Organic-Inorganic Semiconductor Heterojunction P3HT@Ag2NCN Composite Film as a Recyclable SERS Substrate for Molecule Detection Application
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
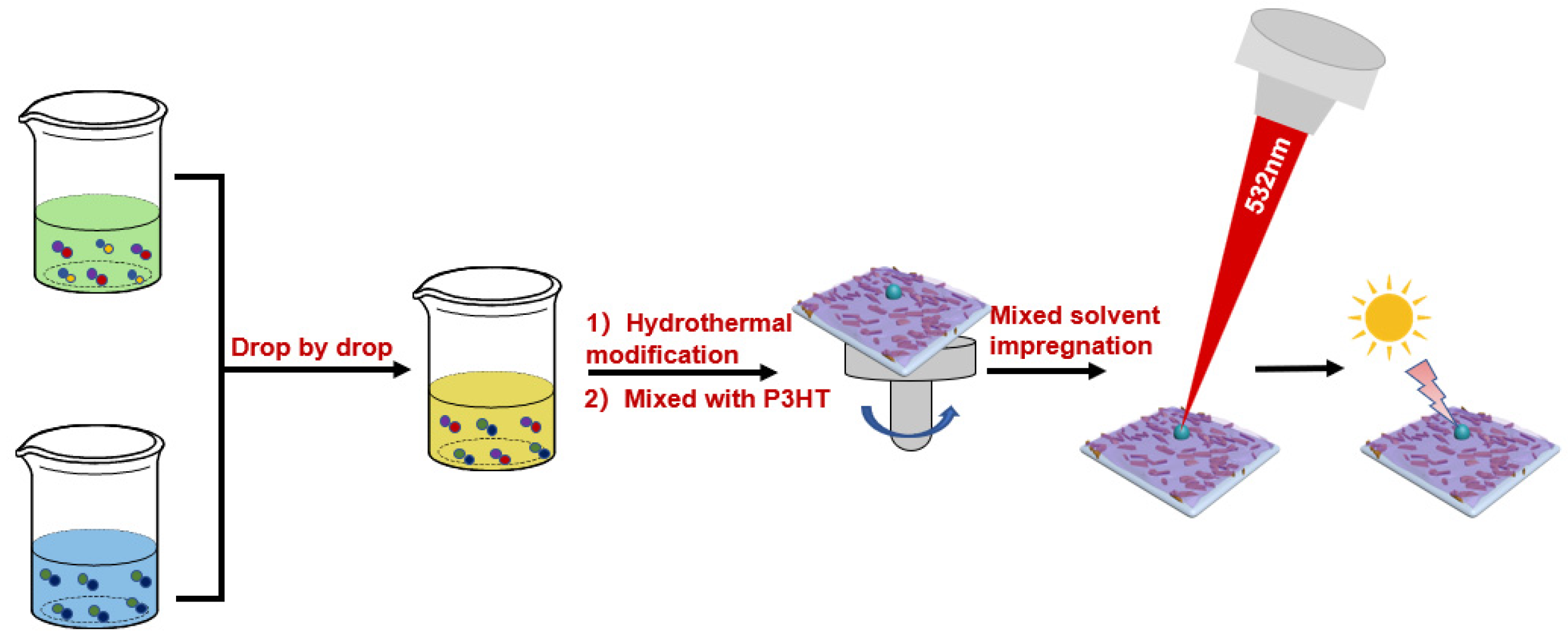
2.2. Preparation of P3HT@Ag2NCN Composite Film
2.3. Raman Spectroscopy
2.4. Characterization Techniques
2.5. SERS Enhancement Factor (EF)
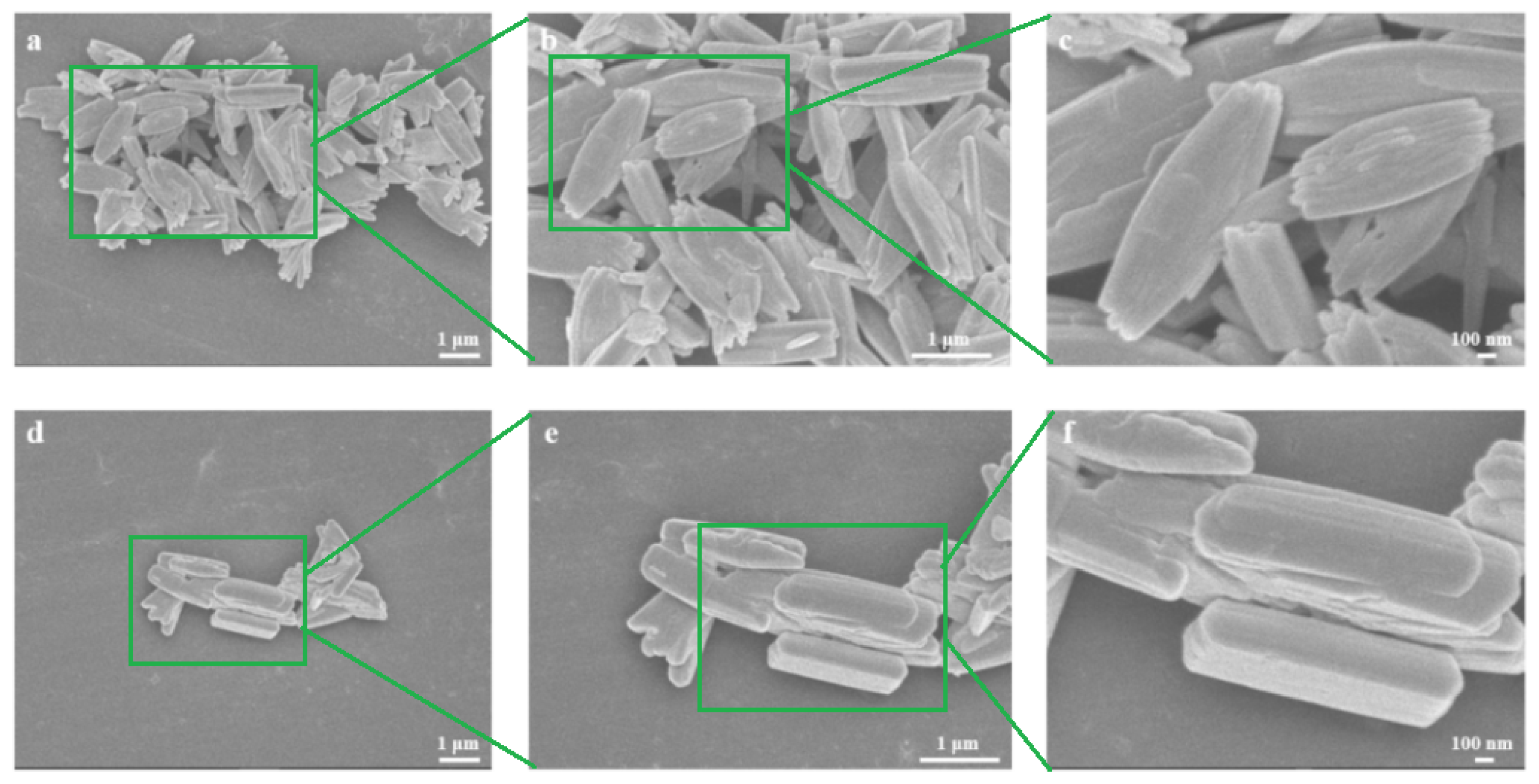
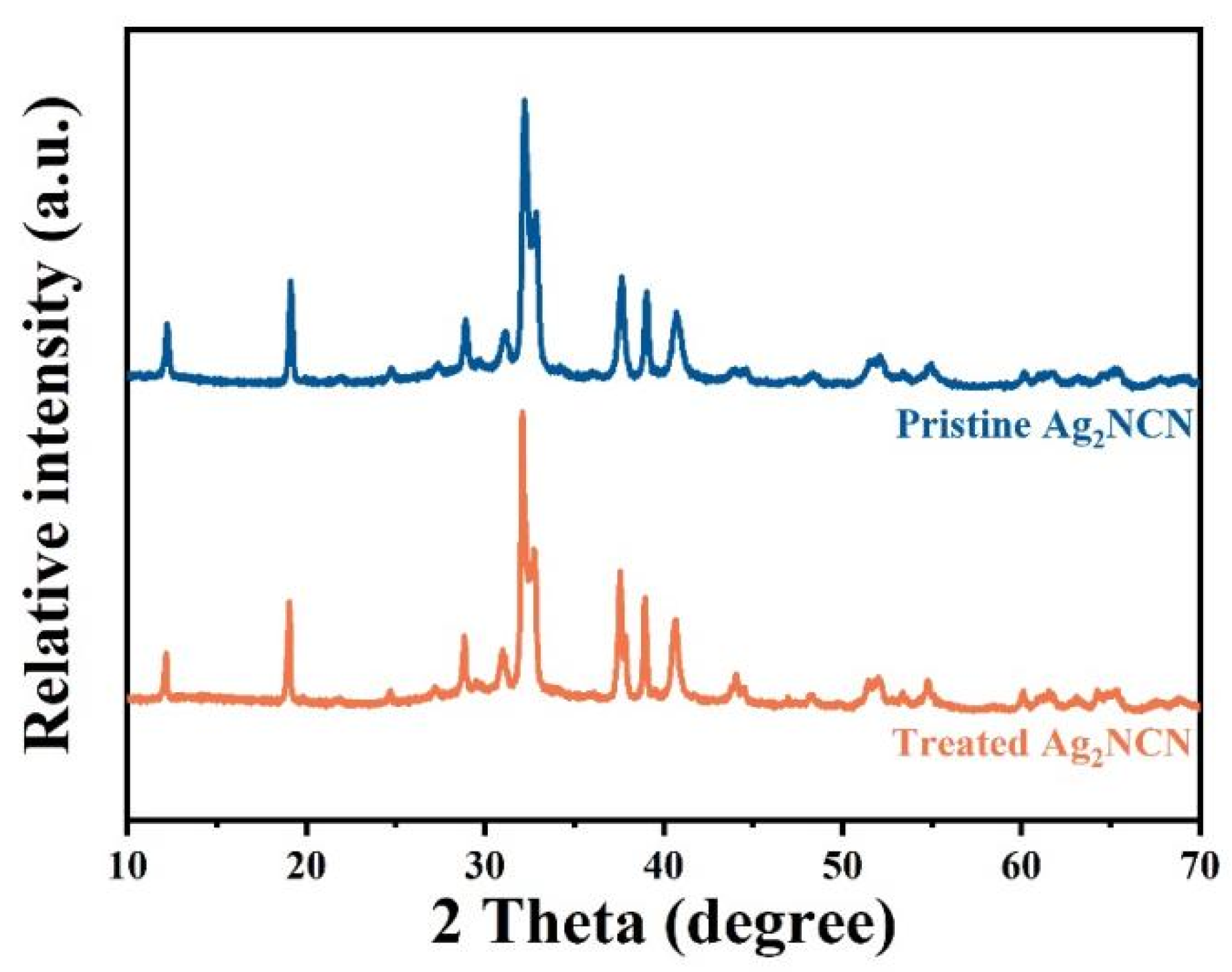
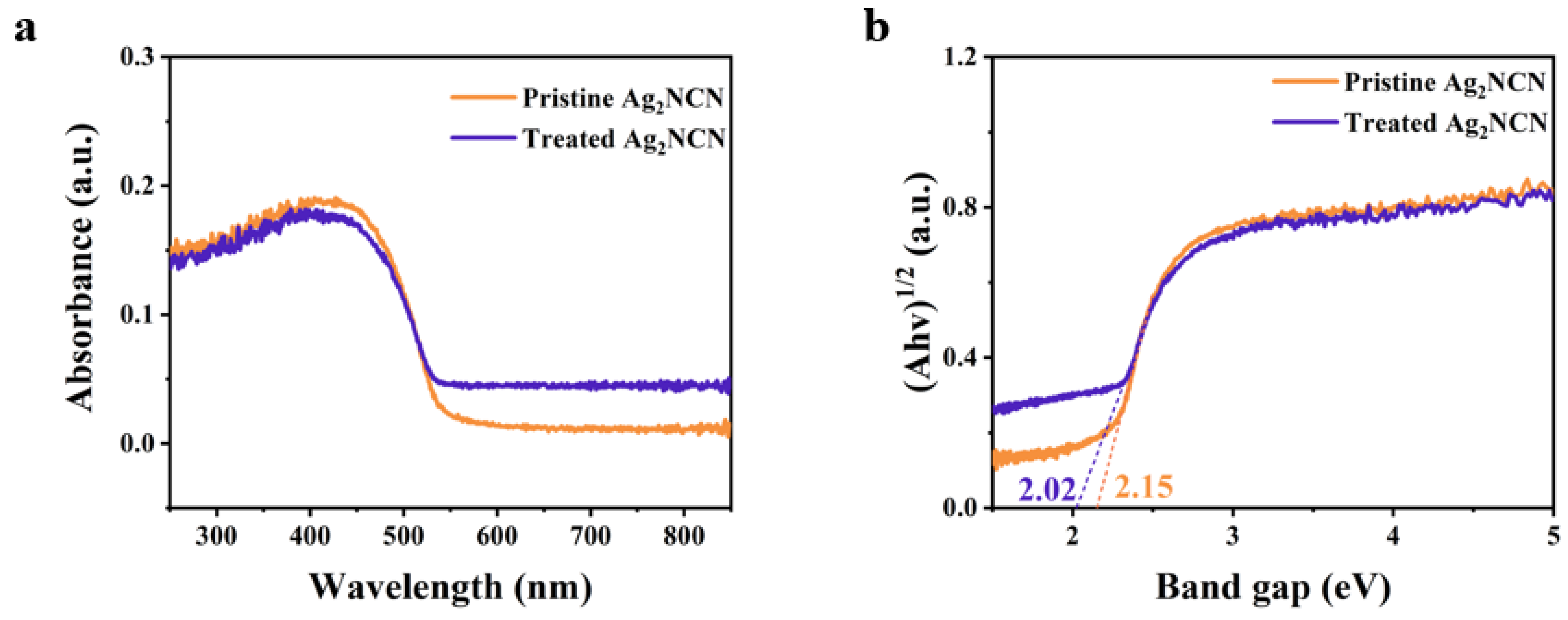
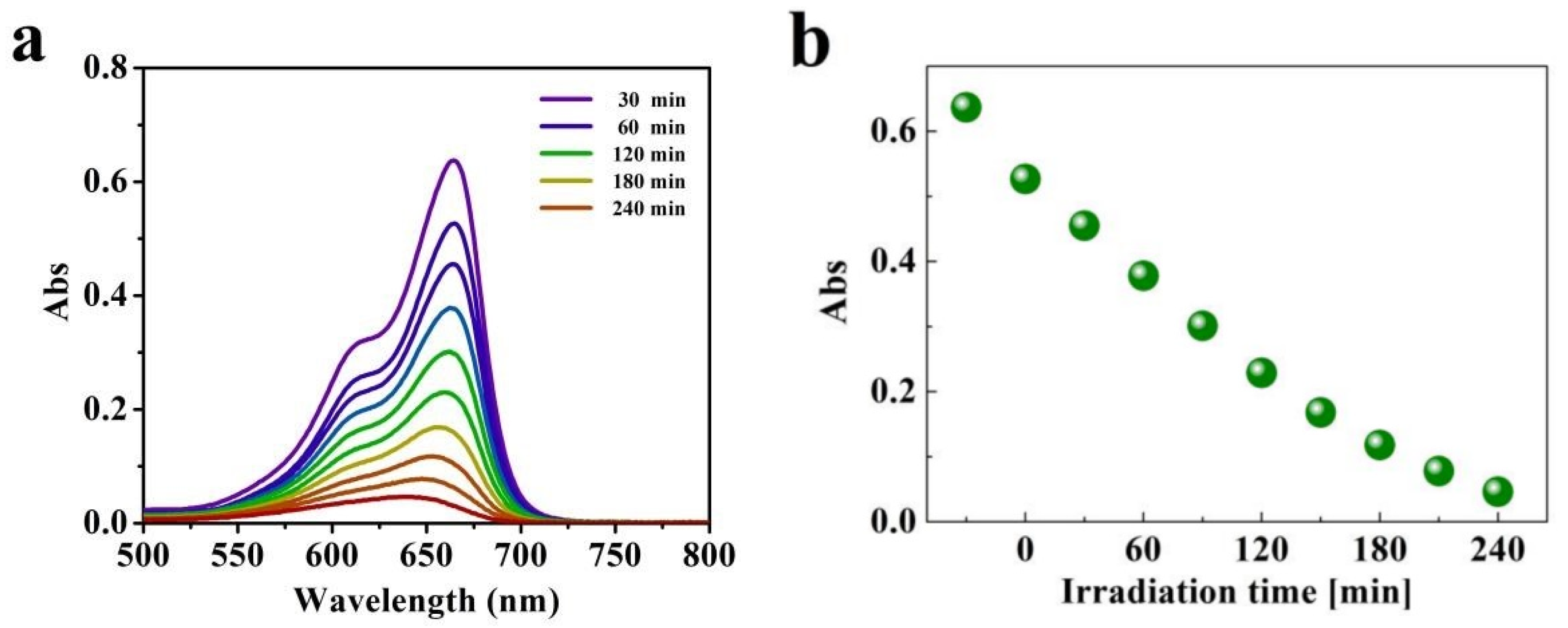
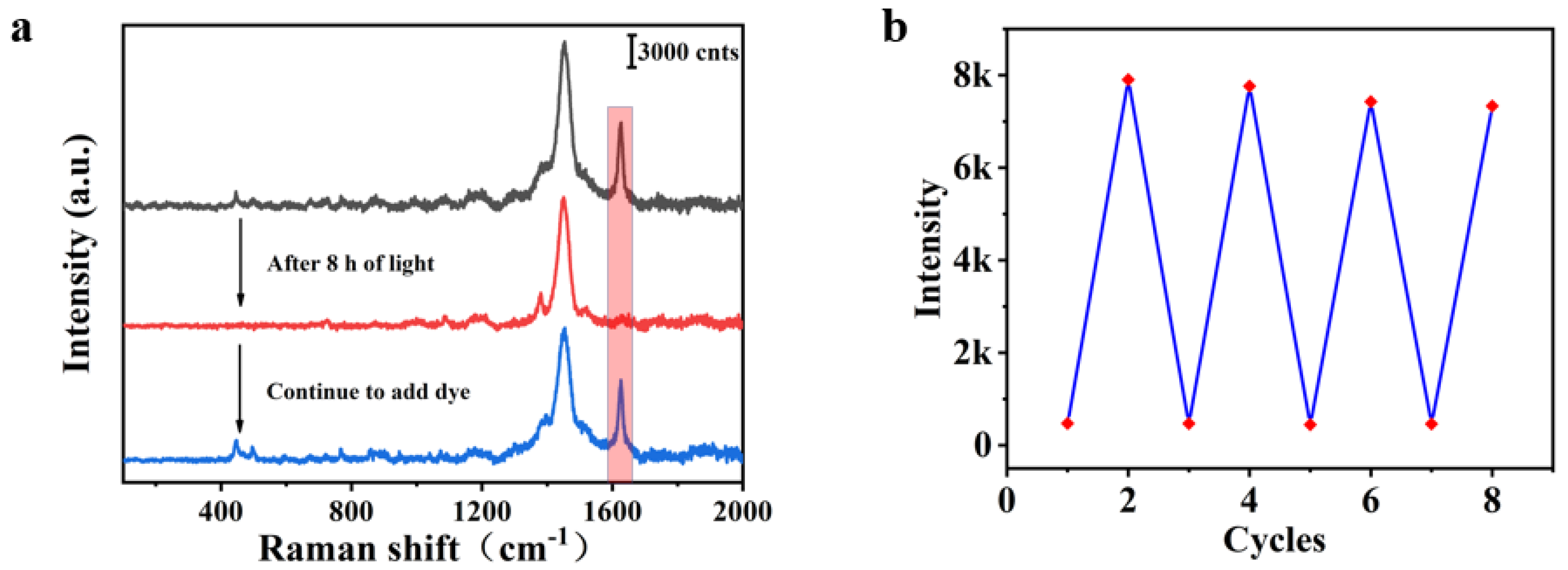
3. Result and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Li, D.; Yao, D.; Li, C.; Luo, Y.H.; Liang, A.H.; Wen, G.Q.; Jiang, Z.L. Nanosol SERS Quantitative Analytical Method: A Review. TrAC-Trends Anal. Chem. 2020, 127, 115885. [Google Scholar] [CrossRef]
- Feng, E.; Tian, Y. Surface-Enhanced Raman Scattering of Self-Assembled Superstructures. Chem. Res. Chin. Univ. 2021, 37, 989–1007. [Google Scholar] [CrossRef]
- Zhou, X.; Hu, Z.; Yang, D.; Yang, D.T.; Xie, S.X.; Jiang, Z.J.; Niessner, R.; Haisch, C.; Zhou, H.B.; Sun, P.H. Bacteria Detection: From Powerful Sers to Its Advanced Compatible Techniques. Adv. Sci. 2020, 7, 2001739. [Google Scholar] [CrossRef]
- Zhao, X.; Campbell, S.; Wallace, G.Q.; Claing, A.; Bazuin, C.G.; Masson, J.F. Branched Au Nanoparticles on Nanofibers for Surface-Enhanced Raman Scattering Sensing of Intracellular pH and Extracellular pH Gradients. ACS Sens. 2020, 5, 2155–2167. [Google Scholar] [CrossRef]
- Chen, X.; Lin, H.; Xu, T.; Lai, K.; Han, X.; Lin, M. Cellulose Nanofibers Coated with Silver Nanoparticles as A Flexible Nanocomposite for Measurement of Flusilazole Residues in Oolong Tea by Surface-Enhanced Raman Spectroscopy. Food Chem. 2020, 315, 126276. [Google Scholar] [CrossRef]
- Zhang, H.H.; Xu, L.; Xu, Y.; Huang, G.; Zhao, X.Y.; Lai, Y.Q.; Shi, T.F. Enhanced Self-Organized Dewetting of Ultrathin Polymer Blend Film for Large-Area Fabrication of SERS Substrate. Sci. Rep. 2016, 6, 38337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tong, L.; Zhu, T.; Liu, Z. Approaching the Electromagnetic Mechanism of Surface-Enhanced Raman Scattering: From Self-Assembled Arrays to Individual Gold Nanoparticles. Chem. Soc. Rev. 2011, 40, 1296–1304. [Google Scholar] [CrossRef]
- Wu, D.Y.; Liu, X.M.; Duan, S.; Xu, X.; Ren, B.; Lin, S.H.; Tian, Z.Q. Chemical Enhancement Effects in SERS Spectra: A Quantum Chemical Study of Pyridine Interacting with Copper, Silver, Gold and Platinum Metals. J. Phys. Chem. C 2008, 112, 4195–4204. [Google Scholar] [CrossRef]
- Dawson, P.; Alexander, K.B.; Thompson, J.R.; Haas, J.W.; Ferrell, T.L. Influence of Metal Grain-Size on Surface-Enhanced Raman-Scattering. Phys. Rev. B 1991, 44, 6372–6381. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Meng, G.; Zheng, P.; Huang, Q.; Li, Z.B.; Hu, X.Y.; Wang, X.J.; Huang, Z.L.; Li, F.D.; Wu, N.Q. A Hierarchically Ordered Array of Silver-Nanorod Bundles for Surface-Enhanced Raman Scattering Detection of Phenolic Pollutants. Adv. Mater. 2016, 28, 4871–4876. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.P.; Wang, Z.L.; Xu, L.; Zhang, X.; Lu, G. Quantum Plasmonics in Nanorods: A Time-Dependent Orbital-Free Density Functional Theory Study with Thousands of Atoms. J. Phys. Chem. C 2020, 124, 945–951. [Google Scholar] [CrossRef]
- Xiang, H.P.; Zu, J.X.; Jiang, H.W.; Xu, L.; Lu, G.; Zhang, X. Understanding Quantum Plasmonic Enhancement in Nanorod Dimers from Time-Dependent Orbital-Free Density Functional Theory. J. Phys. Chem. C 2022, 126, 5046–5054. [Google Scholar] [CrossRef]
- Jin, L.; She, G.; Wang, X.; Yuan, R.; Dai, Y.Q.; Lu, Y.H.; Wang, P. Enhancing the Sers Performance of Semiconductor Nanostructures through a Facile Surface Engineering Strategy. Appl. Surf. Sci. 2014, 320, 591–595. [Google Scholar] [CrossRef]
- Li, K.; Wang, Y.; Jiang, K.; Ren, Y.; Dai, Y.; Lu, Y.; Wang, P. Free-Standing Ag Triangle Arrays a Configurable Vertical Gap for Surface Enhanced Raman Spectroscopy. Nanotechnology 2017, 28, 385401. [Google Scholar] [CrossRef]
- Jen, Y.J.; Lin, M.J.; Cheang, H.L.; Chan, T.L. Obliquely Deposited Titanium Nitride Nanorod Arrays as Surface-Enhanced Raman Scattering Substrates. Sensors 2019, 19, 4765. [Google Scholar] [CrossRef] [Green Version]
- Wen, S.P.; Miao, X.R.; Fan, G.C.; Xu, T.T.; Jiang, L.P.; Wu, P.; Cai, C.; Zhu, J.J. Aptamer-Conjugated Au Nanocage/SiO2 Core-Shell Bifunctional Nanoprobes with High Stability and Biocompatibility for Cellular Sers Imaging and near-Infrared Photothermal Therapy. ACS Sens. 2019, 4, 301–308. [Google Scholar] [CrossRef]
- Glass, D.; Cortes, E.; Ben-Jaber, S.; Brick, T.; Peveler, J.; Blackman, C.; Howle, C.; Quesada-Cabrera, R.; Parkin, I.; Maier, S. Dynamics of Photo-Induced Surface Oxygen Vacancies in Metal-Oxide Semiconductors Studied under Ambient Conditions. Adv. Sci. 2019, 6, 1901841. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Song, K.; Li, X.; Yang, M.; Han, X.X.; Yang, L.B.; Zhao, B. Double Metal Co-Doping of TiO2 Nanoparticles for Improvement of Their Sers Activity and Ultrasensitive Detection of Enrofloxacin: Regulation Strategy of Energy Levels. Chemistryselect 2017, 2, 3099–3105. [Google Scholar] [CrossRef]
- Quan, Y.; Su, R.; Yang, S.; Chen, L.; Wei, M.B.; Liu, H.L.; Yang, H.J.; Gao, M.; Li, B.Z. In-Situ Surface-Enhanced Raman Scattering Based on MTi20 Nanoflowers: Monitoring and Degradation of Contaminants. J. Hazard. Mater. 2021, 412, 125209. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, X.; Li, P.; Han, R.; Liu, Y.W.; Han, X.X.; Zhao, B. Surface-Enhanced Raman Scattering (SERS) as a Probe for Detection of Charge-Transfer between TiO2 and Cds Nanoparticles. New J. Chem. 2019, 43, 230–237. [Google Scholar] [CrossRef]
- Yang, B.; Jin, S.; Guo, S.; Park, Y.; Chen, L.; Zhao, B.; Jung, Y.M. Recent Development of SERS Technology: Semiconductor-Based Study. ACS Omega 2019, 4, 20101–20108. [Google Scholar] [CrossRef] [PubMed]
- Balcytis, A.; Ryu, M.; Seniutinas, G.; Juodkazyte, J.; Cowie, B.; Stoddart, P.; Zamengo, M.; Morikawac, J.; Juodkazis, S. Black-CuO: Surface-Enhanced Raman Scattering and Infrared Properties. Nanoscale 2015, 7, 18299–18304. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Qin, X.; Jiang, X.; Gong, M.; Yin, D.; Zhang, Y.J.; Zhao, B. Sers Investigation of Ciprofloxacin Drug Molecules on TiO2 Nanoparticles. Phys. Chem. Chem. Phys. 2015, 17, 17809–17815. [Google Scholar] [CrossRef] [PubMed]
- Han, X.X.; Ji, W.; Zhao, B.; Ozaki, Y. Semiconductor-Enhanced Raman Scattering: Active Nanomaterials and Applications. Nanoscale 2017, 9, 4847–4861. [Google Scholar] [CrossRef]
- Yilmaz, M.; Babur, E.; Ozdemir, M.; Gieseking, R.; Dede, Y.; Tamer, U.; Schatz, G.; Facchetti, A.; Usta, H.; Demirel, G. Nanostructured Organic Semiconductor Films for Molecular Detection with Surface-Enhanced Raman Spectroscopy. Nat. Mater. 2017, 16, 918–924. [Google Scholar] [CrossRef]
- Wang, T.; Lu, Y.; Xu, L.; Chen, Z.J. π-Conjugated poly(3-hexylthiophene- 2,5-diyl) thin film as a SERS substrate for molecule detection application. J. Mater. Sci. 2022, 57, 16965–16973. [Google Scholar] [CrossRef]
- Demirel, G.; Usta, H.; Yilmaz, M.; Celik, M.; Alidagibe, H.; Buyukserind, F. Surface-Enhanced Raman Spectroscopy (SERS): An Adventure from Plasmonic Metals to Organic Semiconductors as Sers Platforms. J. Mater. Chem. C 2018, 6, 5314–5335. [Google Scholar] [CrossRef]
- Ganesh, S.; Venkatakrishnan, K.; Tan, B. Quantum scale organic semiconductors for SERS detection of DNA methylation and gene expression. Nat. Commun. 2020, 11, 1135. [Google Scholar] [CrossRef] [Green Version]
- Demirel, G.; Gieseking, R.; Ozdemir, R.; Kahmann, S.; Loi, M.; Schatz, G.; Facchetti, A.; Usta, H. Molecular engineering of organic semiconductors enables noble metalcomparable SERS enhancement and sensitivity. Nat. Commun. 2019, 10, 5502. [Google Scholar] [CrossRef] [Green Version]
- Razzell-Hollis, J.; Thiburce, Q.; Tsoi, W.; Kim, J. Interfacial Chemical Composition and Molecular Order in Organic Photovoltaic Blend Thin Films Probed by Surface-Enhanced Raman Spectroscopy. ACS Appl. Mater. Interfaces 2016, 8, 31469–31481. [Google Scholar] [CrossRef]
- Shi, C.; Qin, L.; Wu, S. Highly Sensitive Sers Detection and Photocatalytic Degradation of 4-Ami- Nothiophenol by a Cost-Effective Cobalt Metal-Organic Framework-Based Sandwich-Like Sheet. Chem. Eng. J. 2021, 422, 129970. [Google Scholar] [CrossRef]
- Singh, J.; Manna, A.K.; Soni, R.K. Bifunctional Au-TiO2 Thin Films with Enhanced Photocatalytic Activity and Sers Based Multiplexed Detection of Organic Pollutant. J. Mater. Sci.-Mater. Electron. 2019, 30, 16478–16493. [Google Scholar] [CrossRef]
- Liu, L.; Yang, H.; Ren, X.; Tang, J.; Li, Y.F.; Zhang, X.Q.; Cheng, Z.H. Au-Zno Hybrid Nanoparticles Exhibiting Strong Charge-Transfer-Induced Sers for Recyclable Sers-Active Substrates. Nanoscale 2015, 7, 5147–5151. [Google Scholar] [CrossRef]
- Wu, J.Y.; Hsieh, C.H.; Feria, D.N.; Shen, J.L. PAA/ZnO Raspberry-Shaped Composite Microspheres Decorated with Ag Nanoparticles as Cleanable SERS Substrates. ACS Omega 2020, 5, 29795–29800. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Lodhi, D.K.; Singh, J.P. Highly Sensitive Multifunctional Recyclable Ag-TiO2 Nanorod SERS Substrates for Photocatalytic Degradation and Detection of Dye Molecules. RSC Adv. 2016, 6, 45120–45126. [Google Scholar] [CrossRef]
- Stavytska, M.S.; Salvador, M.; Kulkarni, A.; Ginger, D.S.; Kelley, A.M. Plasmonic Enhancement of Raman Scattering from the Organic Solar Cell Material P3HT/PCBM by Triangular Silver Nanoprisms. J. Phys. Chem. C 2011, 115, 20788–20794. [Google Scholar] [CrossRef]
- Yang, B.; Wang, Y.; Guo, S.; Jin, S.; Park, E.; Chen, L.; Jung, Y.M. Charge Transfer Study for Semiconductor and Semiconductor/Metal Composites Based on Surface-Enhanced Raman Scattering. Bull. Korean Chem. Soc. 2021, 42, 1411–1418. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, L.; Xi, M.; Feng, Q.; Jiang, C.Y.; Fong, H. Electrospun TiO2 Nanofelt Surface-Decorated with Ag Nanoparticles as Sensitive and UV-Cleanable Substrate for Surface Enhanced Raman Scattering. ACS Appl. Mater. Interfaces 2014, 6, 5759–5767. [Google Scholar] [CrossRef]
- Wei, Q.Y.; Dong, Q.R.; Sun, D.W.; Pu, H.B. Synthesis of recyclable SERS platform based on MoS2@TiO2@Au heterojunction for photodegradation and identification of fungicides. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 285, 121895. [Google Scholar] [CrossRef]
- Wang, Y.; Li, M.S.; Wang, D.G.; Han, C.P.; Li, J.J.; Wu, C.Y.; Xu, K. Fabrication of highly uniform Ag nanoparticle-TiO2 nanosheets array hybrid as reusable SERS substrates. Colloid Interface Sci. Commun. 2020, 39, 100324. [Google Scholar] [CrossRef]
- Tao, Q.; Li, S.; Ma, C.Y.; Liu, K.; Zhang, Q.Y. A highly sensitive and recyclable SERS substrate based on Ag-nanoparticle-decorated ZnO nanoflowers in ordered arrays. Dalton Trans. 2015, 44, 3447–3453. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhang, H.H.; Lu, Y.Y.; An, L.J.; Shi, T.F. The effects of solvent polarity on the crystallization behavior of thin π-conjugated polymer film in solvent mixtures investigated by grazing incident X-ray diffraction. Polymer 2020, 190, 122259. [Google Scholar] [CrossRef]
- Sui, C.; Wang, K.G.; Wang, S.; Ren, J.Y.; Bai, X.H.; Bai, J.T. SERS activity with tenfold detection limit optimization on a type of nanoporous aao-based complex multilayer substrate. Nanoscale 2016, 8, 5920–5927. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.Q.; Wang, Y.S.C.; Li, Z.Y.; Song, T.; Long, B.; Ali, A.; Deng, G. Controllable Synthesis of Porous Silver Cyanamide Nanocrystals with Tunable Morphologies for Selective Photocatalytic CO2 Reduction into CH4. J. Colloid Interface Sci. 2021, 593, 152–161. [Google Scholar] [CrossRef]

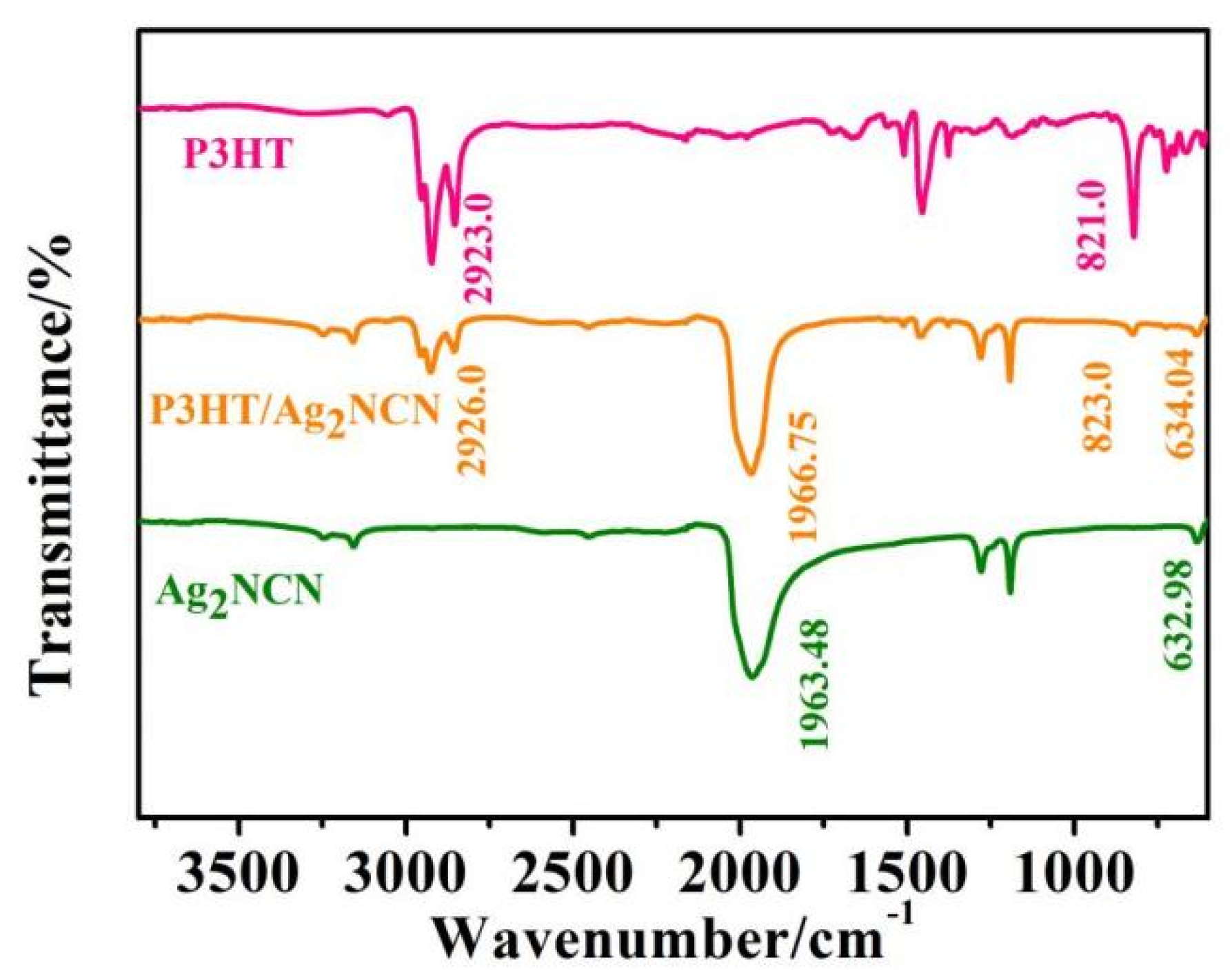
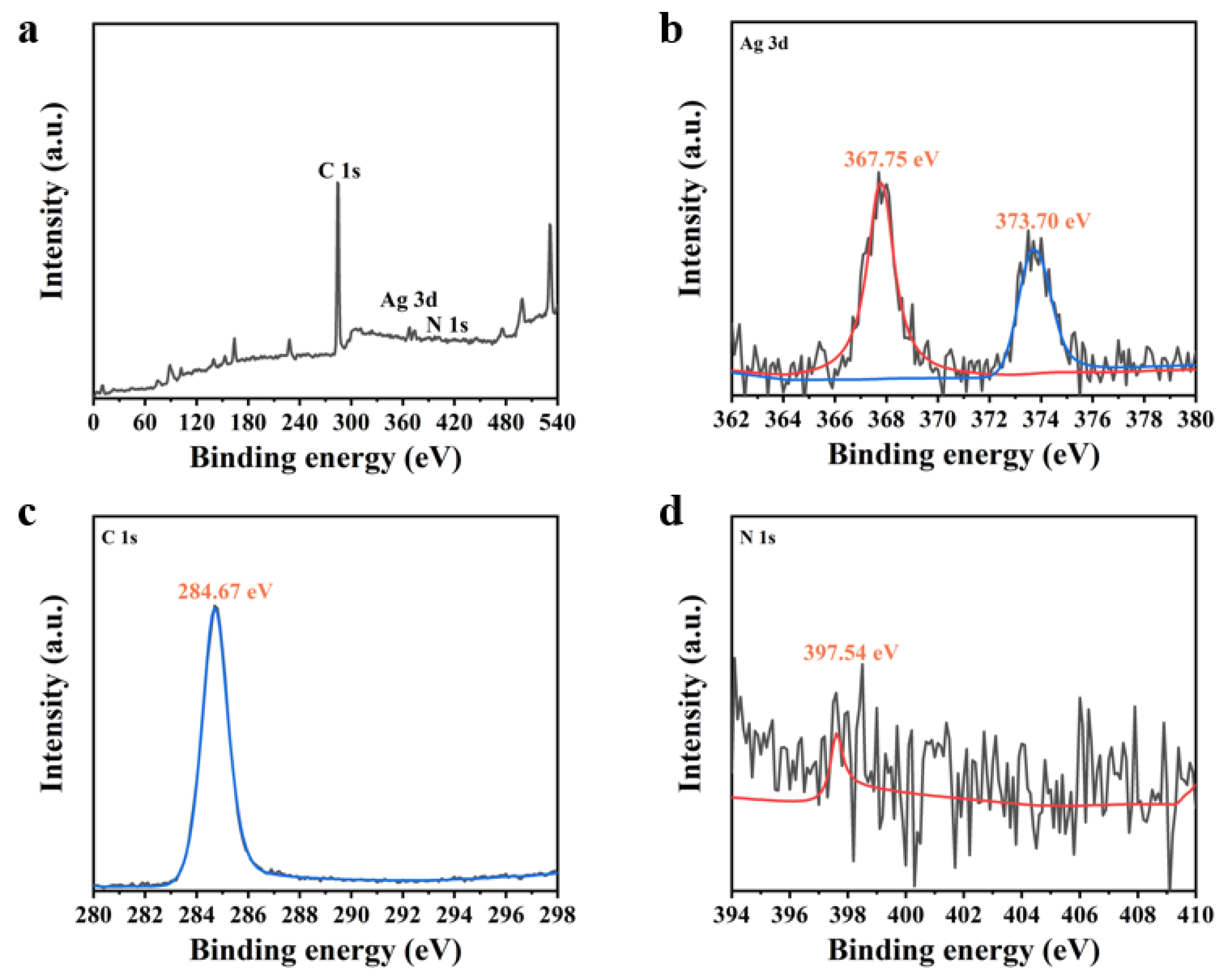
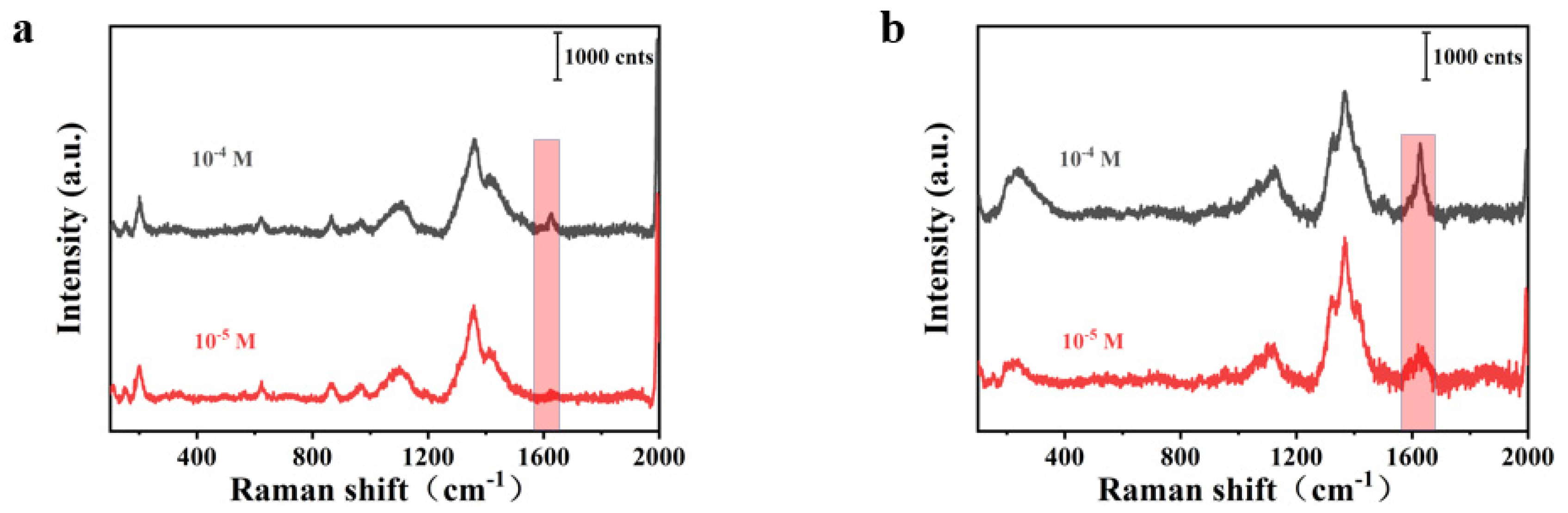
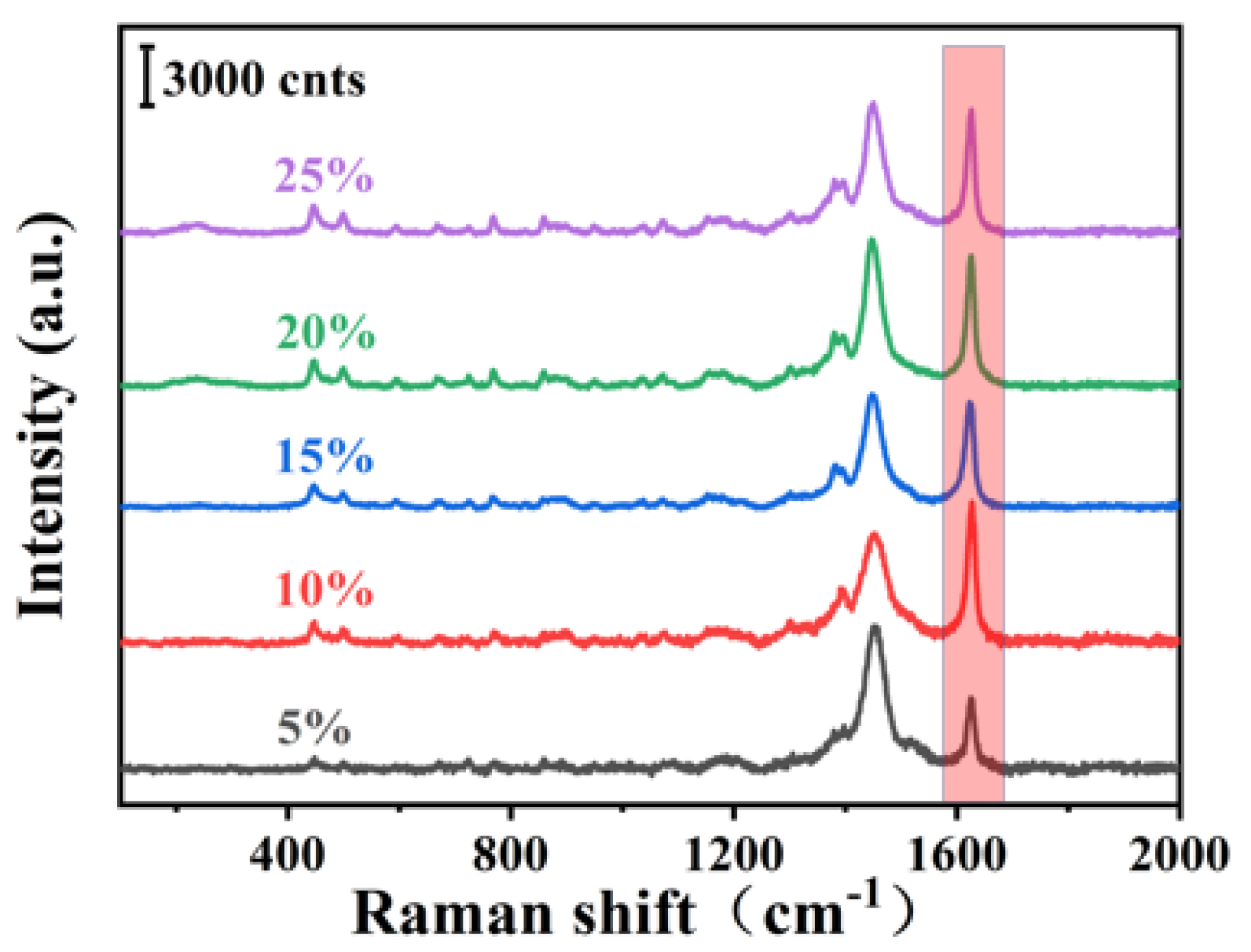

| Element | Wt% | Wt% Sigma |
|---|---|---|
| C | 9.45 | 0.27 |
| N | 11.15 | 0.41 |
| Ag | 76.56 | 0.61 |
| S | 2.84 | 0.26 |
| Total | 100.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; Wang, T.; Li, X.; Chen, Z. Organic-Inorganic Semiconductor Heterojunction P3HT@Ag2NCN Composite Film as a Recyclable SERS Substrate for Molecule Detection Application. Chemosensors 2022, 10, 469. https://doi.org/10.3390/chemosensors10110469
Xu L, Wang T, Li X, Chen Z. Organic-Inorganic Semiconductor Heterojunction P3HT@Ag2NCN Composite Film as a Recyclable SERS Substrate for Molecule Detection Application. Chemosensors. 2022; 10(11):469. https://doi.org/10.3390/chemosensors10110469
Chicago/Turabian StyleXu, Lin, Tao Wang, Xuan Li, and Zhengjian Chen. 2022. "Organic-Inorganic Semiconductor Heterojunction P3HT@Ag2NCN Composite Film as a Recyclable SERS Substrate for Molecule Detection Application" Chemosensors 10, no. 11: 469. https://doi.org/10.3390/chemosensors10110469




