Gold Nanoparticle Conjugated Water Soluble Multiwall Carbon Nanotubes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis and Soxhlet Purification of Raw Product from Fly Ash
2.2. Oxidative Treatment of Soxhlet Purified Product Using Concentrated HNO3
2.3. Synthesis of Gold Nanoparticle Modified wsMWCNTs
3. Results and Discussion
3.1. Elemental Analyses of wsMWCNTs
3.2. Structural Studies of wsMWCNT before and after GNP Conjugation Using Raman Spectroscopy
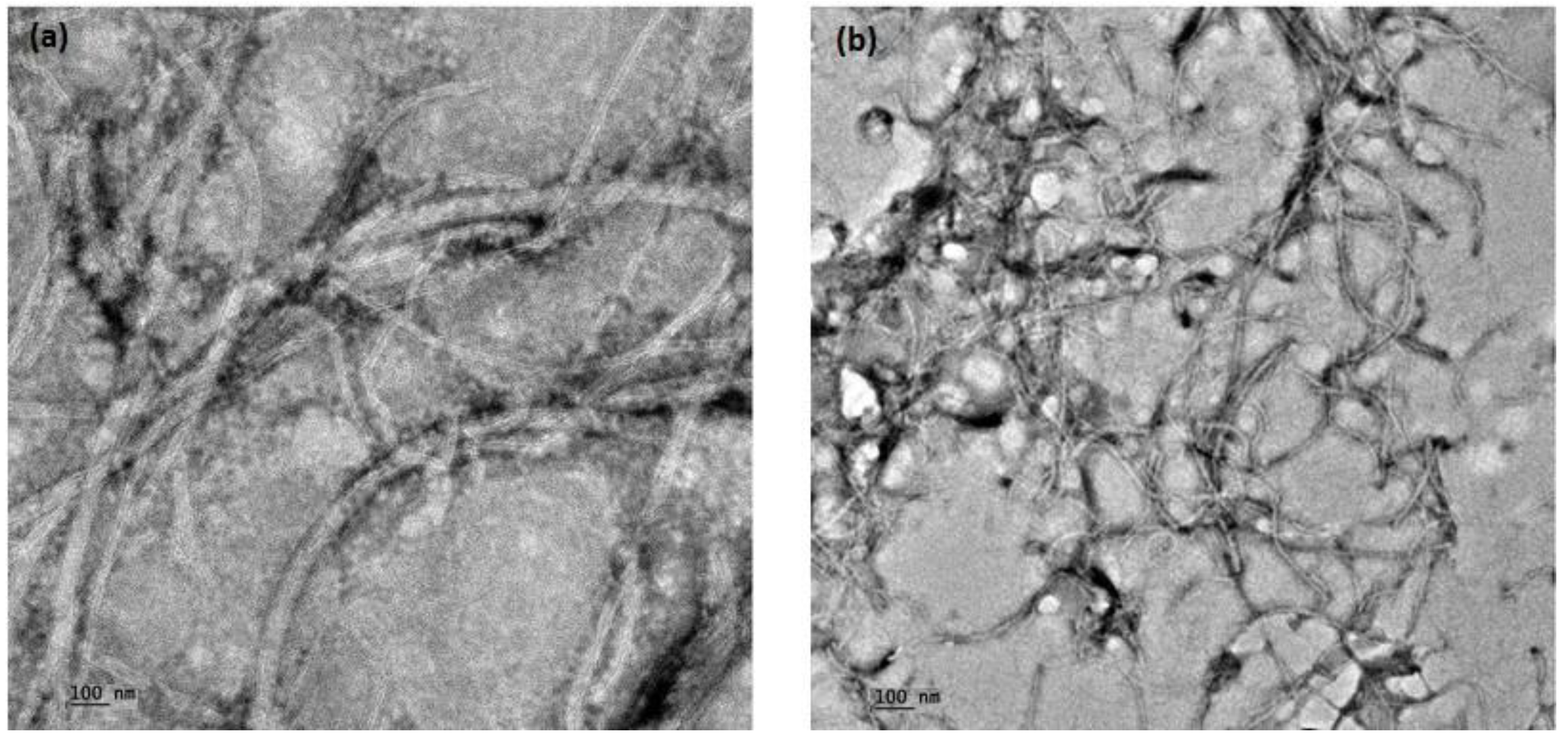
3.3. TEM Investigation of wsMWCNT before and after GNP Conjugation
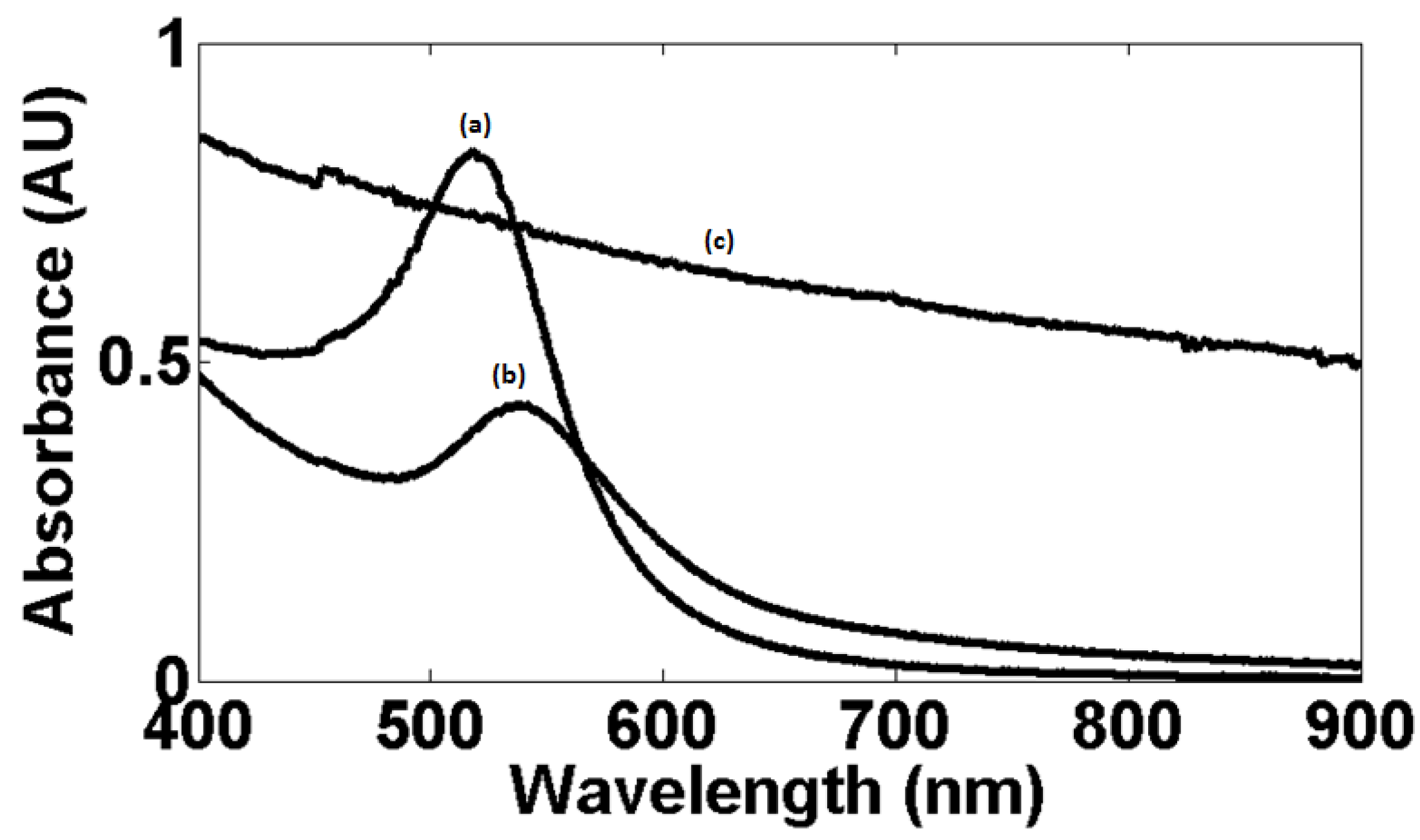
3.4. Absorption Spectroscopic Study of wsMWCNT before and after GNP Conjugation
3.5. Fluorescence Study of wsMWCNT before and after GNP Conjugation
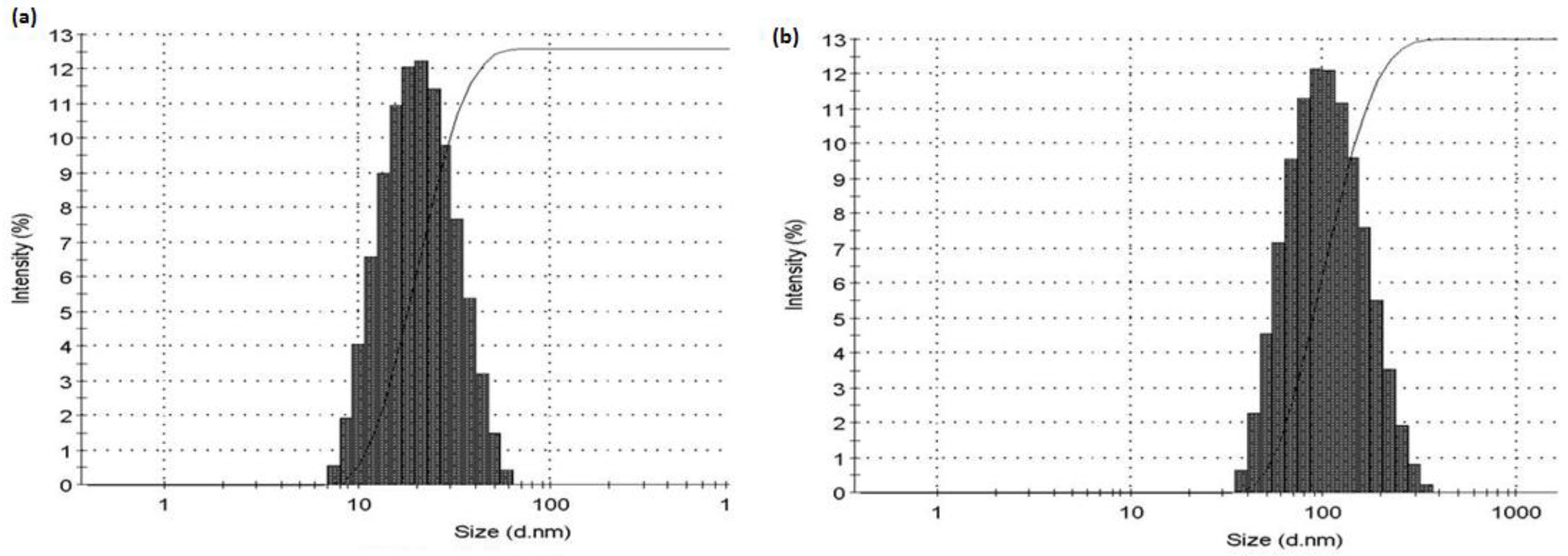
3.6. Photo Co-Relation Spectroscopic Study of wsMWCNT before and after GNP Conjugation
4. Conclusions
Funding
Conflicts of Interest
References
- Kroto, H.W.; Heath, J.R.; O’Brien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfulleren. Nature 1985, 318, 162. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Dresselhaus, G.; Eklund, P.D. Science of Fullerenes and Carbon Nanotubes; Academic Press: San Diego, CA, USA, 1996. [Google Scholar]
- Ugarte, D. Curling and closure of graphitic networks under electron-beam irradiation. Nature 1992, 359, 707. [Google Scholar] [CrossRef] [PubMed]
- Ebbesen, T.W.; Ajayan, P.M. Large-scale synthesis of carbon nanotubes. Nature 1992, 358, 220. [Google Scholar] [CrossRef]
- Eklund, P.C.; Pradhan, B.K.; Kim, U.J.; Xiong, Q.; Fischer, J.E.; Friedman, A.D.; Holloway, B.C.; Jordan, K.; Smith, K.M.W. Large-scale production of single-walled carbon nanotubes using ultrafast pulses from a free electron laser. Nano Lett. 2002, 2, 561. [Google Scholar] [CrossRef]
- Nikolaev, P.; Bronikowski, M.J.; Bradley, R.K.; Rohmund, F.; Colbert, D.T.; Smith, K.A.; Smalley, R.E. Gas-phase catalytic growth of single-walled carbon nanotubes from carbon monoxide. Chem. Phys. Lett. 1999, 313, 91. [Google Scholar] [CrossRef]
- Boccaccini, A.R.; Cho, J.; Roether, J.A.; Thomas, B.J.C.; Minay, E.J.; Shaffer, M.S.P. Electrophoretic deposition of carbon nanotubes. Carbon 2006, 44, 3149. [Google Scholar] [CrossRef]
- Yuan, L.; Saito, K.; Pan, C.; Williams, F.A.; Gordon, A.S. Nanotubes from methane flames. Chem. Phys. Lett. 2001, 340, 237. [Google Scholar] [CrossRef]
- Terrones, M.; Grobert, N.; Olivares, J.; Zhang, J.P.; Terrones, H.; Kordatos, K.; Hsu, W.K.; Hare, J.P.; Townsend, P.D.; Prassides, K.; et al. Controlled production of aligned-nanotube bundles. Nature 1997, 388, 52. [Google Scholar] [CrossRef]
- Li, W.Z.; Xie, S.S.; Qian, L.X.; Chang, B.H.; Zou, B.S.; Zhou, W.Y.; Zhao, R.A.; Wang, G. Large-scale synthesis of aligned carbon nanotubes. Science 1996, 274, 1701–1703. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.F.; Huang, Z.P.; Xu, J.W.; Wang, W.J.; Bush, P.; Siegel, M.P.; Provencio, P.N. Synthesis of large arrays of well-aligned carbon nanotubes on glass. Science 1998, 282, 1105–1107. [Google Scholar] [CrossRef] [PubMed]
- Merkulov, V.L.; Melechko, A.V.; Guillorm, M.A.; Lowndes, D.H.; Simpson, M.L. Alignment mechanism of carbon nanofibers produced by plasma-enhanced chemical-vapor deposition. Appl. Phys. Lett. 2001, 79, 2970–2972. [Google Scholar] [CrossRef]
- Shiratori, Y.; Hiraoka, H.; Takeuchi, Y.; Itoh, S.; Yamamoto, M. One-step formation of aligned carbon nanotube field emitters at 400 °C. Appl. Phys. Lett. 2003, 82, 2485–2487. [Google Scholar] [CrossRef]
- Küttel, O.M.; Groening, O.; Emmenegger, C.; Schlapbach, L. Electron field emission from phase pure nanotube films grown in a methane/hydrogen plasma. Appl. Phys. Lett. 1998, 73, 2113–2115. [Google Scholar] [CrossRef]
- Thess, A.; Lee, R.; Nikolaev, P.; Dai, H.J.; Petit, P.; Robert, J.; Xu, C.H.; Lee, H.Y.; Kim, S.G.; Colbert, D.T.; et al. Crystalline Ropes of Metallic Carbon Nanotubes. Science 1996, 273, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Hata, K.; Futaba, D.N.; Mizuno, K.; Namai, T.; Yumura, M.; Iijima, S. Water-assisted highly efficient synthesis of impurity-free single-walled carbon nanotubes. Science 2004, 306, 1362–1364. [Google Scholar] [CrossRef] [PubMed]
- Dunens, O.M.; MacKenzie, K.J.; Harris, A.T. Synthesis of multiwalled carbon nanotubes on fly ash derived catalysts. Environ. Sci. Technol. 2009, 43, 7889–7894. [Google Scholar] [CrossRef] [PubMed]
- Yasui, A.; Kamiya, Y.; Sugiyama, S.; Ono, S.; Noda, H.; Ichikawa, Y. Synthesis of carbon nanotubes on fly ashes by chemical vapor deposition processing. IEEJ Trans. Electr. Electron. Eng. 2009, 4, 787–789. [Google Scholar] [CrossRef]
- Kim, K.H.; Jo, W.H. A strategy for enhancement of mechanical and electrical properties of polycarbonate/multi-walled carbon nanotube composites. Carbon 2009, 4, 1126–1134. [Google Scholar] [CrossRef]


© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhattacharyya, T. Gold Nanoparticle Conjugated Water Soluble Multiwall Carbon Nanotubes. Chemosensors 2018, 6, 36. https://doi.org/10.3390/chemosensors6030036
Bhattacharyya T. Gold Nanoparticle Conjugated Water Soluble Multiwall Carbon Nanotubes. Chemosensors. 2018; 6(3):36. https://doi.org/10.3390/chemosensors6030036
Chicago/Turabian StyleBhattacharyya, Tamoghna. 2018. "Gold Nanoparticle Conjugated Water Soluble Multiwall Carbon Nanotubes" Chemosensors 6, no. 3: 36. https://doi.org/10.3390/chemosensors6030036



