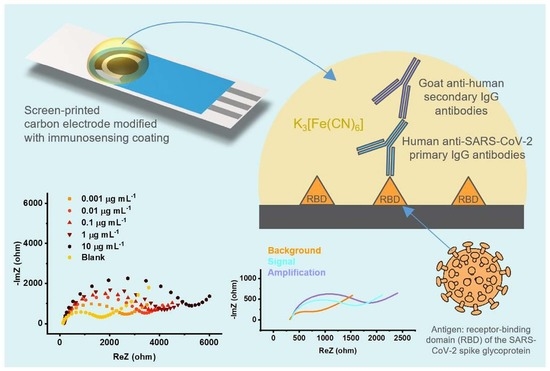
RBD-Modified Polyaniline-Based Label-Free Immunosensor for Sensitive Impedimetric Detection of Anti-SARS-CoV-2 Antibodies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Apparatus
2.3. Preparation of the Immunosensor
2.3.1. Electrochemical Polymerization of Aniline
2.3.2. Modification with Glutaraldehyde, Biorecognition Elements, and Glycine
2.4. Impedimetric Immunosensing
3. Results and Discussion
3.1. Synthesis of PANI Film
3.2. Glutaraldehyde Crosslinking
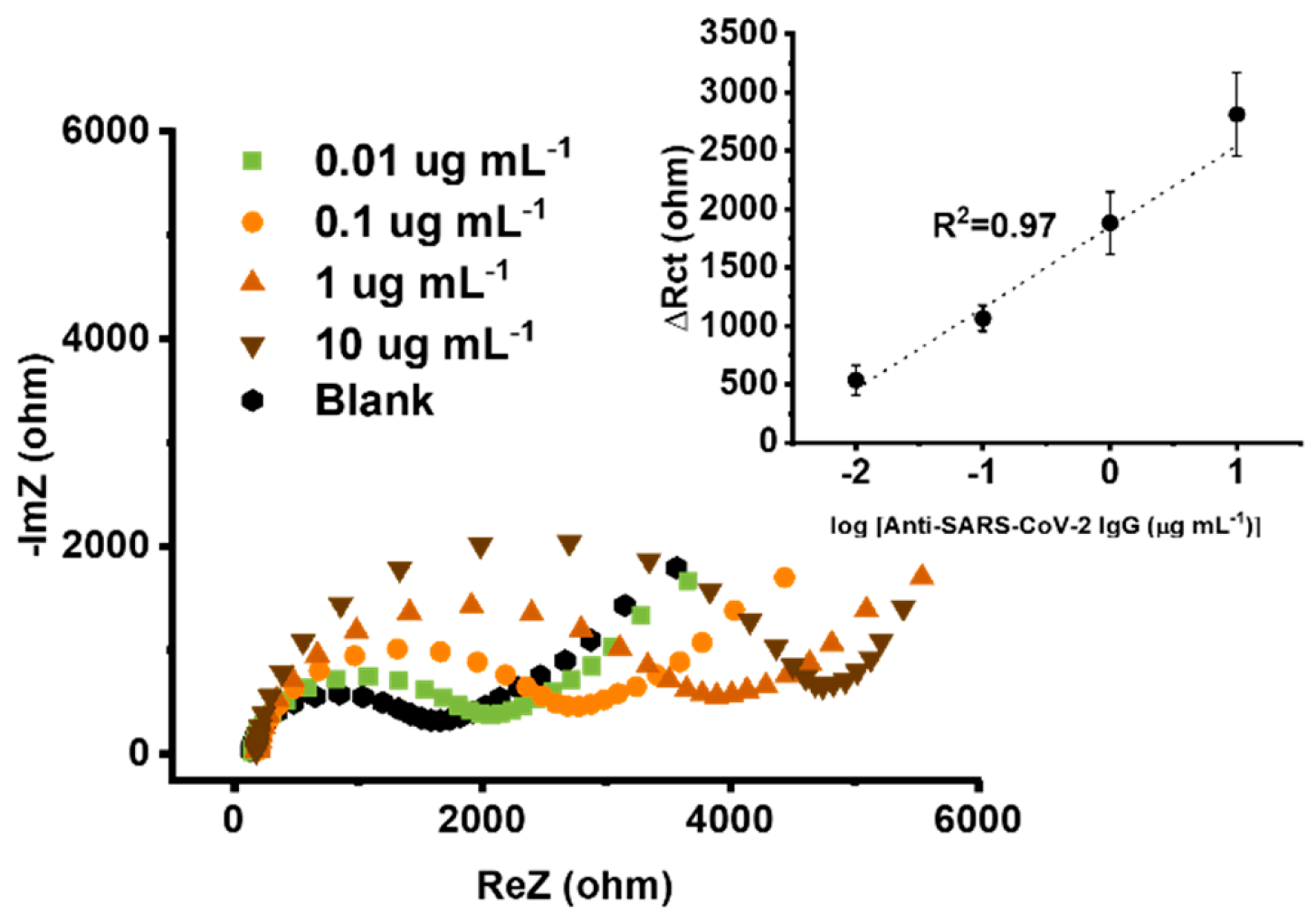
3.3. Electroanalytical Performance
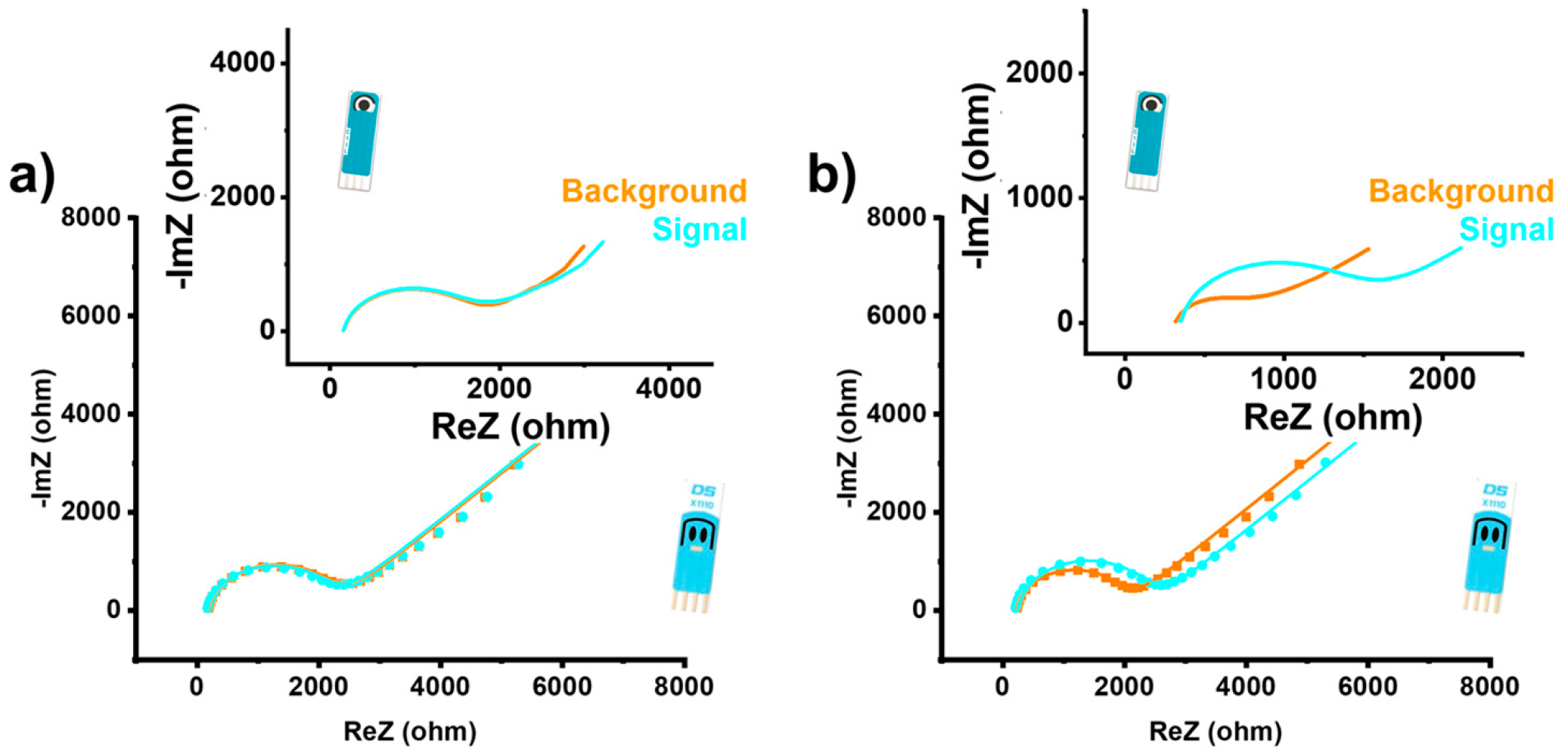
3.4. Practical Application with Dual Electrode
3.5. Performance in the Real Clinical Sample
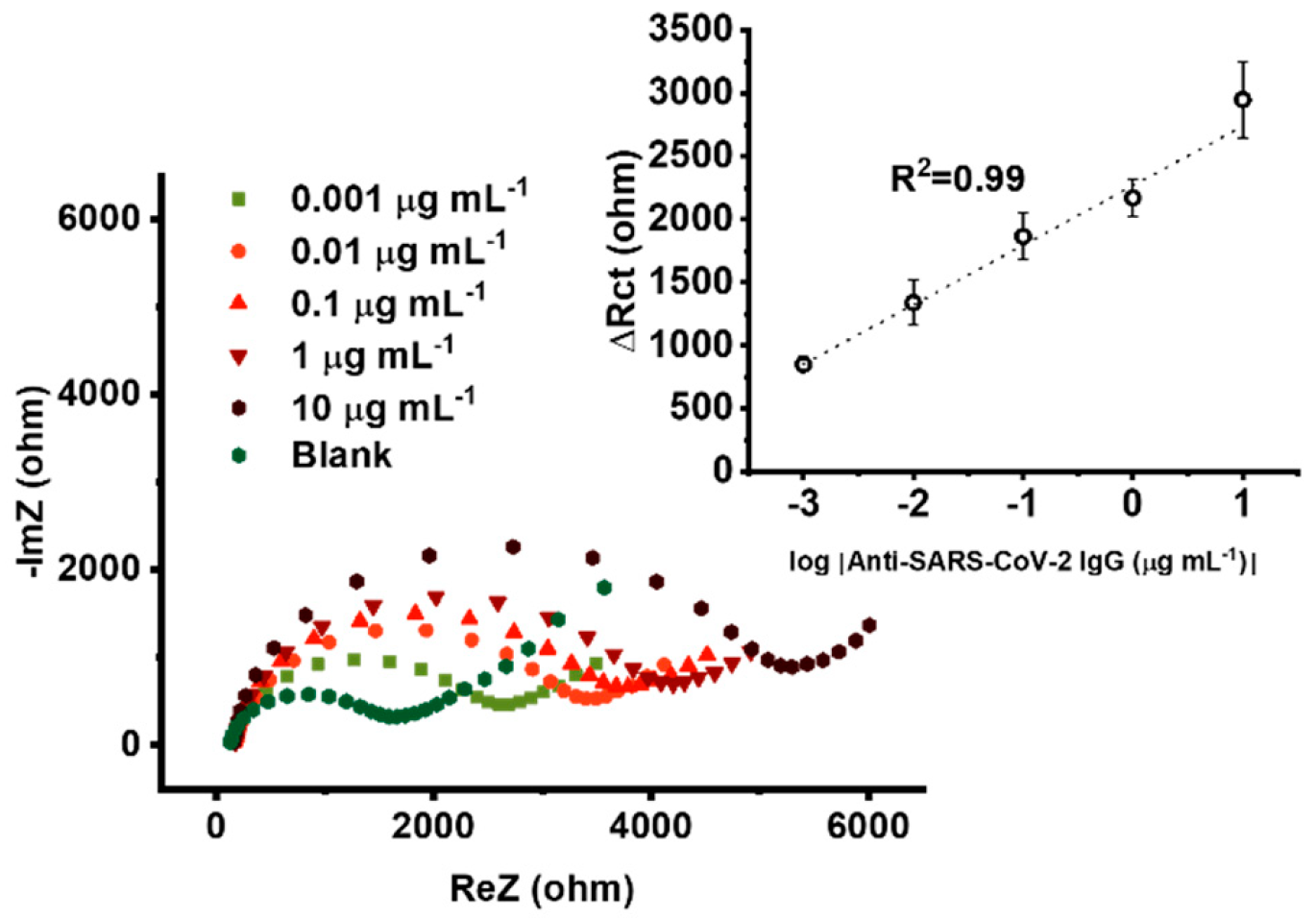
3.6. Amplification Mode
3.7. Comparison with Electrochemical Sensors for Anti-SARS-CoV-2 Antibodies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johns Hopkins. University of Medicine, Coronavirus Resource Center. Available online: https://coronavirus.jhu.edu/map.html (accessed on 7 March 2023).
- Nishiga, M.; Wang, D.W.; Han, Y.; Lewis, D.B.; Wu, J.C. COVID-19 and cardiovascular disease: From basic mechanisms to clinical perspectives. Nat. Rev. Cardiol. 2020, 17, 543–558. [Google Scholar] [CrossRef] [PubMed]
- Corman, V.M.; Landt, O.; Kaiser, M.; Molenkamp, R.; Meijer, A.; Chu, D.K.; Bleicker, T.; Brünink, S.; Schneider, J.; Schmidt, M.L.; et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance 2020, 25, 2000045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Elden, L.J.R.; van Loon, A.M.; van Alphen, F.; Hendriksen, K.A.W.; Hoepelman, A.I.M.; van Kraaiji, M.G.J.; Oosterheert, J.-J.; Schipper, P.; Schuurman, R.; Nijhuis, M. Frequent detection of human coronaviruses in clinical specimens from patients with respiratory tract infection by use of a novel real-time reverse transcriptase polymerase chain reaction. J. Infect. Dis. 2004, 189, 652–657. [Google Scholar] [CrossRef]
- Gootenberg, J.S.; Abudayyeh, O.O.; Lee, J.W.; Essletzbichler, P.; Dy, A.J.; Joung, J.; Verdine, V.; Donghia, N.; Daringer, N.M.; Freije, C.A.; et al. Nucleic acid detection with CRISPR-Cas13a/C2c2. Science 2017, 356, 438–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, B.; Xu, J.; Liu, Y.; Peng, H.; Feng, W.; Cao, Y.; Wu, J.; Xiao, H.; Pabbaraju, K.; Tipples, G.; et al. Isothermal amplification and ambient visualization in a single tube for the detection of SARS-CoV-2 using loop-mediated amplification and CRISPR technology. Anal. Chem. 2020, 92, 16204–16212. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Qian, C.; Pang, Y.; Li, M.; Yang, Y.; Ma, H.; Zhao, M.; Qian, F.; Yu, H.; Liu, Z.; et al. opvCRISPR: One-pot visual RT-LAMP-CRISPR platform for SARS-cov-2 detection. Biosen. Bioelectron. 2020, 172, 112766. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, Z.; Zhai, X.; Li, Y.; Lin, L.; Zhao, H.; Bian, L.; Li, P.; Yu, L.; Wu, Y.; et al. Rapid and sensitive detection of anti-SARS-CoV-2 IgG, using lanthanide-doped nanoparticles-based lateral flow immunoassay. Anal. Chem. 2020, 92, 7226–7231. [Google Scholar] [CrossRef] [PubMed]
- MacMullan, M.A.; Ibrayeva, A.; Trettner, K.; Deming, L.; Das, S.; Tran, F.; Moreno, J.R.; Casian, J.G.; Chellamuthu, P.; Kraft, J.; et al. ELISA detection of SARS-CoV-2 antibodies in saliva. Sci. Rep. 2020, 10, 20818. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, I.A.; Hassan, A.; Oliveira, O.N., Jr.; Crespilho, F.N. On the challenges for the diagnosis of SARS-CoV-2 based on a review of current methodologies. ACS Sens. 2020, 5, 3655–3677. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, W.; Lu, X.; Gooding, J.J.; Li, Q.; Qu, K. Monitoring the progression of loop-mediated isothermal amplification using conductivity. Anal. Biochem. 2014, 466, 16–18. [Google Scholar] [CrossRef] [PubMed]
- Chaibun, T.; Puenpa, J.; Ngamdee, T.; Boonapatcharoen, N.; Athamanolap, P.; O’Mullane, A.P.; Vongpunsawad, S.; Poovorawan, Y.; Lee, S.Y.; Lertanantawong, B. Rapid electrochemical detection of coronavirus SARS-CoV-2. Nat. Comm. 2021, 12, 802. [Google Scholar] [CrossRef] [PubMed]
- Fabiani, L.; Saroglia, M.; Galatà, G.; De Santis, R.; Fillo, S.; Luca, V.; Faggioni, G.; D’Amore, N.; Regalbuto, E.; Salvatori, P.; et al. Magnetic beads combined with carbon black-based screen-printed electrodes for COVID-19: A reliable and miniaturized electrochemical immunosensor for SARS-CoV-2 detection in saliva. Biosen. Bioelectron. 2021, 171, 112686. [Google Scholar] [CrossRef] [PubMed]
- Rashed, M.Z.; Kopechek, J.A.; Priddy, M.C.; Hamorsky, K.T.; Palmer, K.E.; Mittal, N.; Valdez, J.; Flynn, J.; Williams, S.J. Rapid detection of SARS-CoV-2 antibodies using electrochemical impedance-based detector. Biosen. Bioelectron. 2021, 171, 112709. [Google Scholar] [CrossRef]
- Vadlamani, B.S.; Uppal, T.; Verma, S.C.; Misra, M. Functionalized TiO2 nanotube-based electrochemical biosensor for rapid detection of SARS-CoV-2. Sensors 2020, 20, 5871. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, H.; Mahmud, A.; Chang, D.; Das, J.; Gomis, S.; Chen, J.B.; Wang, H.; Been, T.; Yip, L.; Coomes, E.; et al. Detection of SARS-CoV-2 viral particles using direct, reagent-free electrochemical sensing. J. Am. Chem. Soc. 2021, 143, 1722–1727. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Liu, F.; Xie, W.; Zhou, T.C.; OuYang, J.; Jin, L.; Li, H.; Zhao, C.Y.; Zhang, L.; Wei, J.; et al. Ultrasensitive supersandwich-type electrochemical sensor for SARS-CoV-2 from the infected COVID-19 patients using a smartphone. Sens. Actuators B Chem. 2021, 327, 128899. [Google Scholar] [CrossRef]
- Jaewjaroenwattana, J.; Phoolcharoen, W.; Pasomsub, E.; Teengam, P.; Chailapakul, O. Electrochemical paper-based antigen sensing platform using plant-derived monoclonal antibody for detecting SARS-CoV-2. Talanta 2023, 251, 123783. [Google Scholar] [CrossRef] [PubMed]
- Adeel, M.; Asif, K.; Alshabouna, F.; Canzonieri, V.; Rahman, M.M.; Ansari, S.A.; Guder, F.; Rizzolio, F.; Daniele, S. Label-free electrochemical aptasensor for the detection of SARS-CoV-2 spike protein based on carbon cloth sputtered gold nanoparticles. Biosens. Bioelectron. X 2022, 12, 100256. [Google Scholar] [CrossRef] [PubMed]
- Mavrikou, S.; Moschopoulou, G.; Tsekouras, V.; Kintzios, S. Development of a portable, ultra-rapid and ultra-sensitive cell-based biosensor for the direct detection of the SARS-CoV-2 S1 spike protein antigen. Sensors 2020, 20, 3121. [Google Scholar] [CrossRef]
- Cancino-Bernardi, J.; Comparetti, E.J.; Ferreira, N.N.; Miranda, R.R.; Tuesta, M.M.; Sampaio, I.; da Costa, P.I.; Zucolotto, V. A SARS-CoV-2 impedimetric biosensor based on the immobilization of ACE-2 receptor-containing entire cell membranes as the biorecognition element. Talanta 2023, 253, 124008. [Google Scholar] [CrossRef]
- Sandoval Bojórquez, D.I.; Janićijević, Z.; Palestina Romero, B.; Oliveros Mata, E.S.; Laube, M.; Feldmann, A.; Kegler, A.; Drewitz, L.; Fowley, C.; Pietzsch, J.; et al. Impedimetric Nanobiosensor for the Detection of SARS-CoV-2 Antigens and Antibodies. ACS Sens. 2023, 8, 576–586. [Google Scholar] [CrossRef] [PubMed]
- Seo, G.; Lee, G.; Kim, M.J.; Baek, S.H.; Choi, M.; Ku, K.B.; Lee, C.-S.; Jun, S.; Park, D.; Kim, H.G.; et al. Rapid detection of COVID-19 causative virus (SARS-CoV-2) in human nasopharyngeal swab specimens using field-effect transistor-based biosensor. ACS Nano 2020, 14, 5135–5142. [Google Scholar] [CrossRef] [Green Version]
- Raziq, A.; Kidakova, A.; Boroznjak, R.; Reut, J.; Öpik, A.; Syritski, V. Development of a portable MIP-based electrochemical sensor for detection of SARS-CoV-2 antigen. Biosens. Bioelectron. 2021, 178, 113029. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, P.; Singhal, A.; Yadav, S.; Kumar, N.; Murali, S.; Sanghi, S.K.; Khan, R. Rapid diagnosis of SARS-CoV-2 using potential point-of-care electrochemical immunosensor: Toward the future prospects. Int. Rev. Immunol. 2021, 40, 126–142. [Google Scholar] [CrossRef] [PubMed]
- Mahshid, S.S.; Flynn, S.E.; Mahshid, S. The potential application of electrochemical biosensors in the COVID-19 pandemic: A perspective on the rapid diagnostics of SARS-CoV-2. Biosen. Bioelectron. 2021, 176, 112905. [Google Scholar] [CrossRef] [PubMed]
- AAT Bioquest. Buffer Preparations and Recipes. Available online: https://www.aatbio.com/resources/buffer-preparations-and-recipes/ (accessed on 14 May 2020).
- Dhand, C.; Das, M.; Datta, M.; Malhotra, B.D. Recent advances in polyaniline based biosensors. Biosen. Bioelectron. 2011, 26, 2811–2821. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Xu, B.; Ye, L.; Di, Z.; Zhang, J.; Jin, Q.; Zhao, J.; Xue, J.; Chen, X. Immobilization of biomolecules on cysteamine-modified polyaniline film for highly sensitive biosensing. Talanta 2014, 120, 462–469. [Google Scholar] [CrossRef]
- Sai, V.V.R.; Mahajan, S.; Contractor, A.Q.; Mukherji, S. Immobilization of antibodies on polyaniline films and its application in a piezoelectric immunosensor. Anal. Chem. 2006, 78, 8368–8373. [Google Scholar] [CrossRef]
- De Melo, J.V.; Bello, M.E.; De Azevedo, W.M.; De Souza, J.M.; Diniz, F.B. The effect of glutaraldehyde on the electrochemical behavior of polyaniline. Electrochim. Acta 1999, 44, 2405–2412. [Google Scholar] [CrossRef]
- Grothe, R.A.; Lobato, A.; Mounssef, B.; Tasić, N.; Braga, A.A.; Maldaner, A.O.; Maldaner, A.O.; Aldous, L.; Paixão, T.R.L.C.; Gonçalves, L.M. Electroanalytical profiling of cocaine samples by means of an electropolymerized molecularly imprinted polymer using benzocaine as the template molecule. Analyst 2021, 146, 1747–1759. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.W.; Kwon, S.R.; Lutkenhaus, J.L. Polyaniline nanofiber/electrochemically reduced graphene oxide layer-by-layer electrodes for electrochemical energy storage. J. Mater. Chem. A 2015, 7, 3757. [Google Scholar] [CrossRef]
- Coetzee, J.; Van der Merwe, C.F. Some characteristics of the buffer vehicle in glutaraldehyde-based fixatives. J. Microsc. 1987, 146, 143–155. [Google Scholar] [CrossRef]
- Luisetto, M.; Tarro, G.; Edbey, K.; Khan, F.A.; Yesvi, A.R.; Nili, B.A.; Fiazza, C.; Mashori, G.R.; Latyshev, O.V. Coronavirus COVID-19 surface properties: Electrical charges status. Int. J. 2021, 2766, 3264. [Google Scholar] [CrossRef]
- Al Sarkhi, A.K. Hypothesis: The electrical properties of coronavirus. Electromagn. Biol. Med. 2020, 39, 433–436. [Google Scholar] [CrossRef]
- Torrente-Rodríguez, R.M.; Lukas, H.; Tu, J.; Min, J.; Yang, Y.; Xu, C.; Rossiter, H.B.; Gao, W. SARS-CoV-2 RapidPlex: A Graphene-Based Multiplexed Telemedicine Platform for Rapid and Low-Cost COVID-19 Diagnosis and Monitoring. Matter 2020, 3, 1981–1998. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.A.; Hu, C.; Jahan, S.; Yuan, B.; Saleh, M.S.; Ju, E.; Gao, S.-J.; Panat, R. Sensing of COVID-19 Antibodies in Seconds via Aerosol Jet Nanoprinted Reduced-Graphene-Oxide-Coated 3D Electrodes. Adv. Mater. 2021, 33, 2006647. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Xiao, Z.; Wang, T.; Wang, J.; Bie, L.; Saleh, L.; Frey, K.; Zhang, L.; Wang, J. Rapid and sensitive multiplex detection of COVID-19 antigens and antibody using electrochemical immunosensor-/aptasensor-enabled biochips. Chem. Commun. 2022, 58, 7285–7288. [Google Scholar] [CrossRef] [PubMed]
- Liustrovaite, V.; Drobysh, M.; Rucinskiene, A.; Baradoke, A.; Ramanaviciene, A.; Plikusiene, I.; Samukaite-Bubniene, U.; Viter, R.; Chen, C.-F.; Ramanavicius, A. Towards an electrochemical immunosensor for the detection of antibodies against SARS-CoV-2 spike protein. J. Electrochem. Soc. 2022, 169, 037523. [Google Scholar] [CrossRef]
- Shoute, L.C.; Abdelrasoul, G.N.; Ma, Y.; Duarte, P.A.; Edwards, C.; Zhuo, R.; Zeng, J.; Feng, Y.; Charlton, C.L.; Kanji, J.N.; et al. Label-free impedimetric immunosensor for point-of-care detection of COVID-19 antibodies. Microsyst. Nanoeng. 2023, 9, 3. [Google Scholar] [CrossRef]
- Yakoh, A.; Pimpitak, U.; Rengpipat, S.; Hirankarn, N.; Chailapakul, O.; Chaiyo, S. Paper-based electrochemical biosensor for diagnosing COVID-19: Detection of SARS-CoV-2 antibodies and antigen. Biosens. Bioelectron. 2021, 176, 112912. [Google Scholar] [CrossRef] [PubMed]
- Rossen, R.D.; Schade, A.L.; Butler, W.T.; Kasel, J.A. The proteins in nasal secretion: A longitudinal study of the gammaA-globulin, gammaG-globulin, albumin, siderophilin, and total protein concentrations in nasal washings from adult male volunteers. J. Clin. Investig. 1966, 45, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Leeman, M.; Choi, J.; Hansson, S.; Storm, M.U.; Nilsson, L. Proteins and antibodies in serum, plasma, and whole blood—Size characterization using asymmetrical flow field-flow fractionation (AF4). Anal. Bioanal. Chem. 2018, 410, 4867–4873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, X.; Li, C.; Huang, A.; Xia, S.; Lu, S.; Shi, Z.; Lu, L.; Jiang, S.; Yang, Z.; Wu, Y.; et al. Potent binding of 2019 novel coronavirus spike protein by a SARS coronavirus-specific human monoclonal antibody. Emerg. Microbes Infect. 2020, 9, 382–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Sensing Principle | Substrate | Recognition Element | Target | Matrix | Integrated Negative Control | RSD | Limit of Detection (LOD) | Linear Range | Advantages | Disadvantages |
|---|---|---|---|---|---|---|---|---|---|---|
| Impedance spectroscopy [14] | Commercial electrochemical 16-well plate (ACEA Biosciences xCELLigence system) | SARS-CoV-2 RBD | Human anti-SARS-CoV-2 IgG antibodies | 100× diluted human serum | Yes (1% milk solution) | Not reported | Not reported | Not reported | Label-free, Simple operation High sample, throughput, Negative control | High cost, Yes/no readout, Some key performance parameters not tested |
| Impedance spectroscopy [22] | Glass substrate with polydimethylsiloxane (PDMS) and Au nanowires | SARS-CoV-2 RBD | anti-SARS-CoV-2 IgG antibodies | 100× diluted human plasma | No | Not reported as RSD | 0.99 pg/mL | 10−6–10−16 g mL−1 | Label-free, Simultaneous detection of antigens and antibodies | Some key parameters not tested, Complex architecture |
| Amperometry (consumption of H2O2 by HRP-labeled secondary antibodies) [37] | Lab-made laser-engraved graphite electrodes | SARS-CoV-2 spike protein (S1) | Human anti-SARS-CoV-2 IgG and IgM antibodies | 100× diluted human serum | No | 8.4% for IgGs and 6.0% for IgMs | Not reported | 1–500 ng mL−1 for both IgGs and IgMs | Simple operation, Multiplex, platform for several analytes (antigen, antibodies, C-reactive protein) | LOD not tested, Observed interference from SARS-CoV nucleocapsid protein |
| Impedance spectroscopy [38] | Aerosol jet nanoprinted reduced-graphene-oxide-coated 3D electrodes | SARS-CoV-2 spike protein (S1) and RBD | Rabbit anti-SARS-CoV-2 IgG antibodies | Rabbit serum and fetal bovine serum (dilution not reported) | No | ± 6.01% (12.02% in total, author’s note) | 2.8 × 10−15 M (4.2 × 10−4 ng mL−1 *) with S1 and 16.9 × 10−15 M (2.5 × 10−3 ng mL−1 *) with RBD as the recognition element (estimated from noise) | Two ranges: 1 × 10−12 –100 × 10−12 M (0.15–15 ng mL−1 *) and 100 × 10−12 –20 × 10−9 M (15–1000 ng mL−1 *) | Label-free, Fast response, Recoverable (one device can be used for several samples) | Complex architecture, construction process and operation, High cost, Operation in human samples may be different, Limit of detection estimated from blank/noise |
| Cyclic voltammetry [39] | Au nanoparticles modified screen-printed carbon electrodes | SARS-CoV-2 spike protein (S1) | anti-SARS-CoV-2 IgG antibodies | PBS | No | Not reported | 1.28 pg mL−1 | fg mL−1 to ng mL−1 | Label-free, Fast response, Broad-dynamic range, Low cost Simple operation | No selectivity study reported |
| Cyclic voltammetry and Impedance [40] Spectroscopy | Lab-fabricated Au electrodes on microscope slides | SARS-CoV-2 spike protein | anti-SARS-CoV-2 spike antibodies | PBS and serum | No | Not reported | 0.38 μg mL−1 and 0.30 μg mL−1 | 4.5–22.5 μg mL−1 | Label-free, Simple operation | Narrow operating range, No selectivity study reported, Large custom-made electrodes, Common self-assembled monolayer protocol (SAM) |
| Impedance spectroscopy [41] | Lab-made gold interdigitated microelectrode array | Trimeric SARS-CoV-2 spike protein | Total anti-SARS-CoV-2 spike antibodies | Human serum | No | Not reported | 0.4 BAU mL−1 (upper detection limit >100 BAU mL−1) *BAU corresponds to binding antibody units | 1–100 BAU mL−1 | Non-Faradaic mode of operation (capacitive sensing), Label-free | Complex fabrication procedure, Common self-assembled monolayer protocol (SAM) |
| Square-wave voltammetry (attenuation of a redox probe signal after antibody binding) [42] | Lab-made, paper-based screen-printed graphene electrodes | SARS-CoV-2 RBD | Human anti-SARS-CoV-2 IgG and IgM antibodies | Human serum (dilution not reported) | No | 4.2% for IgGs and 3.3% for IgMs | 0.96 ng mL−1 for IgGs and 0.14 ng mL−1 for IgMs (estimated from noise) | 1–1000 ng mL−1 (logarithm scale) for both IgGs and IgMs | Simple operation, Multiplex platform for several analytes (antigen and antibodies), Low cost | Limit of detection estimated from blank/noise |
| Impedance spectroscopy [this study] | Commercial screen-printed carbon electrodes | SARS-CoV-2 RBD | Human anti-SARS-CoV-2 IgG antibodies Kd = 6.3 nM [45] | 20× diluted human serum | Yes (BSA) | ca. 10% for 0.1 μg mL−1 data point | 26 pM (3.9 ng mL−1) and 0.9 pM (0.13 ng mL−1) in amplification mode | 0.01–10 μg mL−1 and 0.001–10 10 μg mL−1 in amplification mode | Label-free, Simple operation, Simple to prepare, Integrated negative control unit, Possible signal amplification | Some degree of non-specific binding when using the amplification protocol |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romih, T.; Tasić, N.; Bibič, L.; Beltram, A.; Fazarinc, I.; Benčina, M.; Jerala, R.; Hočevar, S.B. RBD-Modified Polyaniline-Based Label-Free Immunosensor for Sensitive Impedimetric Detection of Anti-SARS-CoV-2 Antibodies. Chemosensors 2023, 11, 222. https://doi.org/10.3390/chemosensors11040222
Romih T, Tasić N, Bibič L, Beltram A, Fazarinc I, Benčina M, Jerala R, Hočevar SB. RBD-Modified Polyaniline-Based Label-Free Immunosensor for Sensitive Impedimetric Detection of Anti-SARS-CoV-2 Antibodies. Chemosensors. 2023; 11(4):222. https://doi.org/10.3390/chemosensors11040222
Chicago/Turabian StyleRomih, Tea, Nikola Tasić, Lea Bibič, Ajda Beltram, Ika Fazarinc, Mojca Benčina, Roman Jerala, and Samo B. Hočevar. 2023. "RBD-Modified Polyaniline-Based Label-Free Immunosensor for Sensitive Impedimetric Detection of Anti-SARS-CoV-2 Antibodies" Chemosensors 11, no. 4: 222. https://doi.org/10.3390/chemosensors11040222