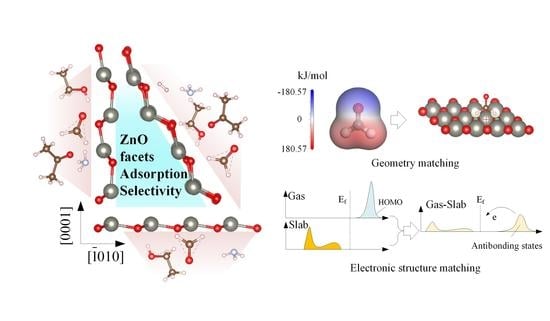
Facet-Dependent Gas Adsorption Selectivity on ZnO: A DFT Study
Abstract
:1. Introduction
2. Computation Methods
3. Results
3.1. Surface Properties
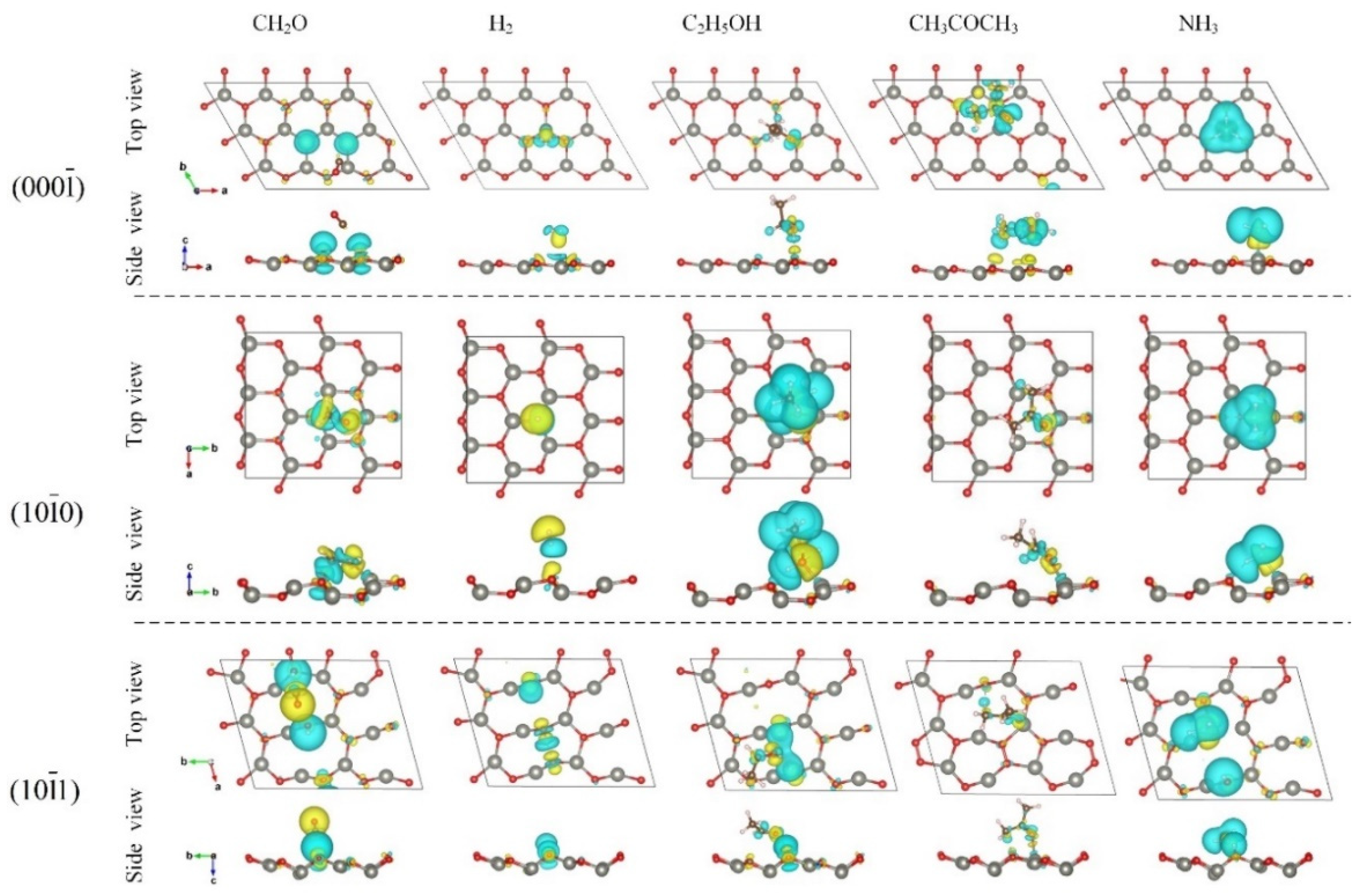
3.2. Adsorption Configuration
3.3. Adsorption Energy and Charge Transfer
4. Selectivity Analysis and Mechanism Discussion
4.1. Polarity
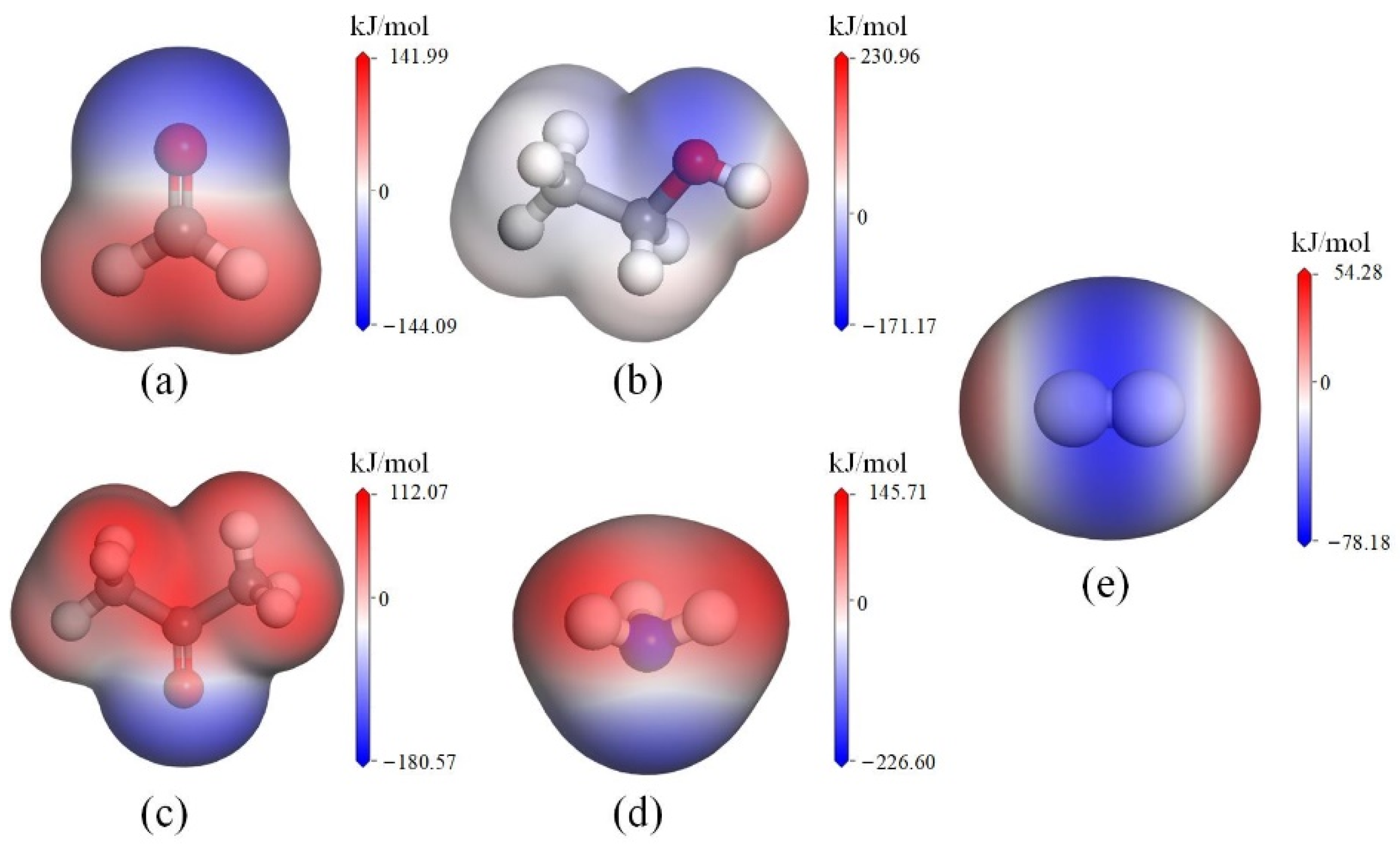
4.2. Geometric Matching and Electrostatic Interactions
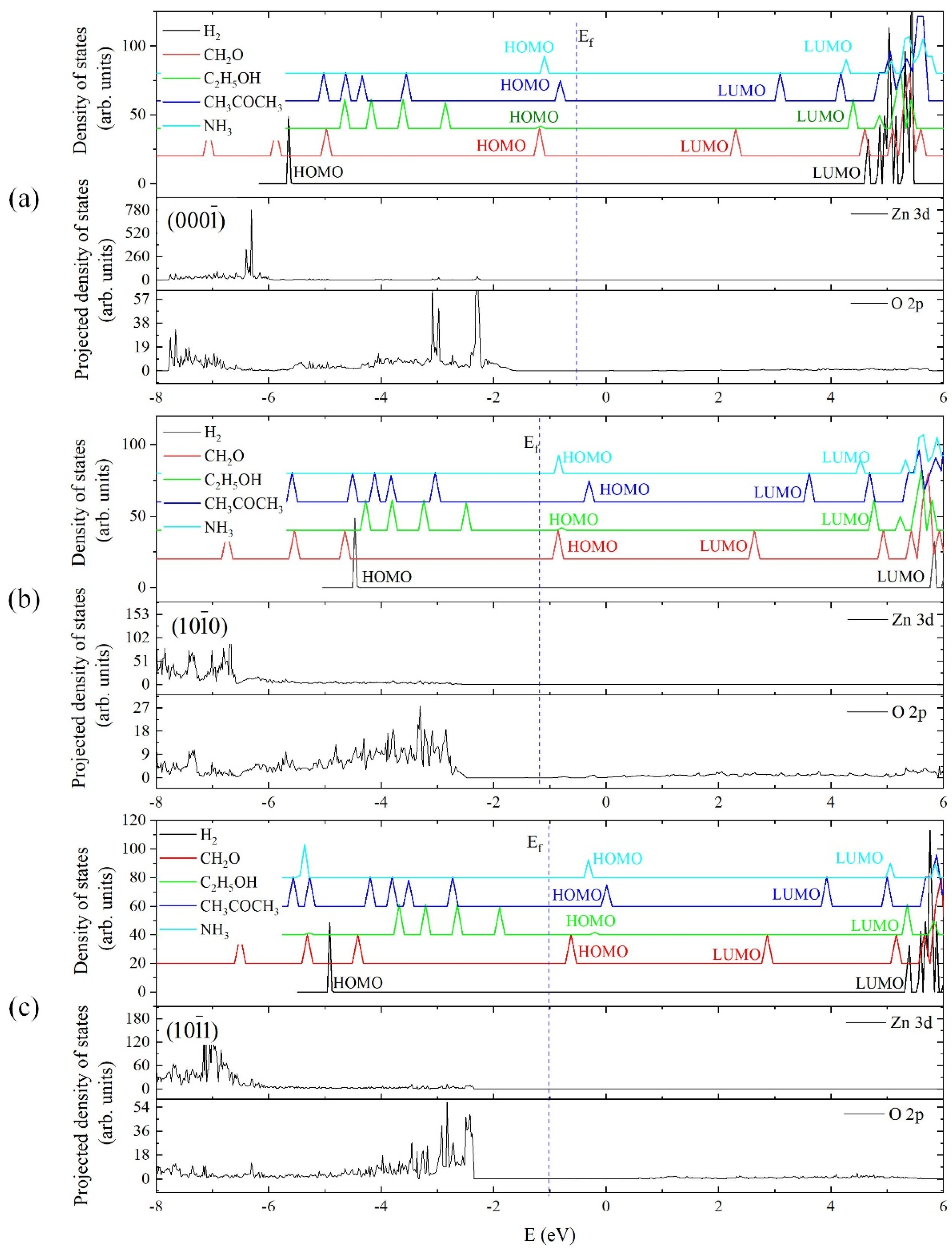
4.3. Electronic Structure Matching
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, B.; Li, K.; Luo, Y.; Gao, L.; Duan, G. Sulfur spillover driven by charge transfer between AuPd alloys and SnO2 allows high selectivity for dimethyl disulfide gas sensing. Chem. Eng. J. 2021, 420, 129881. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, L.; Cheng, B.; Fan, J.; Yu, J. A high-response formaldehyde sensor based on fibrous Ag-ZnO/In2O3 with multi-level heterojunctions. J. Hazard. Mater. 2021, 413, 125352. [Google Scholar] [CrossRef]
- Shokrzadeh, L.; Mohammadi, P.; Mahmoudian, M.R.; Basirun, W.J.; Bahreini, M. L-glycine-assisted synthesis of SnO2/Pd nanoparticles and their application in detection of biodeteriorating fungi. Mater. Chem. Phys. 2020, 240, 122172. [Google Scholar] [CrossRef]
- Zhou, X.; Zou, Y.; Ma, J.; Cheng, X.; Li, Y.; Deng, Y.; Zhao, D. Cementing mesoporous ZnO with silica for controllable and switchable gas sensing selectivity. Chem. Mater. 2019, 31, 8112–8120. [Google Scholar] [CrossRef]
- Kim, T.-H.; Jeong, S.-Y.; Moon, Y.K.; Lee, J.-H. Dual-mode gas sensor for ultrasensitive and highly selective detection of xylene and toluene using Nb-doped NiO hollow spheres. Sens. Actuators B Chem. 2019, 301, 127140. [Google Scholar] [CrossRef]
- Broek, J.; Abegg, S.; Pratsinis, S.; Güntner, A. Highly selective detection of methanol over ethanol by a handheld gas sensor. Nat. Commun. 2019, 10, 4220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suematsu, K.; Oyama, T.; Mizukami, W.; Hiroyama, Y.; Watanabe, K.; Shimanoe, K. Selective detection of toluene using pulse-driven SnO2 micro gas sensors. ACS Appl. Electron. Mater. 2020, 2, 2913–2920. [Google Scholar] [CrossRef]
- Potyrailo, R.A. Multivariable sensors for ubiquitous monitoring of gases in the era of internet of things and industrial internet. Chem. Rev. 2016, 116, 11877–11923. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lei, Y. Pt-CeO2 nanofibers based high-frequency impedancemetric gas sensor for selective CO and C3H8 detection in high-temperature harsh environment. Sens. Actuators B Chem. 2013, 188, 1141–1147. [Google Scholar] [CrossRef]
- Ganesh, R.S.; Durgadevi, E.; Navaneethan, M.; Patil, V.; Ponnusamy, S.; Muthamizhchelvan, C.; Kawasaki, S.; Patil, P.; Hayakawa, Y. Tuning the selectivity of NH3 gas sensing response using Cu-doped ZnO nanostructures. Sens. Actuators A Phys. 2018, 269, 331–341. [Google Scholar] [CrossRef]
- Song, L.; Dou, K.; Wang, R.; Leng, P.; Luo, L.; Xi, Y.; Kaun, C.-C.; Han, N.; Wang, F.; Chen, Y. Sr-doped cubic In2O3/rhombohedral In2O3 homojunction nanowires for highly sensitive and selective breath ethanol sensing: Experiment and DFT simulation studies. ACS Appl. Mater. Interfaces 2019, 12, 1270–1279. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.-Q.; Ji, H.-M.; Wang, D.-H.; Bai, X.; Sun, X.-H.; Jin, Z.-G. Exposed facets induced enhanced acetone selective sensing property of nanostructured tungsten oxide. J. Mater. Chem. A 2014, 2, 13602–13611. [Google Scholar] [CrossRef]
- Qin, N.; Xiang, Q.; Zhao, H.; Zhang, J.; Xu, J. Evolution of ZnO microstructures from hexagonal disk to prismoid, prism and pyramid and their crystal facet-dependent gas sensing properties. CrystEngComm 2014, 16, 7062–7073. [Google Scholar] [CrossRef]
- Zhao, S.; Shen, Y.; Xia, Y.; Pan, A.; Li, Z.; Carraro, C. Maboudian, Synthesis and gas sensing properties of NiO/ZnO heterostructured nanowires. J. Alloys Compd. 2021, 877, 160189. [Google Scholar] [CrossRef]
- Xu, J.; Xue, Z.; Qin, N.; Cheng, Z.; Xiang, Q. The crystal facet-dependent gas sensing properties of ZnO nanosheets: Experimental and computational study. Sens. Actuators B Chem. 2017, 242, 148–157. [Google Scholar] [CrossRef]
- Zhou, T.; Chen, S.; Wang, X.; Xie, C.; Zeng, D. Catalytic activation of cobalt doping sites in ZIF-71-coated ZnO nanorod arrays for enhancing gas-sensing performance to acetone. ACS Appl. Mater. Interfaces 2020, 12, 48948–48956. [Google Scholar] [CrossRef]
- Nikolic, M.V.; Milovanovic, V.; Vasiljevic, Z.Z.; Stamenkovic, Z. Semiconductor gas sensors: Materials, technology, design, and application. Sensors 2020, 20, 6694. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, B.; Liu, J.; Pei, C.; Zhao, H.; Shang, Y.; Yang, H. Enhancing gas-sensing property and sensing mechanism at molecule level of the hollow microspheres assembled with ZnO nanoflakes exposing {001} facets. J. Mater. Sci. Mater. Electron. 2020, 31, 6118–6129. [Google Scholar] [CrossRef]
- Han, X.-G.; He, H.-Z.; Kuang, Q.; Zhou, X.; Zhang, X.-H.; Xu, T.; Xie, Z.-X.; Zheng, L.-S. Controlling morphologies and tuning the related properties of nano/microstructured ZnO crystallites. J. Phys. Chem. C 2009, 113, 584–589. [Google Scholar] [CrossRef]
- Ju, D.; Xu, H.; Zhang, J.; Guo, J.; Cao, B. Direct hydrothermal growth of ZnO nanosheets on electrode for ethanol sensing. Sens. Actuators B Chem. 2014, 201, 444–451. [Google Scholar] [CrossRef]
- Tian, H.; Fan, H.; Li, M.; Ma, L. Zeolitic imidazolate framework coated ZnO nanorods as molecular sieving to improve selectivity of formaldehyde gas sensor. ACS Sens. 2016, 1, 243–250. [Google Scholar] [CrossRef]
- Zhou, T.; Sang, Y.; Wang, X.; Wu, C.; Zeng, D.; Xie, C. Pore size dependent gas-sensing selectivity based on ZnO@ ZIF nanorod arrays. Sens. Actuators B Chem. 2018, 258, 1099–1106. [Google Scholar] [CrossRef]
- Kim, J.-H.; Mirzaei, A.; Kim, H.W.; Wu, P.; Kim, S.S. Design of supersensitive and selective ZnO-nanofiber-based sensors for H2 gas sensing by electron-beam irradiation. Sens. Actuators B Chem. 2019, 293, 210–223. [Google Scholar] [CrossRef]
- Jin, W.; Ma, S.; Tie, Z.; Xu, X.; Jiang, X.; Li, W.; Wang, T.; Lu, Y.; Yan, S. Synthesis of monodisperse ZnO hollow six-sided pyramids and their high gas-sensing properties. Mater. Lett. 2015, 159, 102–105. [Google Scholar] [CrossRef]
- Tang, C.; Spencer, M.J.; Barnard, A.S. Activity of ZnO polar surfaces: An insight from surface energies. Phys. Chem. Chem. Phys. 2014, 16, 22139–22144. [Google Scholar] [CrossRef] [PubMed]
- Degler, D.; Weimar, U.; Barsan, N. Current understanding of the fundamental mechanisms of doped and loaded semiconducting metal-oxide-based gas sensing materials. ACS Sens. 2019, 4, 2228–2249. [Google Scholar] [CrossRef]
- Blackman, C. Do We Need “Ionosorbed” Oxygen Species? (Or, “A Surface Conductivity Model of Gas Sensitivity in Metal Oxides Based on Variable Surface Oxygen Vacancy Concentration”). ACS Sens. 2021, 6, 3509–3516. [Google Scholar] [CrossRef]
- Hafner, J. Ab-initio simulations of materials using VASP: Density-functional theory and beyond. J. Comput. Chem. 2008, 29, 2044–2078. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef]
- ChemSpider Search and Share Chemistry. Available online: http://www.chemspider.com/ (accessed on 14 October 2022).
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, W.; Sanville, E.; Henkelman, G. A grid-based Bader analysis algorithm without lattice bias. J. Phys. Condens. Matter 2009, 21, 084204. [Google Scholar] [CrossRef] [PubMed]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Xia, Y.; Pan, A.; Su, Y.-Q.; Zhao, S.; Li, Z.; Davey, A.K.; Zhao, L.; Maboudian, R.; Carraro, C. In-situ synthesized N-doped ZnO for enhanced CO2 sensing: Experiments and DFT calculations. Sens. Actuators B Chem. 2022, 357, 131359. [Google Scholar] [CrossRef]
- Zhou, C.; Kang, J. Electronic structures of ZnO (0001)-Zn and (000−1)-O polar surfaces. J. Mater. Sci. Mater. Electron. 2008, 19, 229–233. [Google Scholar] [CrossRef]
- Göpel, W.; Pollmann, J.; Ivanov, I.; Reihl, B. Angle-resolved photoemission from polar and nonpolar zinc oxide surfaces. Phys. Rev. B 1982, 26, 3144. [Google Scholar] [CrossRef]
- Mora Fonz, D. A Theoretical Study on the Surfaces of Zinc Oxide. Ph.D. Thesis, University College London, London, UK, 2016. [Google Scholar]
- Zhang, J.; Zhang, Y.; Tse, K.; Deng, B.; Xu, H.; Zhu, J. New approaches for calculating absolute surface energies of wurtzite (0001)/(0001¯): A study of ZnO and GaN. J. Appl. Phys. 2016, 119, 205302. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.; Hong, Y.J.; Yoo, J.; Yi, G.C.; Park, G.S.; Kong, K.j.; Chang, H. Surface morphology and growth mechanism of catalyst-free ZnO and Mgx Zn1–x O nanorods. Phys. Status Solidi RRL 2008, 2, 197–199. [Google Scholar] [CrossRef]
- Na, S.-H.; Park, C.-H. First-principles study of the surface of wurtzite ZnO and ZnS-implications for nanostructure formation. J. Korean Phys. Soc. 2009, 54, 867–872. [Google Scholar] [CrossRef]
- Zhuang, H.; Tkalych, A.J.; Carter, E.A. Surface energy as a descriptor of catalytic activity. J. Phys. Chem. C 2016, 120, 23698–23706. [Google Scholar] [CrossRef]
- Johnson, R.D., III. NIST Computational Chemistry Comparison And Benchmark Database, NIST Standard Reference Database Number 101, Release 16a. Available online: http://cccbdb.nist.gov/ (accessed on 14 October 2022).
- Jin, W.; Chen, G.; Duan, X.; Yin, Y.; Ye, H.; Wang, D.; Yu, J.; Mei, X.; Wu, Y. Adsorption behavior of formaldehyde on ZnO (101¯ 0) surface: A first principles study. Appl. Surf. Sci. 2017, 423, 451–456. [Google Scholar] [CrossRef]
- Chiu, C.-Y.; Wu, H.; Yao, Z.; Zhou, F.; Zhang, H.; Ozolins, V.; Huang, Y. Facet-selective adsorption on noble metal crystals guided by electrostatic potential surfaces of aromatic molecules. J. Am. Chem. Soc. 2013, 135, 15489–15500. [Google Scholar] [CrossRef] [PubMed]
- Haynes, W.M.; Lide, D.R.; Bruno, T.J. CRC Handbook of Chemistry and Physics; CRC press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Fang, C.; Orhan, E.; De Wijs, G.; Hintzen, H.; De Groot, R.; Marchand, R.; Saillard, J.-Y. The electronic structure of tantalum (oxy) nitrides TaON and Ta3N5. J. Mater. Chem. 2001, 11, 1248–1252. [Google Scholar] [CrossRef] [Green Version]
- Richter, C. Electron and Exciton Dynamics at ZnO Surfaces. Master’s Thesis, Freie Universität Berlin, Berlin, Germany, 2014. [Google Scholar]
- Deinert, J.-C. Zinc Oxide Surfaces and Interfaces: Electronic Structure and Dynamics of Excited States. Ph.D. Thesis, Technischen Universität Berlin, Berlin, Germany, 2016. [Google Scholar]
- Deinert, J.-C.; Hofmann, O.T.; Meyer, M.; Rinke, P.; Stähler, J. Local aspects of hydrogen-induced metallization of the ZnO (10 1¯0) surface. Phys. Rev. B 2015, 91, 235313. [Google Scholar] [CrossRef]
- Hoffmann, R. Solids and Surfaces: A Chemist’s View of Bonding in Extended Structures; John Wiley & Sons: Hoboken, NJ, USA, 2021. [Google Scholar]
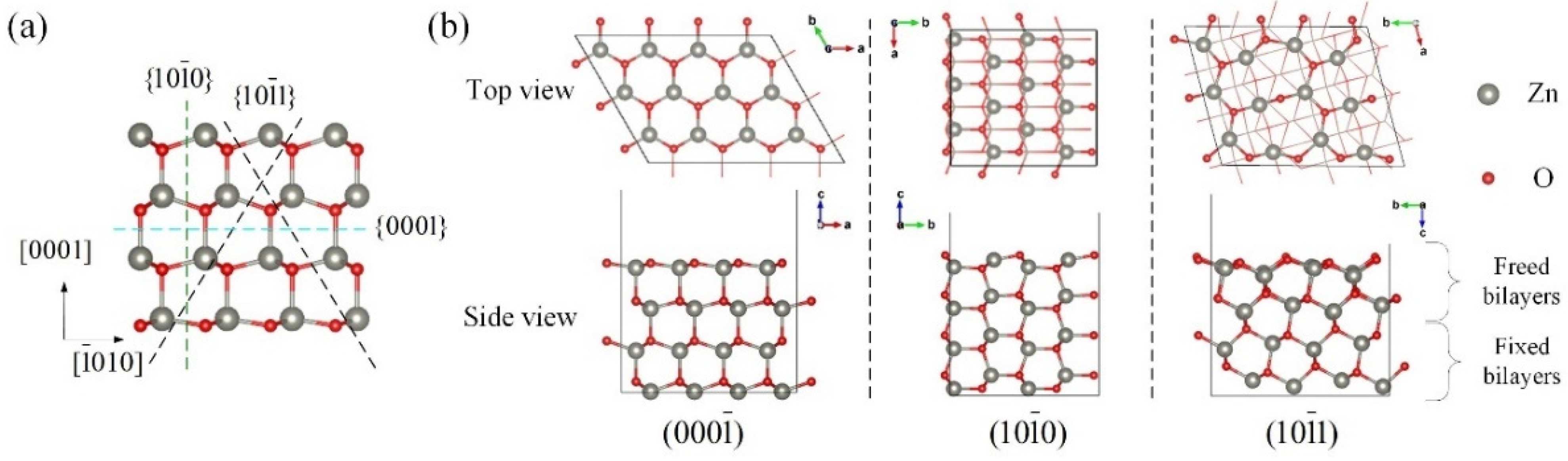
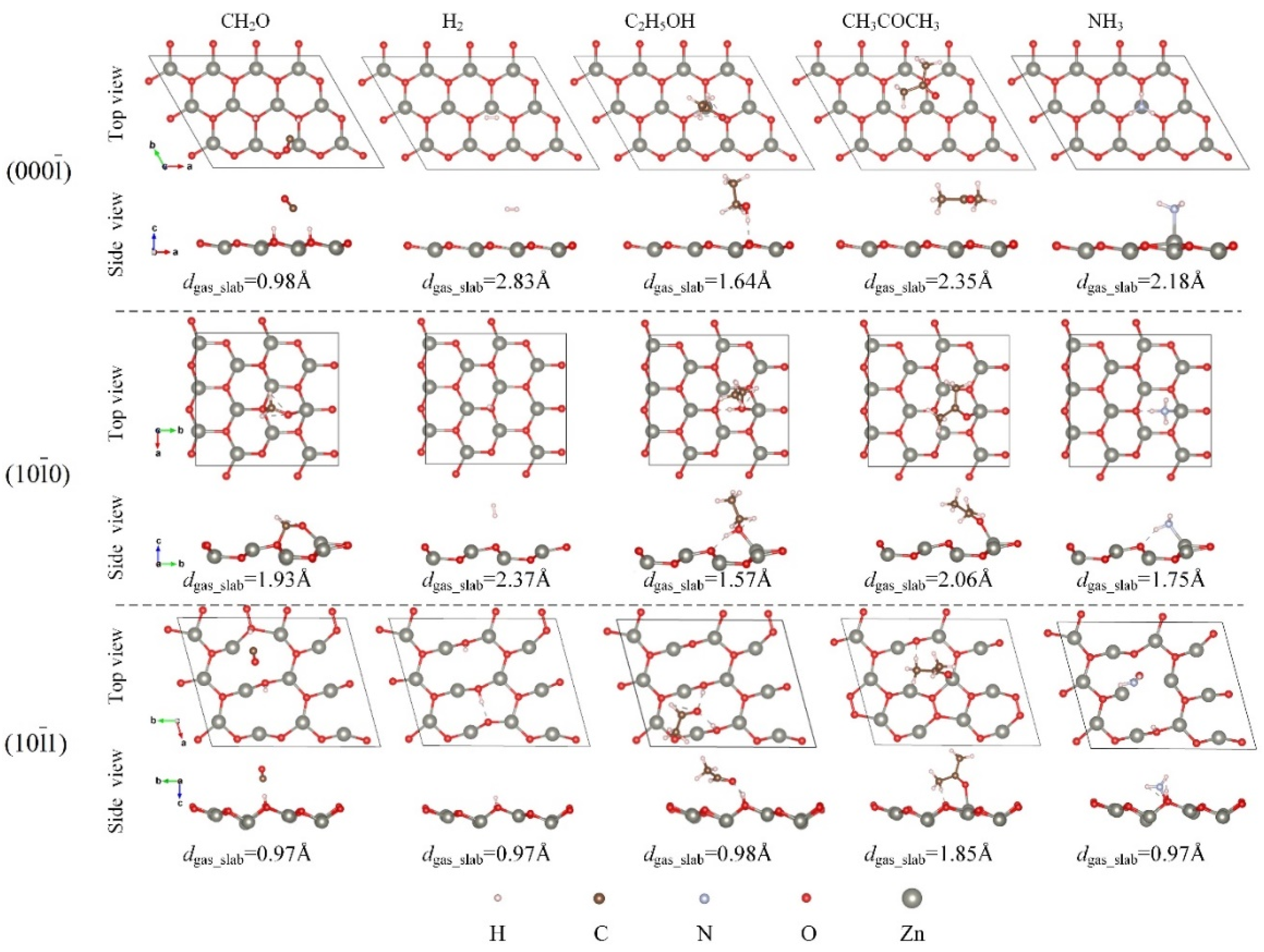
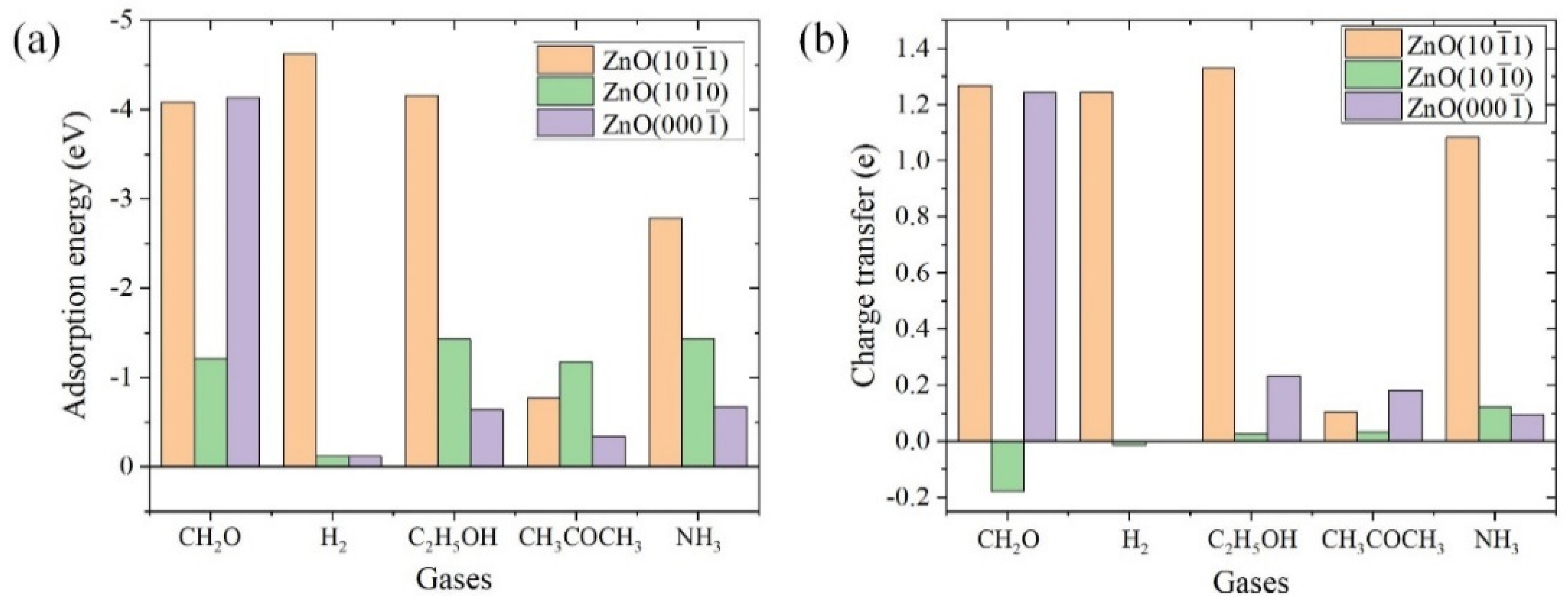

| Surface | Atoms | System Energy (eV) | Coordination Number of O on the 1st Bilayer | Coordination Number of Zn on the 1st Bilayer | Surface Energy (J/m2) | |
|---|---|---|---|---|---|---|
| This Work | Ref. | |||||
| Zn48O48 | −406.12 | 3 | 4 | 1.39 | 1.01 [39]; 0.96 [25] | |
| Zn48O48 | −415.41 | 4, 3 | 4, 3 | 0.87 | 0.91 [40]; 0.82 [39]; 1.12 [41] | |
| Zn48O48 | −395.93 | 3, 2 | 4 | 2.00 | 1.74 [40] | |
| ZnO Facets | Calculated Adsorption Selectivity | Gas Sensing Selectivity by Experiments |
|---|---|---|
| CH2O 1 > NH3, C2H5OH > CH3COCH3 > H2 | C2H5OH (s) 2 [13,15,20], C2H5OH, CH2O (s) [21] | |
| NH3, C2H5OH > CH2O > CH3COCH3 > H2 | CH2O (p) [2]; C2H5OH (s) [13,15,20]; C2H5OH, CH3COCH3 (s) [22]; C2H5OH, CH2O (s) [21]; NH3 (s) [10] | |
| H2 > C2H5OH > CH2O > NH3 > CH3COCH3 | CH2O (p) [2]; C2H5OH (s) [13,24]; NH3 (s) [10]; H2 (p) [23] |
| Gases | CH3COCH3 | CH2O | C2H5OH | NH3 | H2 |
|---|---|---|---|---|---|
| Dipole moment (Debye) | 2.88 | 2.33 | 1.52 | 1.48 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, W.; Xia, Y.; Pan, A.; Luo, Y.; Su, Y.; Zhao, S.; Wang, T.; Zhao, L. Facet-Dependent Gas Adsorption Selectivity on ZnO: A DFT Study. Chemosensors 2022, 10, 436. https://doi.org/10.3390/chemosensors10100436
Jiang W, Xia Y, Pan A, Luo Y, Su Y, Zhao S, Wang T, Zhao L. Facet-Dependent Gas Adsorption Selectivity on ZnO: A DFT Study. Chemosensors. 2022; 10(10):436. https://doi.org/10.3390/chemosensors10100436
Chicago/Turabian StyleJiang, Weile, Yong Xia, Aifei Pan, Yunyun Luo, Yaqiong Su, Sikai Zhao, Tao Wang, and Libo Zhao. 2022. "Facet-Dependent Gas Adsorption Selectivity on ZnO: A DFT Study" Chemosensors 10, no. 10: 436. https://doi.org/10.3390/chemosensors10100436