Simple and Rapid Determination of Ethanol Content in Beer Using an Amperometric Biosensor
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals, Reagents and Solutions
2.2. Instrumentation
2.3. Biosensor Preparation, Measuring Procedure and Sample Processing
2.4. Reference Gas Chromatographic Method
2.5. Statistical Analysis
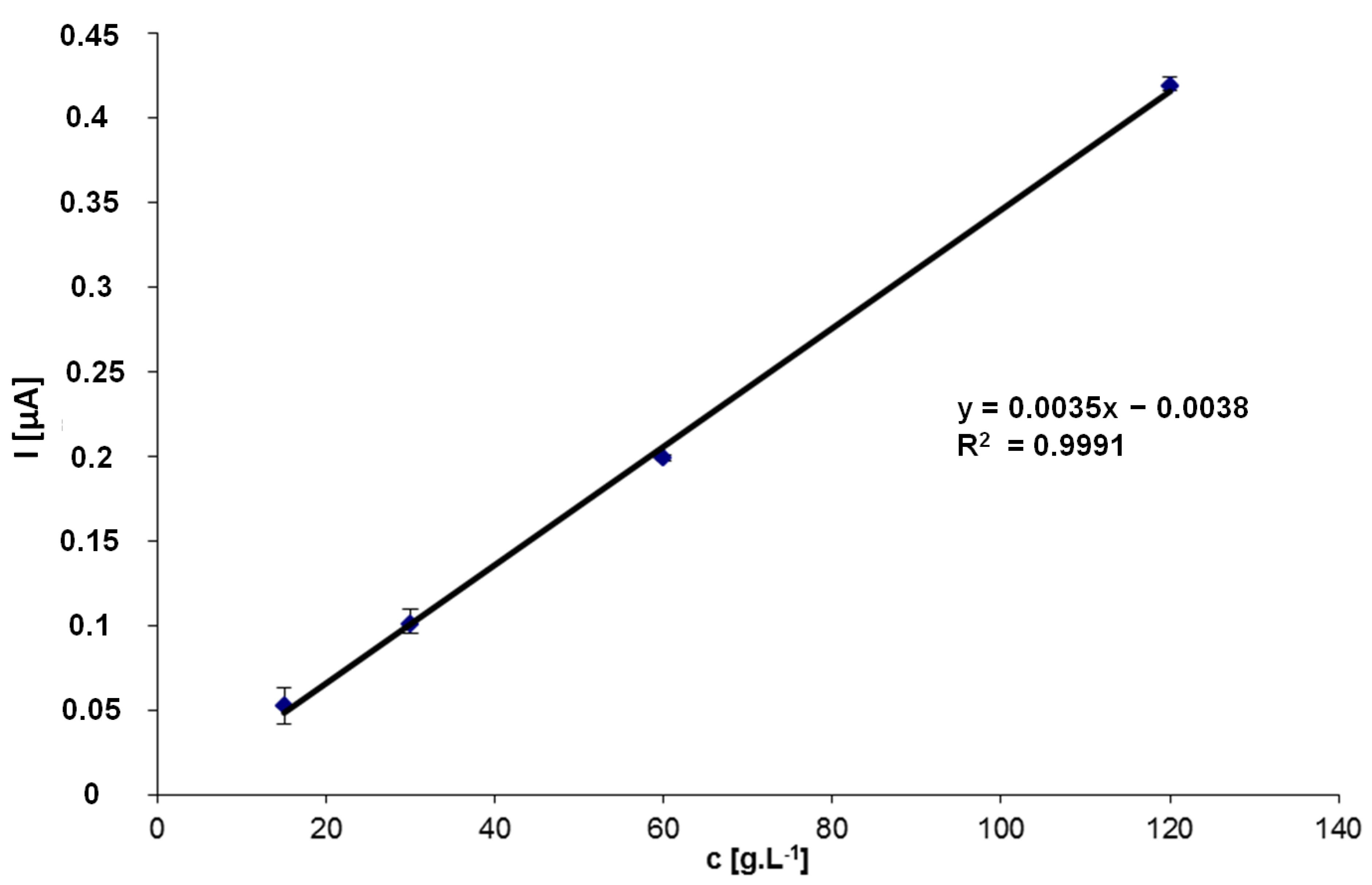
3. Results and Discussion
| Beer Type | Proposed Method | Reference Method | n | u | ucrit | Declared | |
|---|---|---|---|---|---|---|---|
| ± R[%] | [%] | ||||||
| 1 | non-alcoholic | ˂DL | 0.41 ± 0.01 | 3 | – | 0.636 | ≤0.49 |
| 2 | light | 3.8 ± 0.3 | 3.46 ± 0.09 | 0.872 | 4.1 | ||
| 3 | lager | 4.7 ± 0.4 | 3.31 ± 0.08 | 2.896 | 4.9 | ||
| 4 | lager | 5.1 ± 0.2 | 5.28 ± 0.09 | 0.621 | 5.0 | ||
| 5 | lager | 4.1 ± 0.2 | 4.07 ± 0.03 | 0.130 | 4.4 | ||
| 6 | diabetic | 3.8 ± 0.1 | 3.75 ± 0.10 | 0.250 | 4.0 | ||
| 7 | strong lager | 5.7 ± 0.4 | 5.39 ± 0.05 | 0.689 | 6.5 | ||
| 8 | porter | 9.1 ± 0.4 | 7.66 ± 0.05 | 3.200 | 8.0 | ||
| 9 | half dark lager | 5.9 ± 0.2 | 4.35 ± 0.06 | 5.962 | 4.0 | ||
| 10 | lager | 4.7 ± 0.3 | 4.87 ± 0.05 | 0.486 | 4.7 | ||
| 11 | tap lager beer | 4.2 ± 0.1 | 4.25 ± 0.06 | 0.313 | 3.5 | ||
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- České pivo. Available online: http://www.ceskepivo.cz/index.php/Hlavn%C3%AD_strana (accessed on 19 May 2015).
- Gorinstein, S.; Kitov, S.; Sarel, S.; Berman, O.; Berliner, M.; Popovich, G.; Vermus, Y. Changes in the chemical composition of beer during the brewing process as a result of added enzymes. J. Am. Soc. Brewing Chem. 1980, 38, 23–26. [Google Scholar]
- Jandera, P. Methods for the HPLC analysis of phenolic compounds and flavonoids in beer. In Beer in Health and Disease Prevention; Preedy, V.R., Ed.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 1003–1014. [Google Scholar]
- Li, H.L.; Chai, X.S.; Deng, Y.L.; Zhan, H.Y.; Fu, S.Y. Rapid determination of ethanol in fermentation liquor by full evaporation headspace gas chromatography. J. Chrom. A 2009, 1216, 169–172. [Google Scholar] [CrossRef]
- Luque de Castro, M.D.; Gonzales-Rodriguez, J.; Perez-Juan, P. Analytical methods in wineries: Is it time to change? Food Rev. Int. 2005, 21, 231–265. [Google Scholar] [CrossRef]
- Bozkurt, S.S.; Merdivan, E.; Benibol, Y. A fluorescent chemical sensor for ethanol determination in alcoholic beverages. Microchim Acta 2010, 168, 141–145. [Google Scholar] [CrossRef]
- Turner, A.P.F.; Karube, I.; Wilson, G.S. Biosensors: Fundamentals and Applications; Oxford University Press: Oxford, UK, 1987; pp. 180–210. [Google Scholar]
- Nistor, M.; Csöregi, E. Biosensors for food analysis. In Encyclopedia of Sensors; Grimes, C.A., Dickey, E.C., Pishko, M.V., Eds.; American Scientific Publishers: Stevenson Ranch, CA, USA, 2006; Volume 1, pp. 353–369. [Google Scholar]
- Gorton, L.; Bartlett, P.N. NAD(P)-based biosensors. In Bioelectrochemistry. Fundamentals, Experimental Techniques and Applications; Bartlett, P.N., Ed.; Wiley: Chichester, England, UK, 2008; pp. 157–198. [Google Scholar]
- Mishra, A.K.; Mishra, S.B.; Tiwari, A. Nanocomposites and their biosensor applications. In Biosensor Nanomaterials; Li, S.J., Singh, J., Li, H., Banerjee, I.A., Eds.; Wiley-VCH Verlag: Weinheim, Germany, 2011; pp. 255–268. [Google Scholar]
- Zhang, X.E. Screen-printing methods for biosensor production. In Biosensors, 2nd ed.; Cooper, J., Class, T., Eds.; Oxford University Press: Oxford, UK, 2004; pp. 41–54. [Google Scholar]
- Woodward, J.R. Biochemistry and applications of alcohol oxidase from methylotrophic yeast. In Advances in Autotrophic Microbiology and One-Carbon Metabolism; Codd, G.A., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherland, 1990; pp. 193–225. [Google Scholar]
- Azevedo, A.M.; Prayeres, D.M.; Cabral, J.M.; Fonseca, L.P. Ethanol biosensors based on alcohol oxidase. Biosens. Bioelectron. 2005, 21, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Boujtita, M.; Hart, J.P.; Pittson, R. Development of a disposable ethanol biosensor based on a chemically modified screen-printed electrode coated with alcohol oxidase for the analysis of beer. Biosens. Bioelectron. 2000, 15, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Carelli, D.; Centonze, D.; de Giglio, A.; Quinto, M.; Zambonin, P.G. An interference-free first generation alcohol biosensor based on a gold electrode modified by an overoxidised non-conducting polypyrrole film. Anal. Chim. Acta 2006, 565, 27–35. [Google Scholar] [CrossRef]
- Gotoh, M.; Karube, I. Ethanol biosensor using immobilized coenzyme. Anal. Lett. 1994, 27, 273–284. [Google Scholar] [CrossRef]
- Pandey, P.C.; Upadhyay, S.; Upadhyay, B.C.; Pathak, H.C. Ethanol biosensor and electrochemical oxidation of NADH. Anal. Biochem. 1998, 260, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Bala, C.; Rotariu, L.; Magearu, V. Disposable alcohol biosensor based on alcohol dehydrogenase and screen-printed electrodes. Analele Univ. Bucuresti Chim. 2003, 12, 55–60. [Google Scholar]
- Tsai, Y.C.; Huang, J.D.; Chiu, C.C. Amperometric ethanol biosensor based on poly(vinyl alcohol)-multiwalled carbon nanotube—alcohol dehydrogenase biocomposite. Biosens. Bioelectron. 2007, 22, 3051–3056. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Q.; Li, B.H.; Wang, X.; Li, C.N. One-step construction of an electrode modified with electrodeposited Au/SiO2 nanoparticles, and its appliocation to the determination of NADH and ethanol. Microchim. Acta 2010, 171, 399–405. [Google Scholar] [CrossRef]
- Ho, Y.H.; Periasamy, A.P.; Chen, S.M. Amperometric ethanol biosensor based on alcohol dehydrogenase immobilized at poly-l-lysine coated carminic acid functionalized multiwalled carbon nanotube film. Int. J. Electrochem. Sci. 2011, 6, 3922–3937. [Google Scholar]
- Liu, X.Q.; Li, B.H.; Ma, M.; Zhan, G.Q.; Liu, A.X.; Li, C.N. Amperometric sensing of NADH and ethanol using a hybrid film electrode modified with electrochemically fabricated zirconia nanotubes and poly(acid fuchsin). Microchim. Acta 2012, 176, 123–129. [Google Scholar] [CrossRef]
- Kotzian, P.; Brázdilová, P.; Kalcher, K.; Handlíř, K.; Vytřas, K. Oxides of platinum metal group as potential catalysts in carbonaceous amperometric biosensors based on oxidases. Sens. Actuators B Chem. 2007, 124, 297–302. [Google Scholar] [CrossRef]
- Kotzian, P.; Brázdilová, P.; Kalcher, K.; Vytřas, K. Mediators of electron transfer in amperometric enzyme biosensors. In Sensing in Electroanalysis; Vytřas, K., Kalcher, K., Eds.; University of Pardubice Press Centre: Pardubice, Czech Republic, 2007; Volume 2, pp. 181–199. [Google Scholar]
- Kotzian, P.; Brázdilová, S.; Řezková, S.; Kalcher, K.; Vytřas, K. Amperometric glucose biosensor based on rhodium dioxide-modified carbon ink. Electroanalysis 2006, 18, 1499–1504. [Google Scholar] [CrossRef]
- Polan, V.; Soukup, J.; Vytřas, K. Screen-printed carbon electrodes modified by rhodium dioxide and glucose dehydrogenase. Enzyme Res. 2010, 2010, 324184. [Google Scholar] [CrossRef]
- Polan, V.; Vytřas, K. Determination of ethanol in alcoholic drinks using an enzyme biosensor containing alcohol dehydrogenase. In Microorganisms in Industry and Environment. From Scientific and Industrial Research to Consumer Products; Mendez-Vilas, A., Ed.; World Scientific Publishing Co.: Singapore, 2011; pp. 688–692. [Google Scholar]
- Lord, E. The use of range in place of standard deviation in the t-test. Biometrika 1947, 34, 41–67. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polan, V.; Eisner, A.; Vytřas, K. Simple and Rapid Determination of Ethanol Content in Beer Using an Amperometric Biosensor. Chemosensors 2015, 3, 169-177. https://doi.org/10.3390/chemosensors3020169
Polan V, Eisner A, Vytřas K. Simple and Rapid Determination of Ethanol Content in Beer Using an Amperometric Biosensor. Chemosensors. 2015; 3(2):169-177. https://doi.org/10.3390/chemosensors3020169
Chicago/Turabian StylePolan, Vojtěch, Aleš Eisner, and Karel Vytřas. 2015. "Simple and Rapid Determination of Ethanol Content in Beer Using an Amperometric Biosensor" Chemosensors 3, no. 2: 169-177. https://doi.org/10.3390/chemosensors3020169





