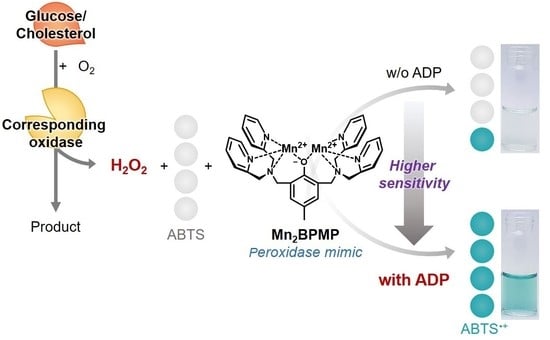
Application of Peroxidase-Mimic Mn2BPMP Boosted by ADP to Enzyme Cascade Assay for Glucose and Cholesterol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Instrumentation
2.2. Effect of Adenosine Phosphates on the Peroxidase-like Activity of Mn2BPMP
2.3. Effect of ADP on the Peroxidase-like Activity of Mn2BPMP
2.4. Colorimetric Detection of H2O2 in the Presence or Absence of ADP
2.5. Colorimetric Detection of Glucose and Selectivity/Interference Experiments in the Presence or Absence of ADP
2.6. Colorimetric Detection of Glucose in Human Serum
2.7. Colorimetric Detection of Cholesterol in the Presence or Absence of ADP
3. Results and Discussion
3.1. Effect of ADP on the Peroxidase-like Activity of Mn2BPMP
3.2. Colorimetric Detection of Glucose Using the ADP/Mn2BPMP/ABTS/GOx System
3.3. Colorimetric Detection of Cholesterol Using the ADP/Mn2BPMP/ABTS/ChOx System
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Delvaux, M.; Walcarius, A.; Demoustier-Champagne, S. Bienzyme HRP-GOx-modified gold nanoelectrodes for the sensitive amperometric detection of glucose at low overpotentials. Biosens. Bioelectron. 2005, 20, 1587–1594. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.W.; Phillips, S.T.; Butte, M.J.; Whitesides, G.M. Patterned Paper as a Platform for Inexpensive, Low-Volume, Portable Bioassays. Angew. Chem.-Int. Ed. 2007, 119, 1340–1342. [Google Scholar] [CrossRef]
- Han, M.; Liu, S.; Bao, J.; Dai, Z. Pd nanoparticle assemblies-as the substitute of HRP, in their biosensing applications for H2O2 and glucose. Biosens. Bioelectron. 2012, 31, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, T.P.; Thorpe, G.H.G.; Carter, T.J.N.; Groucutt, C.; Kricka, L.J. Enhanced luminescence procedure for sensitive determination of peroxidase-labelled conjugates in immunoassay. Nature 1983, 305, 158–159. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, J.; Lin, J. 3,3’-diaminobenzidine (DAB)-H2O2-HRP voltammetric enzyme-linked immunoassay for the detection of carcionembryonic antigen. Bioelectrochemistry 2008, 72, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Zeng, G.-M.; Shen, G.-L.; Li, Y.-P.; Zhang, Y.; Huang, D.-L. Rapid Detection of Picloram in Agricultural Field Samples Using a Disposable Immunomembrane-Based Electrochemical Sensor. Environ. Sci. Technol. 2008, 42, 1207–1212. [Google Scholar] [CrossRef] [PubMed]
- Mir, M.; Vreeke, M.; Katakis, I. Different strategies to develop an electrochemical thrombin aptasensor. Electrochem. Commun. 2006, 8, 505–511. [Google Scholar] [CrossRef]
- Zhang, D.W.; Sun, C.J.; Zhang, F.T.; Xu, L.; Zhou, Y.L.; Zhang, X.X. An electrochemical aptasensor based on enzyme linked aptamer assay. Biosens. Bioelectron. 2012, 31, 363–368. [Google Scholar] [CrossRef]
- Bai, L.; Chai, Y.; Yuan, R.; Yuan, Y.; Xie, S.; Jiang, L. Amperometric aptasensor for thrombin detection using enzyme-mediated direct electrochemistry and DNA-based signal amplification strategy. Biosens. Bioelectron. 2013, 50, 325–330. [Google Scholar] [CrossRef]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef]
- Su, L.; Qin, W.; Zhang, H.; Rahman, Z.U.; Ren, C.; Ma, S.; Chen, X. The peroxidase/catalase-like activities of MFe2O4 (M = Mg, Ni, Cu) MNPs and their application in colorimetric biosensing of glucose. Biosens. Bioelectron. 2015, 63, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, W.; Liu, A.L.; Hong, L.; Deng, H.H.; Lin, X.H. Comparison of the peroxidase-like activity of unmodified, amino-modified, and citrate-capped gold nanoparticles. ChemPhysChem 2012, 13, 1199–1204. [Google Scholar] [CrossRef] [PubMed]
- Ni, P.; Dai, H.; Wang, Y.; Sun, Y.; Shi, Y.; Hu, J.; Li, Z. Visual detection of melamine based on the peroxidase-like activity enhancement of bare gold nanoparticles. Biosens. Bioelectron. 2014, 60, 286–291. [Google Scholar] [CrossRef]
- Zhang, J.W.; Zhang, H.T.; Du, Z.Y.; Wang, X.; Yu, S.H.; Jiang, H.L. Water-stable metal-organic frameworks with intrinsic peroxidase-like catalytic activity as a colorimetric biosensing platform. Chem. Commun. 2014, 50, 1092–1094. [Google Scholar] [CrossRef] [PubMed]
- Ai, L.; Li, L.; Zhang, C.; Fu, J.; Jiang, J. MIL-53(Fe): A metal-organic framework with intrinsic peroxidase-like catalytic activity for colorimetric biosensing. Chem. Eur. J. 2013, 19, 1515–14108. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Li, Q.; Zhou, Z.; Ma, C.; Song, Y.; Xu, F.; Wang, L. A sensitive fluorescent assay for thiamine based on metal-organic frameworks with intrinsic peroxidase-like activity. Anal. Chim. Acta 2015, 856, 90–95. [Google Scholar] [CrossRef]
- Dong, Y.L.; Zhang, H.G.; Rahman, Z.U.; Su, L.; Chen, X.J.; Hu, J.; Chen, X.G. Graphene oxide-Fe3O4 magnetic nanocomposites with peroxidase-like activity for colorimetric detection of glucose. Nanoscale 2012, 4, 3969–3976. [Google Scholar] [CrossRef]
- Tao, Y.; Lin, Y.; Huang, Z.; Ren, J.; Qu, X. Incorporating graphene oxide and gold nanoclusters: A synergistic catalyst with surprisingly high peroxidase-like activity over a broad pH range and its application for cancer cell detection. Adv. Mater. 2013, 25, 2594–2599. [Google Scholar] [CrossRef]
- Li, R.; Zhen, M.; Guan, M.; Chen, D.; Zhang, G.; Ge, J.; Gong, P.; Wang, C.; Shu, C. A novel glucose colorimetric sensor based on intrinsic peroxidase-like activity of C60-carboxyfullerenes. Biosens. Bioelectron. 2013, 47, 502–507. [Google Scholar] [CrossRef]
- Wang, Q.; Ma, K.; Yu, Z.; Ding, J.; Hu, Q.; Liu, Q.; Sun, H.; Wen, D.; Liu, Q.; Kong, J. The peroxidase-like catalytic activity of ferrocene and its application in the biomimetic synthesis of microsphere polyaniline. New J. Chem. 2018, 42, 13536–13540. [Google Scholar] [CrossRef]
- Liu, L.; Shi, Y.; Yang, Y.; Li, M.; Long, Y.; Huang, Y.; Zheng, H. Fluorescein as an artificial enzyme to mimic peroxidase. Chem. Commun. 2016, 52, 13912–13915. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Yoo, S.; Kang, S.; Hong, S.; Han, M.S. An [Mn2(bpmp)]3+ complex as an artificial peroxidase and its applications in colorimetric pyrophosphate sensing and cascade-type pyrophosphatase assay. Analyst 2018, 143, 1780–1785. [Google Scholar] [CrossRef]
- Kang, S.; Park, B.Y.; Lee, S.; Lee, N.; Han, M.S. Colorimetric discrimination of nucleoside phosphates based on catalytic signal amplification strategy and its application to related enzyme assays. Analyst 2021, 146, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.-M.; Xu, J.; Shen, H.-X. Positive Effects of ATP on G-Quadruplex-Hemin DNAzyme-Mediated Reactions. Anal. Chem. 2010, 82, 6148–6153. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.M.; Liu, X.F.; Kong, D.M.; Shen, H.X. A simple, post-additional antioxidant capacity assay using adenosine triphosphate-stabilized 2,2’-azinobis(3-ethylbenzothiazoline)-6-sulfonic acid (ABTS) radical cation in a G-quadruplex DNAzyme catalyzed ABTS-H2O2 system. Biosens. Bioelectron. 2012, 35, 407–412. [Google Scholar] [CrossRef]
- Heller, A.; Feldman, B. Electrochemical Glucose Sensors and Their Applications in Diabetes Management. Chem. Rev. 2008, 108, 2482–2505. [Google Scholar] [CrossRef] [Green Version]
- Bourbonnais, R.; Leech, D.; Paice, M.G. Electrochemical analysis of the interactions of laccase mediators with lignin model compounds. Biochim. Biophys. Acta-Gen. Subj. 1998, 1379, 381–390. [Google Scholar] [CrossRef]
- Motonaka, J.; Faulkner, L.R. Determination of cholesterol and cholesterol ester with novel enzyme microsensors. Anal. Chem. 1993, 65, 3258–3261. [Google Scholar] [CrossRef]
- Martin, S.P.; Lamb, D.J.; Lynch, J.M.; Reddy, S.M. Enzyme-based determination of cholesterol using the quartz crystal acoustic wave sensor. Anal. Chim. Acta 2003, 487, 91–100. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, N.; Yoo, S.; Lee, Y.; Han, M.S. Application of Peroxidase-Mimic Mn2BPMP Boosted by ADP to Enzyme Cascade Assay for Glucose and Cholesterol. Chemosensors 2022, 10, 89. https://doi.org/10.3390/chemosensors10020089
Lee N, Yoo S, Lee Y, Han MS. Application of Peroxidase-Mimic Mn2BPMP Boosted by ADP to Enzyme Cascade Assay for Glucose and Cholesterol. Chemosensors. 2022; 10(2):89. https://doi.org/10.3390/chemosensors10020089
Chicago/Turabian StyleLee, Namgeol, Soyeon Yoo, Youngkeun Lee, and Min Su Han. 2022. "Application of Peroxidase-Mimic Mn2BPMP Boosted by ADP to Enzyme Cascade Assay for Glucose and Cholesterol" Chemosensors 10, no. 2: 89. https://doi.org/10.3390/chemosensors10020089