Res. Granthaalayah 2015, 530, 1–7. [Google Scholar]
- Muller, P.; van Bakel, H.; van de Sluis, B.; Holstege, F.; Wijmenga, C.; Klomp, L.W.J. Gene expression profiling of liver cells after copper overload in vivo and in vitro reveals new copper-regulated genes. JBIC 2007, 12, 495–507. [Google Scholar] [CrossRef] [PubMed]
- Sadhra, S.S.; Wheatley, A.D.; Cross, H.J. Dietary exposure to copper in the European union and its assessment for EU regulatory risk assessment. Sci. Total Environ. 2007, 374, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Scheiber, I.F.; Schmidt, M.M.; Dringen, R. Zinc prevents the copper-induced damage of cultured astrocytes. Neurochem. Int. 2010, 57, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Merker, K.; Hapke, D.; Reckzeh, K.; Schmidt, H.; Lochs, H.; Grune, T. Copper related toxic effects on cellular protein metabolism in human astrocytes. BioFactors 2005, 24, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Qian, J.; Jiang, L.; He, S. Fluorescence quenching of quantum dots by gold nanorods and its application to DNA detection. Appl. Phys. Lett. 2009, 94, 063111. [Google Scholar] [CrossRef]
- Chen, Z.M.; Shen, G.Z.; Li, Y.P.; Zhang, P.; Ji, H.W.; Liu, S.C.; Li, C.Y.; Qian, Z.J. A novel biomimetic logic gate for sensitive and selective detection of Pb(II) base on porous alumina nanochannels. Electrochem. Commun. 2015, 60, 83–87. [Google Scholar] [CrossRef]
- Reddi, G.S.; Rao, C.R.M. Analytical techniques for the determination of precious metals in geological and related materials. Analyst 1999, 124, 1531–1540. [Google Scholar] [CrossRef]
- Soodan, R.K.; Pakade, Y.B.; Nagpal, A.; Katnoria, J.K. Analytical techniques for estimation of heavy metals in soil ecosystem: A tabulated review. Talanta 2014, 125, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Ladokhin, A.S. Fluorescence spectroscopy in peptide and protein analysis. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2000. [Google Scholar]
- Sádecká, J.; Tóthová, J. Fluorescence spectroscopy and chemometrics in the food classification &minus: A review. Czech J. Food Sci. 2008, 25, 159–174. [Google Scholar]
- Pinaud, F.; Michalet, X.; Bentolila, L.A.; Tsay, J.M.; Doose, S.; Li, J.J.; Iyer, G.; Weiss, S. Advances in fluorescence imaging with quantum dot bio-probes. Biomaterials 2006, 27, 1679–1687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alivisatos, A.P.; Gu, W.; Larabell, C. Quantum dots as cellular probes. Annu. Rev. Biomed. Eng. 2005, 7, 55–76. [Google Scholar] [CrossRef] [PubMed]
- Larson, D.R. Water-Soluble quantum dots for multiphoton fluorescence imaging in vivo. Science 2003, 300, 1434–1436. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Yang, L.; Petros, J.A.; Marshall, F.F.; Simons, J.W.; Nie, S. In vivo molecular and cellular imaging with quantum dots. Curr. Opin. Biotechnol. 2005, 16, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Frasco, M.F.; Chaniotakis, N. Bioconjugated quantum dots as fluorescent probes for bioanalytical applications. Anal Bioanal Chem. 2010, 396, 223–240. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.T.R.; Winfield, S.A.; Miller, J.N. Relative fluorescence quantum yields using a computer-controlled luminescence spectrometer. Analyst 1983, 108, 1067. [Google Scholar] [CrossRef]
- Grabolle, M.; Spieles, M. Determination of the fluorescence quantum yield of quantum dots: Suitable procedures and achievable uncertainties. Anal. Chem. 2009, 81, 6285–6294. [Google Scholar] [CrossRef]
- Van Dam, B.; Bruhn, B.; Kondapaneni, I.; Dohnal, G.; Wilkie, A.; Křivánek, J.; Valenta, J.; Mudde, Y.; Schall, P.; Dohnalová, K. On a critical artifact in the quantum yield methodology. arXiv 2018, arXiv:1808.00779. [Google Scholar]
- Lei, J.; Ju, J. Fundamentals and bioanalytical applications of functional quantum dots as electrogenerated emitters of chemiluminescence. Trac-Trend Anal Chem. 2011, 30, 1351–1359. [Google Scholar] [CrossRef]
- Huang, S.; Xiao, Q.; Li, R.; Guan, H.L.; Liu, J.; Liu, X.R.; He, Z.K.; Liu, Y. A simple and sensitive method for l-cysteine detection based on the fluorescence intensity increment of quantum dots. Anal. Chim. Acta 2009, 645, 73–78. [Google Scholar] [CrossRef]
- Xu, X.; Liu, X.; Oh, M.; Park, J. Oyster shell as a low-cost adsorbent for removing heavy metal ions from Wastewater. Pol. J. Environ. Stud. 2019, 28, 2949–2959. [Google Scholar] [CrossRef]
- Alqahtani, S.F.; Farooq, W.A.; Ali, S.M.; Tawfik, W. Fabrication and study of structural and optical properties of cadmium telluride quantum dots. J. Optoelectron. Adv. Mater. 2019, 21, 385–394. [Google Scholar]
- Liu, Q.; Zheng, C.; Zhao, H.; Wang, K.; Tao, W. Tumor cell pH detection based on CdSe quantum dots’ fluorescence charateristics. Technol. Health Care 2019, 27, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.X.; Liu, H.Y.; Zhang, J.R.; Zhu, J.J. Ultrasensitive Cu2+ sensing by near-infrared-emitting CdSeTe alloyed quantum dots. Talanta 2010, 80, 2172–2176. [Google Scholar] [CrossRef] [PubMed]
- Sung, T.W.; Lo, Y.L. Highly sensitive and selective sensor based on silica-coated CdSe/ZnS nanoparticles for Cu2+ ion detection. Sens. Actuators B Chem. 2012, 165, 119–125. [Google Scholar] [CrossRef]
- Koneswaran, M.; Narayanaswamy, R. L-Cysteine-capped ZnS quantum dots based fluorescence sensor for Cu2+ ion. Sens. Actuators B Chem. 2009, 139, 104–109. [Google Scholar] [CrossRef]
- Liu, F.C.; Chen, Y.M.; Lin, J.H.; Tseng, W.L. Synthesis of highly fluorescent glutathione-capped ZnxHg1−xSe quantum dot and its application for sensing copper ion. J. Colloid Interface Sci. 2009, 337, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.H.; Han, C.S. Ultrasensitive and selective fluorimetric detection of copper ions using thiosulfate-involved quantum dots. Anal. Chem. 2014, 86, 7209–7213. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Rosenzweig, Z. Luminescent CdS quantum dots as selective ion probes. Anal. Chem. 2002, 74, 5132–5138. [Google Scholar] [CrossRef] [PubMed]
- Sajwan, R.K.; Bagbi, Y.; Sharma, P.; Solanki, P.R. L-cysteine and 3-mercaptopropionic acid capped cadmium selenide quantum dots based metal ion probes. J. Lumin. 2017, 187, 126–132. [Google Scholar] [CrossRef]
- Shang, L.; Dong, S. Silver nanocluster-based fluorescent sensors for sensitive detection of Cu(II). J. Mater. Chem. 2008, 18, 4636. [Google Scholar] [CrossRef]
- Li, H.; Wang, X. Single quantum dot-micelles coated with gemini surfactant for selective recognition of a cation and an anion in aqueous solutions. Sens. Actuators B Chem. 2008, 134, 238–244. [Google Scholar] [CrossRef]


| 4.030 | 21.60 | 24.64 | 75.36 | 0.99 |
| Probes | Limit of Detection | Linear Range | Application | Ref |
|---|---|---|---|---|
| l-Cysteine-capped CdSeTe QDs | 0.007 μM | 0.02–2.0 μM | Not mentioned | [27] |
| Silica-coated CdSe/ZnS QDs | 0.9 μM | 0–10 μM | Not mentioned | [28] |
| l-Cysteine-capped ZnS QDs | 7.1 μM | 0–260 μM | Not mentioned | [29] |
| Glutathione-capped ZnxHg1−xSe QDs | 20 μM | 0.03–5.0 μM | Not mentioned | [30] |
| Hexadecyl trimethylammonium bromide modified CdSe/ZnS QDs | 0.15 nM | 0–600 nM | Deionization water | [31] |
| CdS QDs | 0.1 μM | Not mentioned | Not mentioned | [32] |
| MPA-CdSe QDs | Not mentioned | 4–160 μM | Not mentioned | [33] |
| PMAA (methacrylic acid)-Ag nanocluster | 0.008 μM | 0.01–6.0 μM | Not mentioned | [34] |
| Gemini-coated CdSe/ZnS QDs | 1.1 μM | 0–500 μM | Not mentioned | [35] |
| 3-MPA CdSe QDs | 0.03 μM | 0.03–3 μM | oysters | This work |
| Fluorescence Intensity | Corresponding Concentration (μg/mL) | Mean Concentration (μg/mL) | Concentration Measured by Flame Atomic Absorption Spectrometry (FAAS) (μg/mL) | Relative Error (%) | |
|---|---|---|---|---|---|
| Sample 1 | 855.5 | 0.3512 | 0.3592 (±6.841 × 10−3) | 0.3667 | 2.04 |
| 840.1 | 0.3586 | ||||
| 821.1 | 0.3679 | ||||
| Sample 2 | 732.4 | 0.4174 | 0.4224 (±5.276 × 10−3) | 0.4277 | 1.24 |
| 713.0 | 0.4297 | ||||
| 728.3 | 0.4200 | ||||
| Sample 3 | 595.7 | 0.5183 | 0.5581 (±2.868 × 10−2) | 0.5496 | 1.55 |
| 541.2 | 0.5708 | ||||
| 527.9 | 0.5850 | ||||
| Sample 4 | 762.0 | 0.3998 | 0.4035 (±7.455 × 10−3) | 0.3921 | 2.91 |
| 767.2 | 0.3968 | ||||
| 738.2 | 0.4139 | ||||
| Sample 5 | 709.8 | 0.4318 | 0.4111 (±1.989 × 10−2) | 0.4175 | 1.53 |
| 732.6 | 0.4173 | ||||
| 790.2 | 0.3841 | ||||
| Sample 6 | 971.6 | 0.3023 | 0.2959 (±4.808 × 10−3) | 0.3007 | 1.60 |
| 975.0 | 0.3011 | ||||
| 1022 | 0.2842 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, S.; Lu, Z.; Su, T.; Feng, Y.; Zhou, C.; Hong, P.; Sun, S.; Li, C. High Sensitivity Detection of Copper Ions in Oysters Based on the Fluorescence Property of Cadmium Selenide Quantum Dots. Chemosensors 2019, 7, 47. https://doi.org/10.3390/chemosensors7040047
Jiang S, Lu Z, Su T, Feng Y, Zhou C, Hong P, Sun S, Li C. High Sensitivity Detection of Copper Ions in Oysters Based on the Fluorescence Property of Cadmium Selenide Quantum Dots. Chemosensors. 2019; 7(4):47. https://doi.org/10.3390/chemosensors7040047
Chicago/Turabian StyleJiang, Shiqi, Zifan Lu, Tiantian Su, Yanting Feng, Chunxia Zhou, Pengzhi Hong, Shengli Sun, and Chengyong Li. 2019. "High Sensitivity Detection of Copper Ions in Oysters Based on the Fluorescence Property of Cadmium Selenide Quantum Dots" Chemosensors 7, no. 4: 47. https://doi.org/10.3390/chemosensors7040047




