Detection of Azo Dyes Using Carbon Dots from Olive Mill Wastes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Instruments and Methods
2.2. Materials
3. Results and Discussion
3.1. Synthesis and Characterization of WP-CDs
3.2. Photophysical Properties of WP-CDs
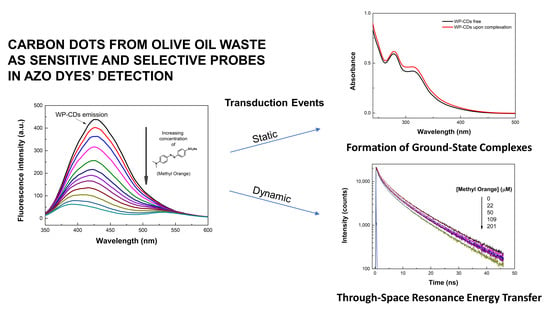
3.3. Sensing of Dyes, Transition Metal Cations, and Anions by WP-CDs
3.4. Sensitivity of WP-CDs toward Methyl Orange Dye
3.5. Quenching Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Summary Color Additives for Use in the United States in Foods, Drugs, Cosmetics and Medical Devices. Available online: https://www.fda.gov/industry/color-additive-inventories/summary-color-additives-use-united-states-foods-drugs-cosmetics-and-medical-devices#ftnote3 (accessed on 10 March 2022).
- Regulation (EC) Nº 1333/2008 of the European Parliament and of the Council of 16 December 2008 on Food Additives. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:02008R1333-20200702#tocId52 (accessed on 10 March 2022).
- Nguyen, T.V.; Vu, D.C.; Pham, V.H.; Pham, T.B.; Pham, V.H.; Bui, H. Improvement of SERS for detection of ultra-low concentration of methyl orange by nanostructured silicon decorated with Ag nanoparticles. Optik 2021, 231, 166431. [Google Scholar] [CrossRef]
- Wu, L.; Pu, H.; Huang, L.; Sun, D.W. Plasmonic nanoparticles on metal-organic framework: A versatile SERS platform for adsorptive detection of new coccine and orange II dyes in food. Food Chem. 2020, 328, 127105. [Google Scholar] [CrossRef] [PubMed]
- Fang, G.; Wu, Y.; Dong, X.; Liu, C.; He, S.; Wang, S. Simultaneous determination of banned acid orange dyes and basic orange dyes in foodstuffs by liquid chromatography-tandem electrospray ionization mass spectrometry via negative/positive ion switching mode. J. Agric. Food Chem. 2013, 61, 3834–3841. [Google Scholar] [CrossRef] [PubMed]
- Ghoreishi, S.M.; Behpour, M.; Golestaneh, M. Simultaneous voltametric determination of Brilliant Blue and Tartrazine in real samples at the surface of a multi-walled carbon nanotube paste electrode. Anal. Methods 2011, 3, 2842–2847. [Google Scholar] [CrossRef]
- Chen, B.; Huang, Y. Dispersive liquid-phase microextraction with solidification of floating organic droplet coupled with high-performance liquid chromatography for the determination of Sudan dyes in foodstuffs and water samples. J. Agric. Food Chem. 2014, 62, 5818–5826. [Google Scholar] [CrossRef] [PubMed]
- Demchenko, A.P. Introduction to Fluorescence Sensing; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Valeur, B.; Berberan-Santos, M.N. Molecular Fluorescence: Principles and Applications, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2012. [Google Scholar]
- Huang, J.; Wang, J.; Li, D.; Chen, P.; Liu, H.-B. Terthiophene-functionalized mesoporous silica-based fluorescence sensor for the detection of trace methyl orange in aqueous media. Microchim. Acta 2021, 188, 410. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, Y.; Zhang, P.; Ge, W. Highly sensitivity, selectivity chemosensor for methyl orange using upconversion NaBiF4: Yb/Tm nanosheets. J. Solid State Chem. 2021, 301, 122307. [Google Scholar] [CrossRef]
- Tropp, J.; Ihde, M.H.; Crater, E.R.; Bell, N.C.; Bhatta, R.; Johnson, I.C.; Bonizzoni, M.; Azoulay, J.D. Sensor Array for the Nanomolar Detection of Azo Dyes in Water. ACS Sens. 2020, 5, 1541–1547. [Google Scholar] [CrossRef] [PubMed]
- Bogireddy, N.K.R.; Lara, J.; Fragoso, L.R.; Agarwal, V. One-step hydrothermal preparation of highly stable N doped oxidized carbon dots for toxic organic pollutants sensing and bioimaging. Chem. Eng. J. 2020, 401, 126097. [Google Scholar] [CrossRef]
- Hu, Y.; Gao, Z. Sensitive detection of Sudan dyes using tire-derived carbon dots as a fluorescent sensor. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 239, 118514. [Google Scholar] [CrossRef] [PubMed]
- Zulfajri, M.; Dayalan, S.; Li, W.-Y.; Chang, C.-J.; Chang, Y.-P.; Huang, G.G. Nitrogen-Doped Carbon Dots from Averrhoa carambola Fruit Extract as a Fluorescent Probe for Methyl Orange. Sensors 2019, 19, 5008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sousa, D.A.; Costa, A.I.; Alexandre, M.R.; Prata, J.V. How an environmental issue could turn into useful high-valued products: The olive mill wastewater case. Sci. Total Environ. 2019, 647, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Sousa, D.A.; Ferreira, L.F.V.; Fedorov, A.A.; Rego, A.M.B.; Ferraria, A.M.; Cruz, A.B.; Berberan-Santos, M.N.; Prata, J.V. Luminescent Carbon Dots from Wet Olive Pomace: Structural insights, photophysical properties and cytotoxicity. Molecules 2022, 27, 6768. [Google Scholar] [CrossRef] [PubMed]
- Tsagaraki, E.; Lazarides, H.N.; Petrotos, K.B. Olive Mill Wastewater Treatment. In Utilization of By-Products and Treatment of Waste in the Food Industry; Oreopoulou, V., Russ, W., Eds.; Springer: New York, NY, USA, 2007. [Google Scholar]
- Panigrahi, S.K.; Mishra, A.K. Inner filter effect in fluorescence spectroscopy: As a problem and as a solution. J. Photochem. Photobiol. C Photochem. Rev. 2019, 41, 100318. [Google Scholar] [CrossRef]
- Long, G.L.; Winefordner, J.D. Limit of detection A closer look at the IUPAC definition. Anal. Chem. 1983, 55, 712A–724A. [Google Scholar] [CrossRef]
- Preus, S. a|e—UV-Vis-IR Spectral Software Version 2.2, FluorTools. Available online: www.fluortools.com (accessed on 20 March 2022).
- Parker, C.A.; Barnes, W.J. Some Experiments with Spectrofluorimeters and Filter Fluorimeters. Analyst 1957, 82, 606–618. [Google Scholar] [CrossRef]
- Christmann, D.R.; Crouch, S.R.; Holland, J.F.; Timnick, A. Correction of right-angle molecular fluorescence measurements for absorption of fluorescence radiation. Anal. Chem. 1980, 52, 291–295. [Google Scholar] [CrossRef]
- Stella, L.; Capodilupo, A.L.; Bietti, M. A reassessment of the association between azulene and [60] fullerene. Possible pitfalls in the determination of binding constants through fluorescence spectroscopy. Chem. Commun. 2008, 39, 4744–4746. [Google Scholar] [CrossRef] [PubMed]
- Dados da Qualidade da Água/Torneiras da Cidade de Lisboa. EPAL, Grupo Águas de Portugal, 2022. Available online: https://www.epal.pt/EPAL/menu/%C3%A1gua/divulga%C3%A7%C3%A3o-de-dados-da-qualidade-da-%C3%A1gua/controlo-legal/torneiras-na-cidade-de-lisboa (accessed on 3 November 2022).
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: New York, NY, USA, 2006; p. 282. [Google Scholar]













| Probes | Method | Mechanism 1 | LOD (μM) | Ref. |
|---|---|---|---|---|
| Silica functionalized with terthiophene | Fluorescence (turn-off) | IFEs or RET | 0.09 | [10] |
| Citric acid/urea CDs | Fluorescence (turn-off) | IFEs | 0.04 | [13] |
| Lanthanide-doped NaBiF4 nanosheets | Fluorescence (turn-off) | IFEs/RET | 4.6 | [11] |
| Conjugated fluorene-based polymers | Fluorescence (turn-off) | IFEs (sensor array) | n/a 2 | [12] |
| Extract of A. carambola and arginine CDs | Fluorescence (turn-off) | IFEs/RET | 0.30 | [15] |
| WP-CDs | Fluorescence (turn-off) | Static & RET | 0.46 3 | This work |
| Acceptor Dye | R0 (Å) 1 |
|---|---|
| Methyl Orange (MO) | 36.9 |
| Methyl Red (MR) | 33.8 |
| Indigo Carmine (IC) | 26.3 |
| Fuchsin (Fch) | 26.2 |
| Methylene Blue (MB) | 24.6 |
| Rhodamine-6G (Rh-6G) | 34.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sousa, D.A.; Berberan-Santos, M.N.; Prata, J.V. Detection of Azo Dyes Using Carbon Dots from Olive Mill Wastes. Chemosensors 2022, 10, 487. https://doi.org/10.3390/chemosensors10110487
Sousa DA, Berberan-Santos MN, Prata JV. Detection of Azo Dyes Using Carbon Dots from Olive Mill Wastes. Chemosensors. 2022; 10(11):487. https://doi.org/10.3390/chemosensors10110487
Chicago/Turabian StyleSousa, Diogo A., Mário N. Berberan-Santos, and José V. Prata. 2022. "Detection of Azo Dyes Using Carbon Dots from Olive Mill Wastes" Chemosensors 10, no. 11: 487. https://doi.org/10.3390/chemosensors10110487