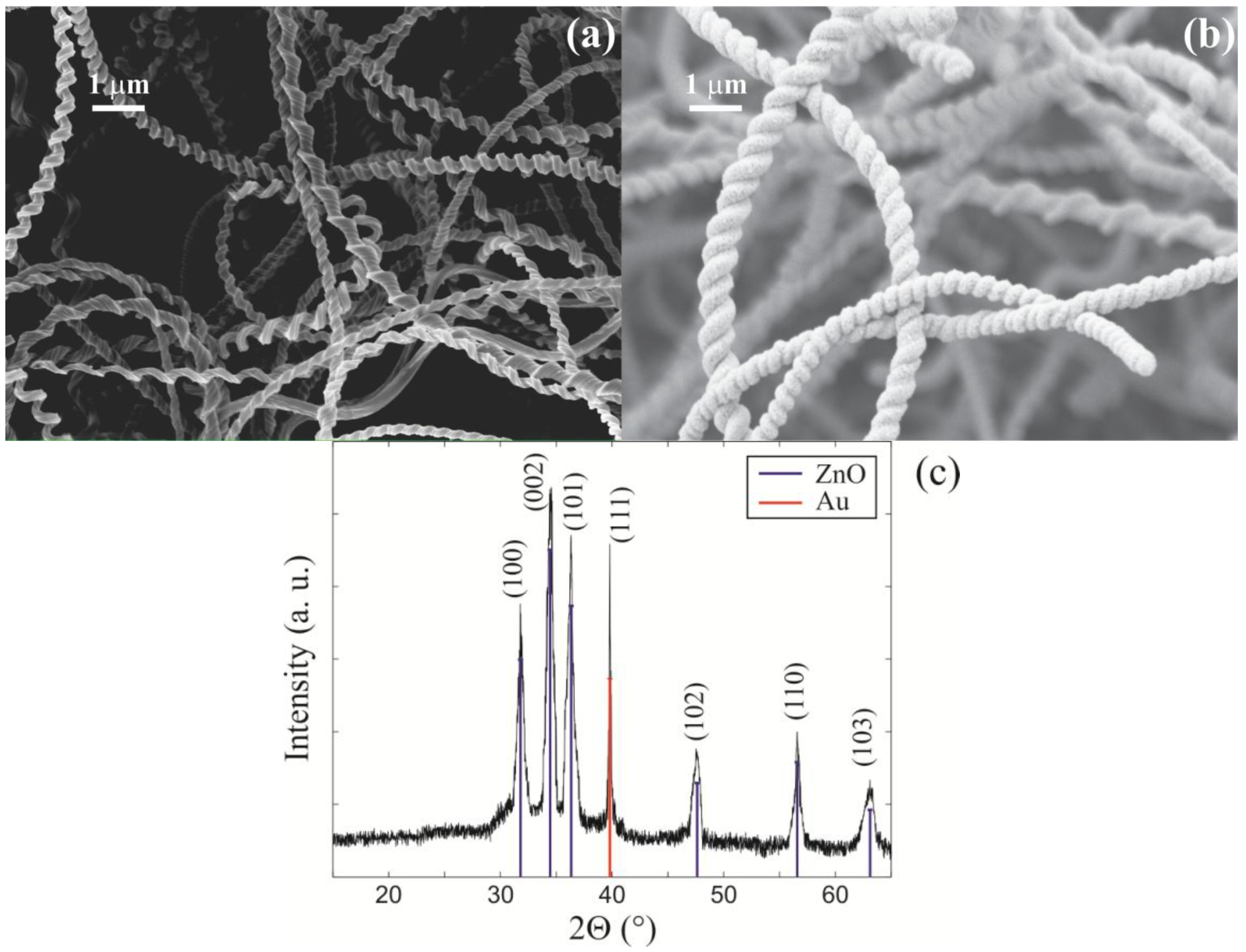
3. Results
The electrical sensor response to toluene, as a function of xenon light bulb sensor surface temperature (T
LB) and vapor-gas mixture temperature (T
V), was observed and analyzed. The first set of experiments was aimed at determining the electrical response of a sensor to toluene vapor as a function of the xenon light bulb sensor surface temperature (T
LB). The experiment was conducted without any heating of the vapor-gas mixture (T
V = 22 °C). The procedure for experimental data acquisition was to allow the sensor to reach steady state resistance in the synthetic air at a given temperature T
LB, at which time it was exposed to room temperature pulses of the vapor-gas mixture. Note, pulses of Ar sans toluene did not produce a significant sensor response. The results for T
LB = 280 °C, 370 °C, and 435 °C are summarized in
Figure 3a–c, respectively. The effect of temperature on the electronic properties of a thin ZnO layer deposited on the silica nanosprings is significantly different relatively to bulk ZnO. Heating bulk ZnO increases the number of carriers in the conduction band, which in its turn, leads to a drop in resistance. However, for a thin ZnO layer on silica nanosprings, where the length scale of surface interactions is comparable to the thickness of the layer, there are two competing processes. Namely, the generation of “free carriers” (decreasing resistance) and oxidation of the ZnO surface (increasing resistance). In the oxidation process, the adsorbed oxygen species can trap “free electrons” from the near-surface ZnO region (redox process) due to thermal activation [
4,
5]. Thermalizing of the electron carriers is needed to overcome the surface potential barrier and to reach the oxygen species on the ZnO surface. As a result of this process, a negative surface charge is formed, which depletes the n-type ZnO semiconductor layer.
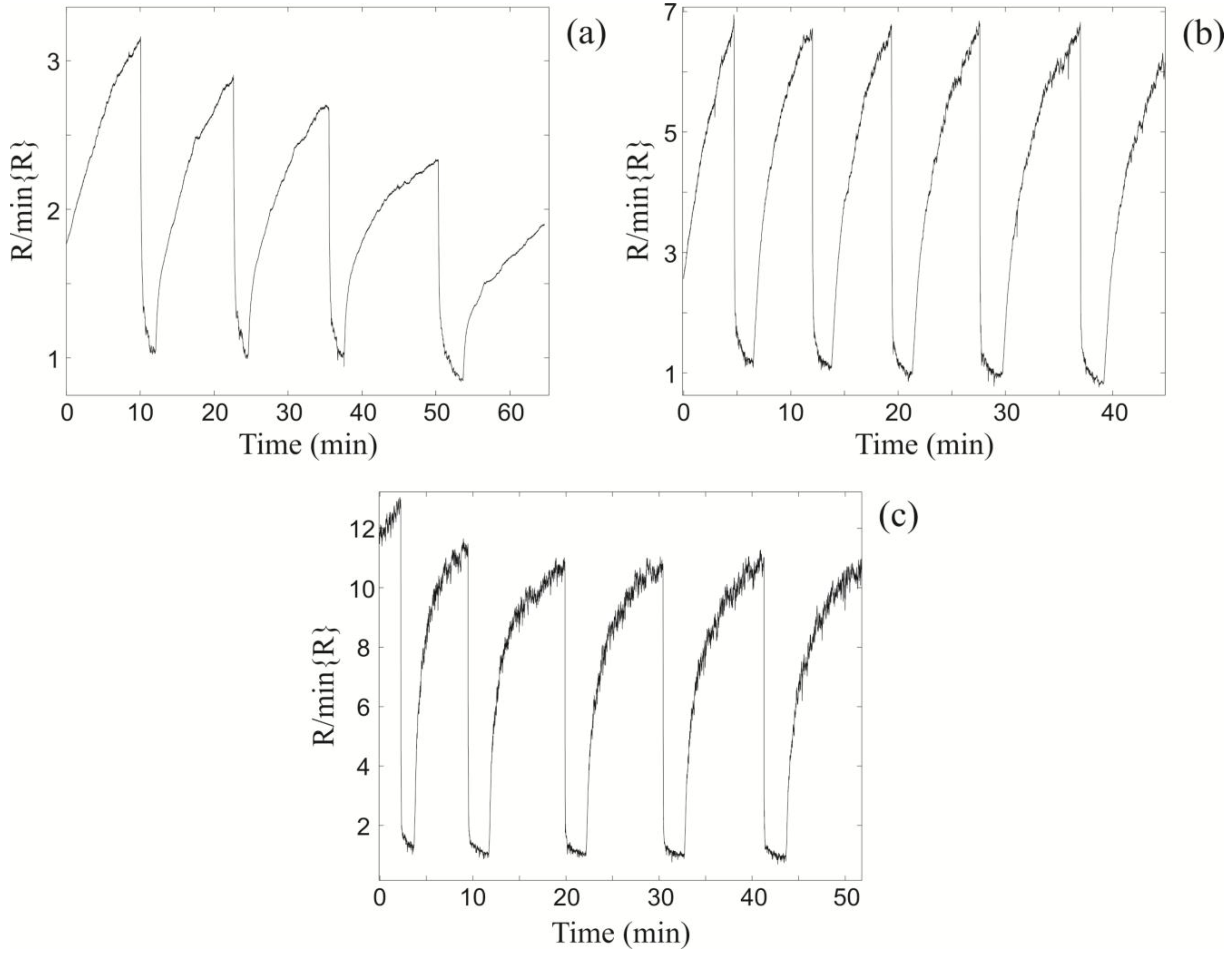
Figure 3.
The relative changes in resistance of the light bulb ZnO nanospring sensor at (a) TLB = 280 °C; (b) TLB = 370 °C; and (c) TLB = 435 °C upon exposure to sequential 1000 ppm toluene pulses at fixed vapor-gas temperature TV = 22 °C.
Figure 3.
The relative changes in resistance of the light bulb ZnO nanospring sensor at (a) TLB = 280 °C; (b) TLB = 370 °C; and (c) TLB = 435 °C upon exposure to sequential 1000 ppm toluene pulses at fixed vapor-gas temperature TV = 22 °C.
Upon exposure to toluene vapor, the resistance changes by a factor of 3 at T
LB = 280 °C (
Figure 3a), 7 at T
LB = 370 °C (
Figure 3b), and 11 at T
LB = 435 °C (
Figure 3c). Note, the xenon light bulb chemiresistor is
not self-refreshing in the synthetic air at T
LB = 280 °C (
Figure 3a), as apparent from systematic decrease in its baseline with sequential exposures. However, at higher temperatures it
is self-refreshing. The optimal (maximum) sensor response to room temperature pulses of toluene is at T
LB = 435 °C (
Figure 3c). For T
LB > 435 °C the sensor response begins to decrease (not shown), which is attributed to surface phonon enhanced desorption of oxygen from the ZnO surface.
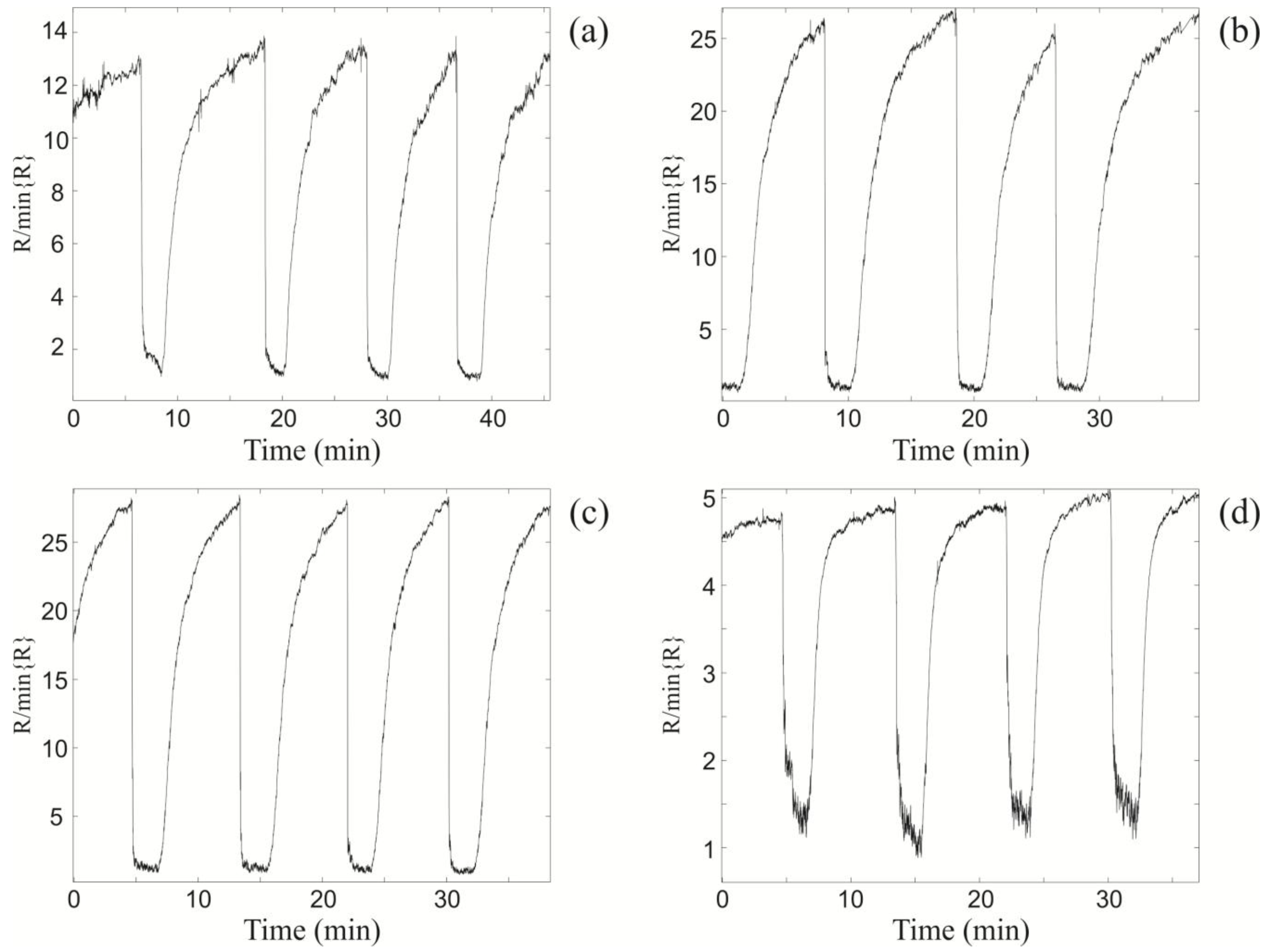
Figure 4.
The relative changes in resistance of the xenon light bulb ZnO nanospring sensor upon exposure to 1,000 ppm consequent toluene pulses at different vapor gas mixture temperatures TV and the optimal light bulb temperature TLB = 435 °C. (a) TV = 100 °C; (b) TV = 200 °C; (c) TV = 250 °C; and (d) TV = 300 °C.
Figure 4.
The relative changes in resistance of the xenon light bulb ZnO nanospring sensor upon exposure to 1,000 ppm consequent toluene pulses at different vapor gas mixture temperatures TV and the optimal light bulb temperature TLB = 435 °C. (a) TV = 100 °C; (b) TV = 200 °C; (c) TV = 250 °C; and (d) TV = 300 °C.
The second set of experiments was aimed at determining the chemiresistor response at T
LB = 435 °C to toluene vapor as a function of the vapor-gas mixture temperature (T
V) (
Figure 4). The sensor resistance characteristics were obtained at vapor-gas mixture temperatures of 100 °C, 200 °C, 250 °C, and 300 °C, which correspond to
Figure 4a–d, respectively. From examination of
Figure 4, it is apparent that the electrical response increases with increasing T
V until the maximum at T
V = 250 °C, at which point the response begins to decrease at subsequently higher temperatures. The maximum relative change in the sensor resistance at T
V = 250 °C and at T
LB = 435 °C is a factor of 27 (
Figure 4c). The drop in sensor response at T
V = 300 °C (
Figure 4d) is due to the atmospheric oxidation of the toluene vapor prior to reaching the light bulb surface. As a consequence, the concentration of unoxidized toluene reaching the ZnO surface is significantly reduced relative to lower gas-vapor temperatures.
4. Discussion
The effects of chemisorption on the electrical transport properties of metal oxides is well documented [
1,
2,
3,
4,
5,
6,
7,
8,
9,
10,
11,
19,
20,
21,
22]. In the case of ZnO
1-δ, where the surface is oxygen deficient, the surface readily oxidized in ambient air to obtain the ideal surface stoichiometry of ZnO. The oxidation process involves either chemisorption (O
2 + e
− = O
2−) or dissociative chemisorption (O
2 + 2e
− = 2O
−), where these act as traps of conduction electrons in the near-surface region of the ZnO layer. It has been reported in the literature [
5,
7] that below 150 °C the molecular adsorption of oxygen dominates, while above 150 °C dissociative chemisorption of oxygen prevails. On the other hand, at relatively high temperatures (T > 435 °C) the excitation of surface phonons leads to enhancement of oxygen desorption from the ZnO surface. The net effect is a decrease of sensor response (sensitivity) when the oxygen desorption rate exceeds the oxygen chemisorption rate.
The result of oxygen chemisorption process is the formation of a surface charge (
QS) depleting the n-type ZnO semiconductor. The width of the depletion layer
L, the Debye screening length
LD, and the height of the surface potential barrier ∆V are related by the following equation [
11,
21]:
where
e is the electron charge,
k is the Boltzmann constant, and
T is the absolute temperature. The Debye screening length is given by the relationship,
where ɛ is the dielectric constant of the material and
N is the carrier density. The surface barrier height ∆
V is determined by the surface charge
QSA schematic representation of the effects of surface charge depletion on the sensor response is illustrated in
Figure 5. The response to toluene is attributed to the toluene oxidation at the ZnO surface and the corresponding reduction of the negative surface charge. This, in turn, decreases the depletion width producing a drop in the sensor resistance. Self-refreshing re-oxidation of the ZnO surface (redox process) returns the chemiresistor characteristics to their baselines.
Figure 5.
(
a) Exponential “RC-circuit” approximation of the sensor response. Energy level schematics (
b) of the bulk ZnO; (
c) at the depleted surface of ZnO; and (
d) upon toluene oxidation on the ZnO surface (arbitrary intrinsic E
i levels correspond to defect states in n-type ZnO). Schematic energy-level diagrams of the polycrystalline ZnO layer (
e) prior to toluene exposure and (
f) after exposure and subsequent toluene oxidation [
1,
2,
3].
Figure 5.
(
a) Exponential “RC-circuit” approximation of the sensor response. Energy level schematics (
b) of the bulk ZnO; (
c) at the depleted surface of ZnO; and (
d) upon toluene oxidation on the ZnO surface (arbitrary intrinsic E
i levels correspond to defect states in n-type ZnO). Schematic energy-level diagrams of the polycrystalline ZnO layer (
e) prior to toluene exposure and (
f) after exposure and subsequent toluene oxidation [
1,
2,
3].
In the case of heating the vapor-gas mixture with a fixed sensor surface temperature T
LB = 435 °C, two processes take place on the surface. The first is simply the filling of unoccupied oxygen sites, while the second is increase of dissociative chemisorption of oxygen that results in ionic oxygen (O
−) species on the ZnO surface. On the one hand, the heating of the synthetic air enables oxygen to activate additional surface sites, thus, increasing the concentration of adsorbed oxygen species. In addition, the gas heating enhances the rate of dissociative chemisorption of oxygen, thus, increasing the concentration of ionic oxygen (O
−) species on the ZnO surface. These changes in the concentration and chemical forms of surface oxygen lead to the increment of the baseline resistance (
Figure 6a) and an enhancement of the sensor sensitivity. On the other hand, the gas heating intensifies the toluene oxidation process in the ambient air prior to reaching the surface. This, in turn, decreases the toluene oxidation rate at the ZnO surface, thereby reducing the sensitivity. The cumulative effect of the three processes on the chemiresistor response is illustrated by
Figure 6b. Namely, the graph demonstrates that the sensor response grows until the temperature of flowing gases reaches 200 °C. Between 200 °C–250 °C the aforementioned effects counterbalance one another such that the sensor response plateaus. Above 250 °C, the pre-oxidation of toluene in the synthetic air prevails, leading to a decline in sensitivity of the sensor. More simply stated, the activation of additional oxygen sites at the ZnO surface enhances the oxidation rate of the analyte, but this is countered by gas phase oxidation of analyte, thereby reducing the number of unoxidized species impinging on the ZnO surface.
Figure 6.
(a) The average baseline resistance of the xenon light bulb ZnO nanospring sensor (TLB = 435 °C) at different vapor gas mixture temperatures TV; (b) The average change in the chemiresistor response to 1,000 ppm toluene vapor at different vapor gas mixture temperatures TV (TLB = 435 °C).
Figure 6.
(a) The average baseline resistance of the xenon light bulb ZnO nanospring sensor (TLB = 435 °C) at different vapor gas mixture temperatures TV; (b) The average change in the chemiresistor response to 1,000 ppm toluene vapor at different vapor gas mixture temperatures TV (TLB = 435 °C).
The self-refreshing re-oxidation (redox process) of the ZnO surface and toluene surface oxidation processes can be described with relatively high precision by the exponential functions ρ↑ and ρ↓, respectively.
where ρ
0 is the pre-exponential factor,
t0 is the starting time for the introduction of toluene vapor into the flow of the synthetic air, and τ
↑ and τ
↓ are the characteristic recovery and response time constants, respectively, which can be determined graphically from the data as shown in
Figure 5a. The time constants τ
↑ and τ
↓ are inversely proportional to the oxygen adsorption rate
Ra and the toluene oxidation rate
Rd on the ZnO surface, respectively.
The rate of the oxygen adsorption on the ZnO surface,
Ra, can be expressed in the same way as any kinetic process,
i.e., as a product of the incident flux
F of O
2 molecules and the sticking coefficient, or sticking probability,
s:
The molecular flux,
F, is given by the Hertz-Knudsen equation,
where
P is the gas pressure,
m is the mass of one oxygen molecule, T
GS is the gas temperature near the surface, which is usually taken as equal to the surface temperature (T
LB). The sticking coefficient
s is usually expressed in an Arrhenius form [
19]
where
A is the pre-exponential factor, E
a is the activation energy of oxygen adsorption on the ZnO surface, and R is the universal gas constant. Hence, the oxygen adsorption rate
Ra can be written as
The catalytic surface oxidation of toluene causes the corresponding desorption (depletion) of oxygen from the ZnO surface. The desorption rate
Rd of an adsorbate from the surface can be described in an Arrhenius form [
20],
where
D is the pre-exponential factor and E
d is the activation energy of oxygen desorption due to the catalytic oxidation of toluene vapor on the ZnO surface.
From relation (5) and Equations (9) and (10) the activation energies E
a and E
d can be expressed in terms of the characteristic time constants and temperature T
LB of the xenon light bulb chemiresistor as follows:
In Equations (11) the superscripts (1) and (2) refer to the different sensor temperatures TLB. The average values of the activation energies Ea and Ed, based on the above analysis, have been determined to be 83 kJ/mol and 87 kJ/mol, respectively.
The graphically determined values of characteristic recovery (τ
↑) and response (τ
↓) time constants as functions of temperatures T
LB and T
V from
Figure 3 and
Figure 4 are summarized in
Table 1. The decrease of both the time constants with increasing xenon light bulb temperature (T
LB) at a fixed vapor temperature of T
V = 22 °C is in good agreement with Arrhenius relationships in Equations (9) and (10) for the oxygen adsorption and desorption rates, respectively. The characteristic response time τ
↑ remains constant for 22 °C < T
V < 250 °C, while the increase of the characteristic recovery time τ
↓ with increasing T
V from 22 °C to 250 °C (at the fixed sensor temperature T
LB = 435 °C) also correlates with Equation (9), under the assumption that the gas temperature
TGS (see Equation (9)) near the ZnO surface rises with the increment of T
V. Heating the vapor-gas mixture reduces the interaction time between oxygen molecules and the ZnO surface and, hence, decreases the oxygen adsorption rate at the ZnO surface. The significant rise of both the time constants τ
↑ and τ
↓ at T
LB = 435 °C and T
V = 300 °C is attributed to the increase in the gas phase oxidation rate of toluene prior to reaching the ZnO surface.
Table 1.
Characteristic recovery (τ↑) and response (τ↓) time constants at different xenon light bulb temperatures (TLB) and vapor gas mixture temperatures (TV).
Table 1.
Characteristic recovery (τ↑) and response (τ↓) time constants at different xenon light bulb temperatures (TLB) and vapor gas mixture temperatures (TV).
| | TLB=280 °C | TLB=370 °C | TLB=435 °C | TLB=435 °C | TLB=435 °C | TLB=435 °C | TLB=435 °C |
|---|
| TV=22 °C | TV=22 °C | TV=22 °C | TV=100 °C | TV=200 °C | TV=250 °C | TV=300 °C |
|---|
| τ↑ (s) | 540 | 42.2 | 11.3 | 12 | 16 | 18 | 73 |
| τ↓ (s) | 80 | 3 | 1.2 | 1.2 | 1.2 | 1.2 | 10 |