The Detection of 27 Fentanyl Compounds in Solid and Liquid Drugs Based on Differential Raman Spectroscopy
Abstract
:1. Introduction
2. Experiments and Materials
3. Results and Discussion
3.1. Preparation and Characterization of SERS-Enhanced Substrates
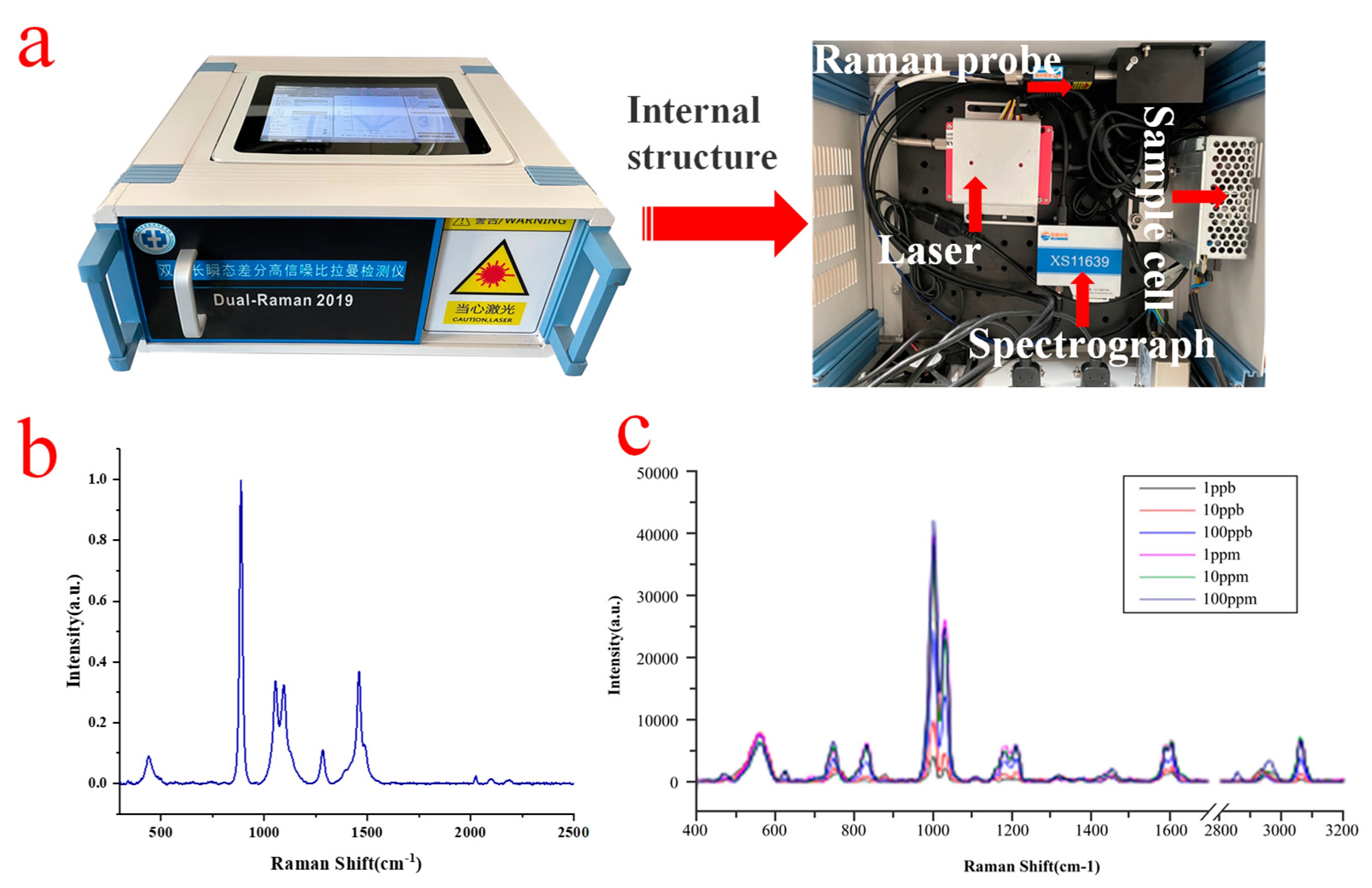
3.2. POCT Device and Optimization of Spectral Raman Detection Technique
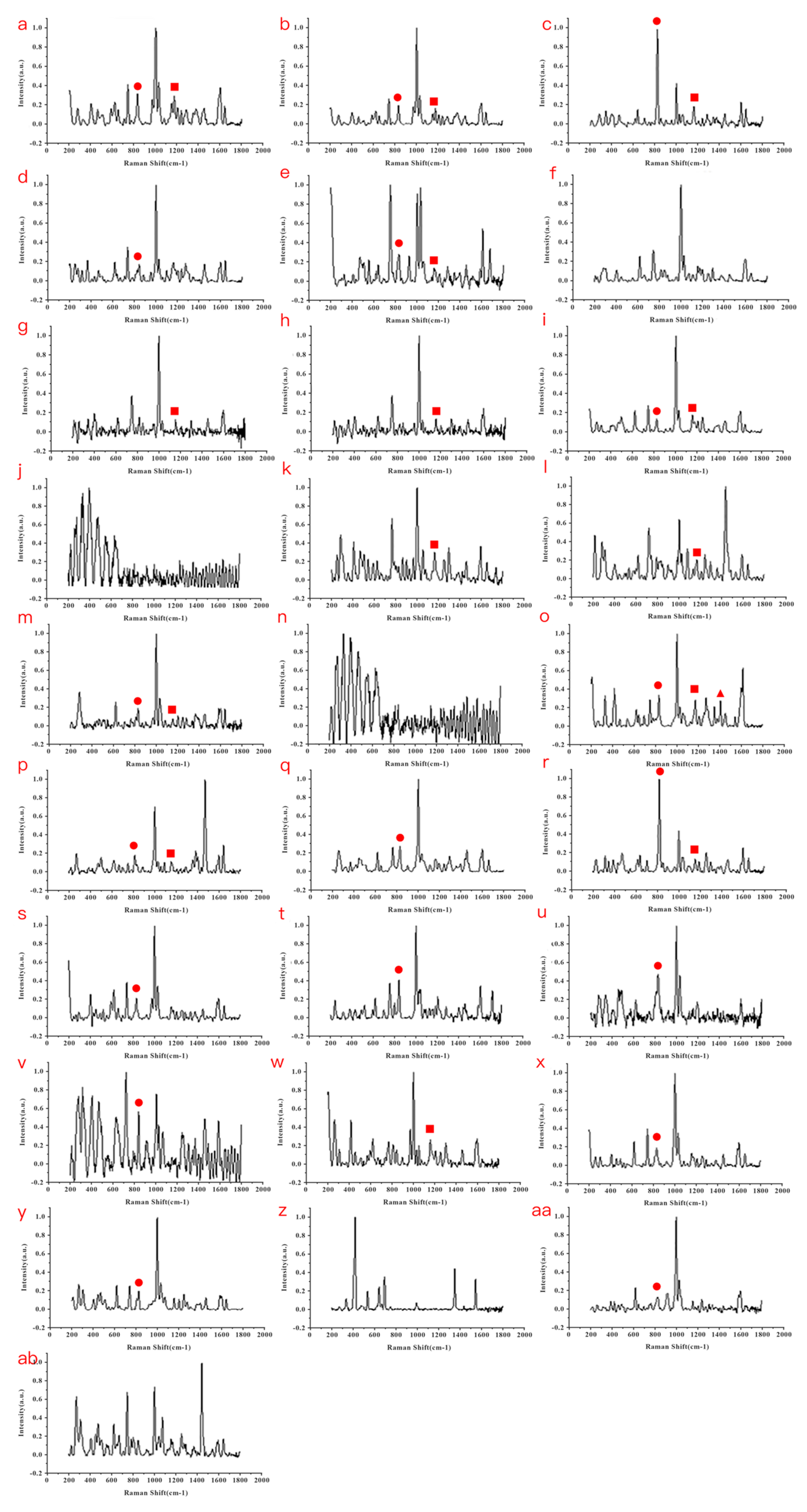
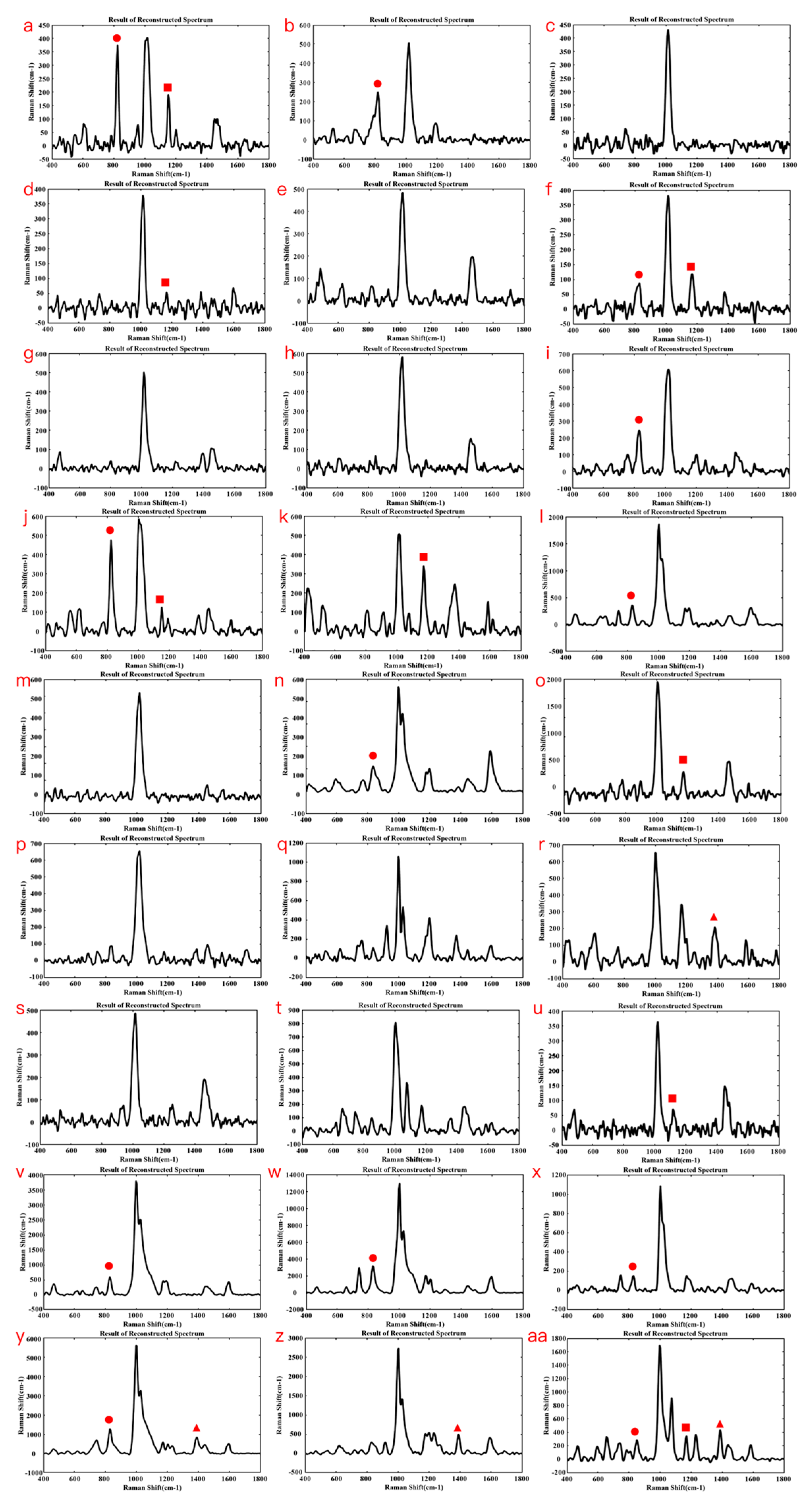
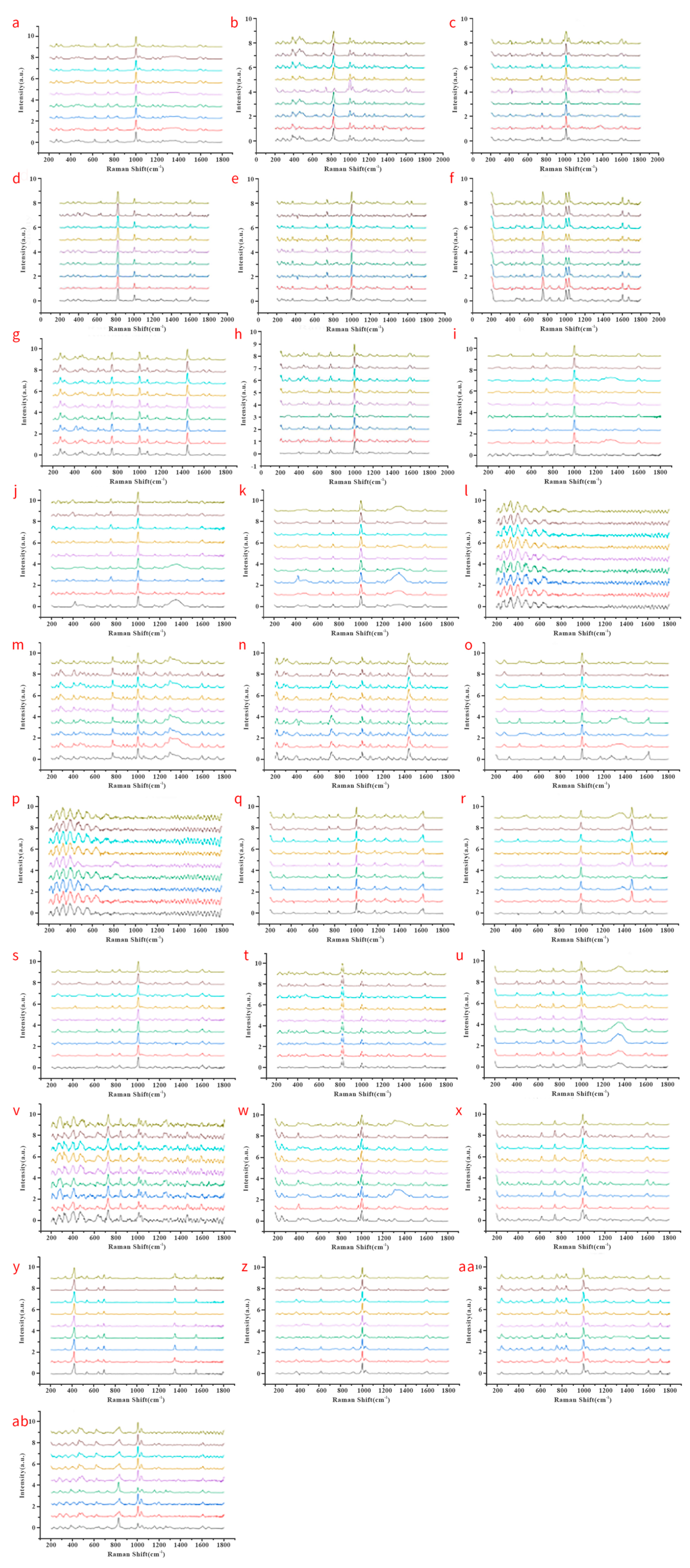
3.3. Establishment of a Fentanyl POCT Detecting Method
3.4. Screening Application of Fentanyl-like Substances by POCT Raman Spectroscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peterson, A.B.; Gladden, R.M.; Delcher, C.; Spies, E.; Garcia-Williams, A.; Wang, Y.; Halpin, J.; Zibbell, J.; McCarty, C.L.; DeFiore-Hyrmer, J. Increases in fentanyl-related overdose deaths—Florida and Ohio, 2013–2015. Morb. Mortal. Wkly. Rep. 2016, 65, 844–849. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Increases in Fentanyl Drug Confiscations and Fentanyl-Related Overdose Fatalities. 26 October 2015. Available online: https://app.overton.io/document.php?policy_document_id=cdc-5fb7370ab681a48a2a4b85bd69b9f3a4&funder_highlight=U.S.%20Department%20of%20Health%20and%20Human%20Services (accessed on 1 January 2022).
- Salani, D.; Mckay, M.; Zdanowicz, M. The deadly trio: Heroin, FentaNYL, and carfentanil. J. Emerg. Nurs. 2020, 46, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Spencer, M.R.; Warner, M.; Bastian, B.A.; Trinidad, J.P.; Hedegaard, H. Drug overdose deaths involving fentanyl, 2011–2016. Natl. Vital Stat. Rep. Cent. Dis. Control Prev. Natl. Cent. Health Stat. Natl. Vital Stat. Syst. 2019, 68, 1–19. [Google Scholar]
- Ebrahimzadeh, H.; Yamini, Y.; Gholizade, A.; Sedighi, A.; Kasraee, S. Determination of fentanyl in biological and water samples using single-drop liquid–liquid–liquid microextraction coupled with high-performance liquid chromatography. Anal. Chim. Acta 2008, 626, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Su, F.H. Determination of Nine Fentanyl Drugs in Hair Samples by GC-MS/MS and LC-MS/MS. ACS Omega 2022, 7, 19176–19182. [Google Scholar] [CrossRef]
- Mochizuki, A.; Nakazawa, H.; Adachi, N.; Takekawa, K.; Shojo, H. Identification and quantification of mepirapim and acetyl fentanyl in authentic human whole blood and urine samples by GC–MS/MS and LC–MS/MS. Forensic Toxicol. 2018, 36, 81–87. [Google Scholar] [CrossRef]
- Gilbert, N.; Antonides, L.H.; Schofield, C.J.; Costello, A.; Kilkelly, B.; Cain, A.R.; Dalziel, P.R.; Horner, K.; Mewis, R.E.; Sutcliffe, O.B. Hitting the Jackpot–development of gas chromatography–mass spectrometry (GC–MS) and other rapid screening methods for the analysis of 18 fentanyl-derived synthetic opioids. Drug Test. Anal. 2020, 12, 798–811. [Google Scholar] [CrossRef]
- Strayer, K.E.; Antonides, H.M.; Juhascik, M.P.; Daniulaityte, R.; Sizemore, I.E. LC-MS/MS-based method for the multiplex detection of 24 fentanyl analogues and metabolites in whole blood at sub ng mL−1 concentrations. ACS Omega 2018, 3, 514–523. [Google Scholar] [CrossRef]
- Swaminathan, S.K.; Fisher, J.; Kandimalla, K.K. Sensitive determination of fentanyl in low-volume serum samples by LC-MS/MS. AAPS PharmSciTech 2018, 19, 2812–2817. [Google Scholar] [CrossRef]
- Guerrieri, D.; Kjellqvist, F.; Kronstrand, R.; Gréen, H. Validation and cross-reactivity data for fentanyl analogs with the immunalysis fentanyl ELISA. J. Anal. Toxicol. 2019, 43, 18–24. [Google Scholar] [CrossRef]
- Abbott, D.L.; Limoges, J.F.; Virkler, K.J.; Tracy, S.J.; Sarris, G.G. ELISA screens for fentanyl in urine are susceptible to false-positives in high concentration methamphetamine samples. J. Anal. Toxicol. 2022, 46, 457–459. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Pan, J.; Xu, X.; Fu, G.; Zhang, L.; Sun, P.; Yan, X.; Liu, F.; Wang, C.; Liu, X. Gold-trisoctahedra-coated capillary-based SERS platform for microsampling and sensitive detection of trace fentanyl. Anal. Chem. 2022, 94, 4850–4858. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Liu, X.; Xie, Y.; Chen, M.; Zhong, H.; Li, M. Quantitative Label-Free SERS Detection of Trace Fentanyl in Biofluids with a Freestanding Hydrophobic Plasmonic Paper Biosensor. Anal. Chem. 2023, 95, 3821–3829. [Google Scholar] [CrossRef]
- Ding, Z.; Wang, C.; Song, X.; Li, N.; Zheng, X.; Wang, C.; Su, M.; Liu, H. Strong π-Metal Interaction Enables Liquid Interfacial Nanoarray–Molecule Co-assembly for Raman Sensing of Ultratrace Fentanyl Doped in Heroin, Ketamine, Morphine, and Real Urine. ACS Appl. Mater. Interfaces 2023, 15, 12570–12579. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Miao, Q.; Zhang, X.; Cui, P.; Wang, C.; Feng, Y.; Gan, L.; Fu, J.; Wang, S.; Dai, Z.; et al. Single-atom sites on perovskite chips for record-high sensitivity and quantification in SERS. Sci. China Mater. 2022, 65, 1601–1614. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, X.; Cui, F.; Liu, J.; Li, X. Development and Application of Fluorescence Suppression Based on Multi Wavelength Raman Spectrometer. Spectrosc. Spectr. Anal. 2022, 42, 86–92. [Google Scholar]
- Mažeikienė, R.; Niaura, G.; Malinauskas, A. Study of deprotonation processes of polyaniline by differential multiwavelength Raman spectroscopy in an electrochemical system. Chemija 2019, 30, 4. [Google Scholar] [CrossRef]
- Oztekin, E.K.; Burton, D.J.; Hahn, D.W. Detection of explosives using differential laser-induced perturbation spectroscopy with a Raman-based probe. Appl. Spectrosc. 2016, 70, 676–687. [Google Scholar] [CrossRef]
- Wang, Y.; Zou, M.; Chen, Y.; Tang, F.; Dai, J.; Jin, Y.; Wang, C.; Xue, F. Ultrasensitive and selective detection of sulfamethazine in milk via a Janus-labeled Au nanoparticle-based surface-enhanced Raman scattering-immunochromatographic assay. Talanta 2023, 267, 125208. [Google Scholar]
- Yu, B.; Ge, M.; Li, P.; Xie, Q.; Yang, L. Development of surface-enhanced Raman spectroscopy application for determination of illicit drugs: Towards a practical sensor. Talanta 2019, 191, 1–10. [Google Scholar] [CrossRef]
- Inscore, F.; Shende, C.; Sengupta, A.; Huang, H.; Farquharson, S. Detection of drugs of abuse in saliva by surface-enhanced Raman spectroscopy (SERS). Appl. Spectrosc. 2011, 65, 1004–1008. [Google Scholar] [CrossRef] [PubMed]
- Stanley, T.H. The fentanyl story. J. Pain 2014, 15, 1215–1226. [Google Scholar] [CrossRef]
- Mirsafavi, R.; Moskovits, M.; Meinhart, C. Detection and classification of fentanyl and its precursors by surface-enhanced Raman spectroscopy. Analyst 2020, 145, 3440–3446. [Google Scholar] [CrossRef]
- Melent’ev, A.; Kataev, S. Metabolism of designer drugs. The fentanyl derivatives. Sudebno-Meditsinskaia Ekspertiza 2015, 58, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Wang, S.; Zhang, Y.; Li, B.; Lu, M.; Qi, X.; Wei, H.; Li, Y.; Zou, M. Surface-enhanced shifted excitation Raman difference spectroscopy for trace detection of fentanyl in beverages. Appl. Opt. 2021, 60, 2354–2361. [Google Scholar] [CrossRef] [PubMed]


| Serial Number | Sample | Type | Substrate | CAS Number | Detection Limit (ppb) |
|---|---|---|---|---|---|
| 1 | 4-fluorobutanyl fentanyl | Standard | methanol | 244195-31-1 | 10 |
| 2 | 4-fluoroisobutyrl fentanyl | Standard | methanol | 244195-32-2 | 10 |
| 3 | Butyryl fentanyl | Standard | methanol | 1169-70-6 | 10 |
| 4 | Isobutylfentanyl hydrochloride | hydrochloride | methanol | 117332-90-87 | 10 |
| 5 | Furanfentanyl hydrochloride | hydrochloride | methanol | 101365-56-4 | 0.1 |
| 6 | Pentanyl fentanyl hydrochloride | hydrochloride | methanol | 117332-91-9 | 25 |
| 7 | β-hydroxythiofentanyl | Standard | methanol | NA | 10 |
| 8 | Cis-3-methylfentanyl hydrochloride | hydrochloride | methanol | 78995-18-3 | 10 |
| 9 | Ocfentanil | Standard | methanol | 10343-69-5 | 10 |
| 10 | To the flufentanil | Standard | methanol | 90736-23-5 | 10 |
| 11 | Sufentanil citrate | Standard | methanol | 60561-17-3 | 10 |
| 12 | Acetyl fentanyl | Standard | methanol | 3258-84-2 | 10 |
| 13 | β-hydroxy-3-methylfentanyl | Standard | methanol | 78995-14-9 | 10 |
| 14 | 4-anilinegroup-N-phenylethyl piperidine | Precursor | methanol | 21409-26-7 | 10 |
| 15 | Remifentanil hydrochloride | hydrochloride | methanol | 132539-07-2 | 10 |
| 16 | Alpha-methylfentanyl hydrochloride | hydrochloride | methanol | 1443-44-3 | 10 |
| 17 | N-phenylethyl-4-piperidone | Precursor | acetonitrile | 39742-60-4 | 10 |
| 18 | Carfentanil | Standard | methanol | 59708-52-0 | 10 |
| 19 | Beta-hydroxyfentanyl | Standard | methanol | 1473-95-6 | 10 |
| 20 | 3-methylthiofentanyl | Standard | methanol | 86052-04-2 | 10 |
| 21 | alfentanil | Standard | methanol | 69049-06-5 | 1 |
| 22 | fentanyl | Standard | methanol | 437-38-7 | 10 |
| 23 | Acetyl alfa methyl fentanyl | Standard | methanol | 101860-00-8 | 10 |
| 24 | Allanyl fentanyl hydrochloride | Standard | methanol | 79279-03-1 | 10 |
| 25 | Thiofentanyl hydrochloride | Standard | methanol | 79278-88-9 | 10 |
| 26 | Alfa methyl thiofentanyl | Standard | methanol | 117332-94-2 | 100 |
| 27 | Tetrahydrofuran fentanyl | Standard | methanol | NA | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Sheng, W.; Liu, X.; Guo, J.; Zhang, X.; Qi, X.; Zou, M.; Wang, C. The Detection of 27 Fentanyl Compounds in Solid and Liquid Drugs Based on Differential Raman Spectroscopy. Chemosensors 2023, 11, 561. https://doi.org/10.3390/chemosensors11110561
Wang Y, Sheng W, Liu X, Guo J, Zhang X, Qi X, Zou M, Wang C. The Detection of 27 Fentanyl Compounds in Solid and Liquid Drugs Based on Differential Raman Spectroscopy. Chemosensors. 2023; 11(11):561. https://doi.org/10.3390/chemosensors11110561
Chicago/Turabian StyleWang, Yufeng, Wanli Sheng, Xiang Liu, Jiajuan Guo, Xun Zhang, Xiaohua Qi, Mingqiang Zou, and Cong Wang. 2023. "The Detection of 27 Fentanyl Compounds in Solid and Liquid Drugs Based on Differential Raman Spectroscopy" Chemosensors 11, no. 11: 561. https://doi.org/10.3390/chemosensors11110561





